Wildlife and Bait Density Monitoring to Describe the Effectiveness of a Rabies Vaccination Program in Foxes
Abstract
1. Introduction and Aims of the Work
2. Study Area Overview
3. Methods
3.1. Bait Delivery
3.2. Fox Monitoring and Transect Fox Density
3.3. Identification of Critical Areas of Insufficient Bait Density
4. Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hankins, D.G.; Rosekrans, J.A. Overview, Prevention, and Treatment of Rabies. Mayo Clin. Proc. 2004, 79, 671–676. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Coleman, P.G.; Fèvre, E.M.; Cleaveland, S. Estimating the public health impact of rabies. Emerg. Infect. Dis. 2004, 10, 140. [Google Scholar] [CrossRef]
- Cochi, S.L.; Dowdle, W.R. Disease Eradication in the 21st Century: Implications for Global Health; MIT Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Wandeler, A.I. The rabies situation in Western Europe. Dev. Biol. 2008, 131, 19–25. [Google Scholar]
- Steck, F.; Wandeler, A.I.; Bichsel, P.; Capt, S.; Schneider, L.G. Oral immunisation of foxes against rabies. Zent. Vet. Reihe B 1982, 29, 372–396. [Google Scholar]
- Wandeler, A.I.; Capt, S.; Hauser, R.; Kappeler, A. Oral Immunization of Wildlife Against Rabies: Concept and First Field Experiments. Clin. Infect. Dis. 1988, 10, S649–S653. [Google Scholar] [CrossRef]
- Johnston, D.H.; Voigt, D.R.; MacInnes, C.D.; Bachmann, P.; Lawson, K.F.; Rupprecht, C.E. An Aerial Baiting System for the Distribution of Attenuated or Recombinant Rabies Vaccines for Foxes, Raccoons, and Skunks. Clin. Infect. Dis. 1988, 10, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Freuling, C.M.; Hampson, K.; Selhorst, T.; Schröder, R.; Meslin, F.X.; Mettenleiter, T.C.; Müller, T. The elimination of fox rabies from Europe: Determinants of success and lessons for the future. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120142. [Google Scholar] [CrossRef] [PubMed]
- Mulatti, P.; Ferrè, N.; Patregnani, T.; Bonfanti, L.; Marangon, S. Geographical information systems in the management of the 2009-2010 emergency oral anti-rabies vaccination of foxes in north-eastern Italy. Geospat. Health 2011, 5, 217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henning, J.; Giorgi, E.; Magalhães, R.J.S.; Tizzani, P.; Viviani, P.; Pejovic, N.; Hrapović, M.; Potzsch, C. Factors influencing the success of aerial rabies vaccination of foxes. Sci. Rep. 2017, 7, 14376. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The oral vaccination of foxes against rabies. In Report of the Scientific Committee on Animal Health and Animal Welfare; European Commission: Bruxelles, Belgium, 23 October 2002; Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scah_out80_en.pdf (accessed on 20 February 2020).
- Neff, D.J. The Pellet-Group Count Technique for Big Game Trend, Census, and Distribution: A Review. J. Wildl. Manag. 1968, 32, 597. [Google Scholar] [CrossRef]
- Langbein, J.; Hutchings, M.; Harris, S.; Stoate, C.; Tapper, S.C.; Wray, S. Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mammal Rev. 1999, 29, 93–116. [Google Scholar] [CrossRef]
- Tizzani, P.; Menzano, A.; Dematteis, A.; Meneguz, P.G. Methodological problems related to spotlight count as a census technique for Lepus europaeus in an alpine environment. Acta Theriol. 2014, 59, 271276. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001; p. 448. [Google Scholar]
- Thomas, L.; Buckland, S.T.; Rexstad, E.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project, 2011. Available online: http://qgis.osgeo.org (accessed on 1 November 2011).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2012; Available online: http://www.R-project.org/ (accessed on 1 September 2012).
- Morters, M.K.; Restif, O.; Hampson, K.; Cleaveland, S.; Wood, J.L.; Conlan, A.J. Evidence-based control of a canine rabies: A critical review of population density reduction. J. Anim. Ecol. 2013, 82, 6–14. [Google Scholar] [CrossRef]
- Mainguy, J.; Rees, E.E.; Canac-Marquis, P.; Bélanger, D.; Fehlner-Gardiner, C.; Séguin, G.; Larrat, S.; Lair, S.; Landry, F.; Côté, N. Oral rabies vaccination of raccoons and striped skunks with 430 ONRAB® baits: Multiple factors influence field immunogenicity. J. Wildl. Dis. 2012, 48, 979–990. [Google Scholar] [CrossRef]
- Smith, G.C.; Wilkinson, D. Modeling control of rabies outbreaks in red fox populations to evaluate culling, vaccination, and vaccination combined with fertility control. J. Wildl. Dis. 2003, 39, 278–286. [Google Scholar] [CrossRef]
- Curtis, A. Using a spatial filter and a geographic information system to improve rabies surveillance data. Emerg. Infect. Dis. 1999, 5, 603–606. [Google Scholar] [CrossRef]
- Bright, P.R.; Schmidt, E.; Elvinger, F.; Weisbarth, M.M.; Bulley, R.F.; Mills, C.L. GIS use in oral rabies vaccine programs. In Proceedings of the Wildlife Damage Management Conferences, State College, PA, USA, 5–10 October 2000; p. 23. [Google Scholar]
- Ramey, C.A.; Mills, K.H.; Robin, M. Overview of the First Use of GPS 1991/GIS 1992 during a Wyoming Skunk Rabies Epizootic; USDA National Wildlife Research Center—Staff Publications: Chicago, IL, USA, January 2007; Paper 771. [Google Scholar]
- Pfeiffer, D.U.; Hugh-Jones, M. Geographical information systems as a tool in epidemiological assessment and wildlife disease management. Rev. Sci. Tech. de l’OIE 2002, 21, 91–102. [Google Scholar] [CrossRef]
- Dzwiki, A.A.; Abbas, I.I. Mapping the Spatial Distribution of Rabies in Kaduna State, Nigeria (1999–2009) Using Geographic Information Systems Technology. Environ. Nat. Resour. J. 2012, 2, 24–31. [Google Scholar]
- Blanton, J.D.; Manangan, A.; Manangan, J.; Hanlon, C.A.; Slate, D.; Rupprecht, C.E. Development of a GIS-based, real-time Internet mapping tool for rabies surveillance. Int. J. Health Geogr. 2006, 5, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Webbon, C.C.; Baker, P.J.; Harris, S. Faecal density counts for monitoring changes in red fox numbers in rural Britain. J. Appl. Ecol. 2004, 41, 768–779. [Google Scholar] [CrossRef]
- Page, R.J.C. Dispersal and population density of the fox (Vulpes vulpes) in an area of London. J. Zool. 1981, 194, 485–491. [Google Scholar] [CrossRef]
- Panek, M.; Bresiński, W. Red foxVulpes vulpes density and habitat use in a rural area of western Poland in the end of 1990s, compared with the turn of 1970s. Acta Thériol. 2002, 47, 433–442. [Google Scholar] [CrossRef]
- Gołdyn, B.; Hromada, M.; Surmacki, A.; Tryjanowski, P. Habitat use and diet of the red foxVulpes vulpes in an agricultural landscape in Poland. Eur. J. Wildl. Res. 2003, 49, 191–200. [Google Scholar] [CrossRef]
- Coman, B.; Robinson, J.; Beaumont, C. Home Range, Dispersal and Density of Red Foxes (Vulpes vulpes L.) In Central Victoria. Wildl. Res. 1991, 18, 215. [Google Scholar] [CrossRef]
- NIRAS IC Consortium. EU-IPA Project “Support for the Control and Eradication of Rabies and Classical Swine Fever in Montenegro—EuropeAid/128207/C/SER/ME”; Final Report; NIRAS IC Consortium: Podgorica, Montenegro, April 2013. [Google Scholar]
- Standing Group of Experts on Rabies in Europe under the GF-TADs Umbrella. First Meeting (SGE RAB1) Brussels, Belgium, 13 February 2019. Available online: https://web.oie.int/RR-Europe/eng/Regprog/docs/docs/SGE%20RAB1/SGE_RAB1_report.pdf (accessed on 1 February 2020).
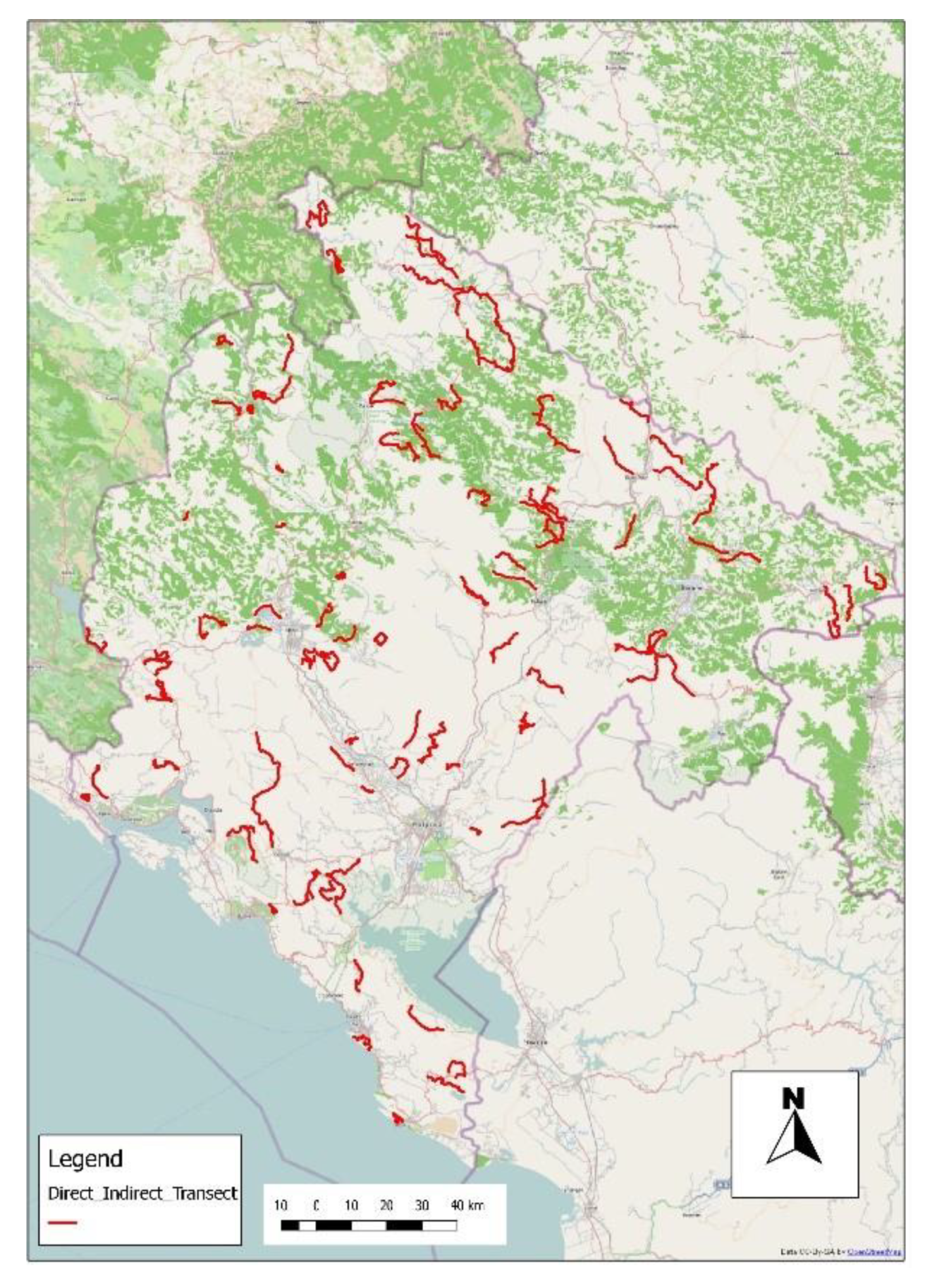
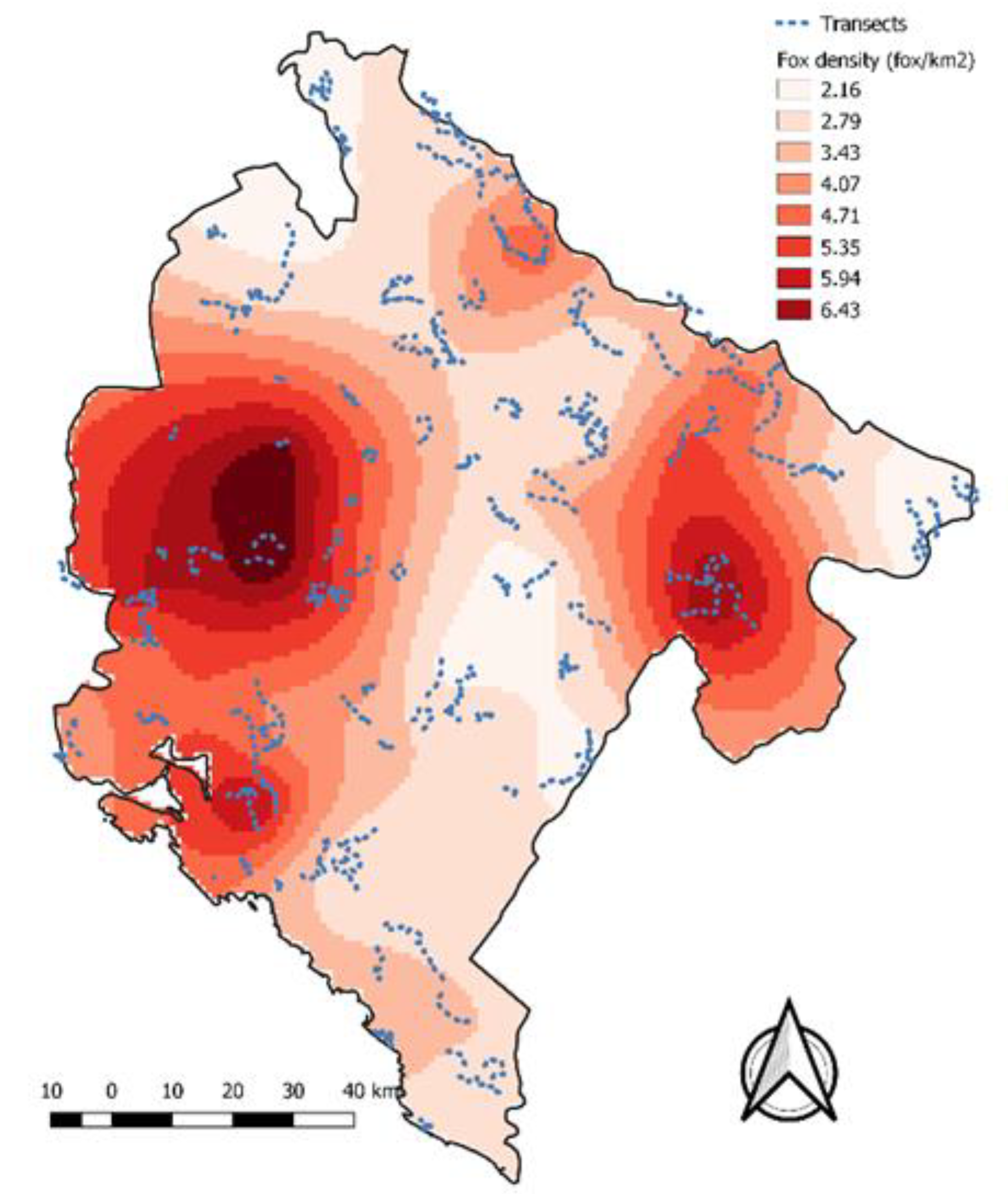
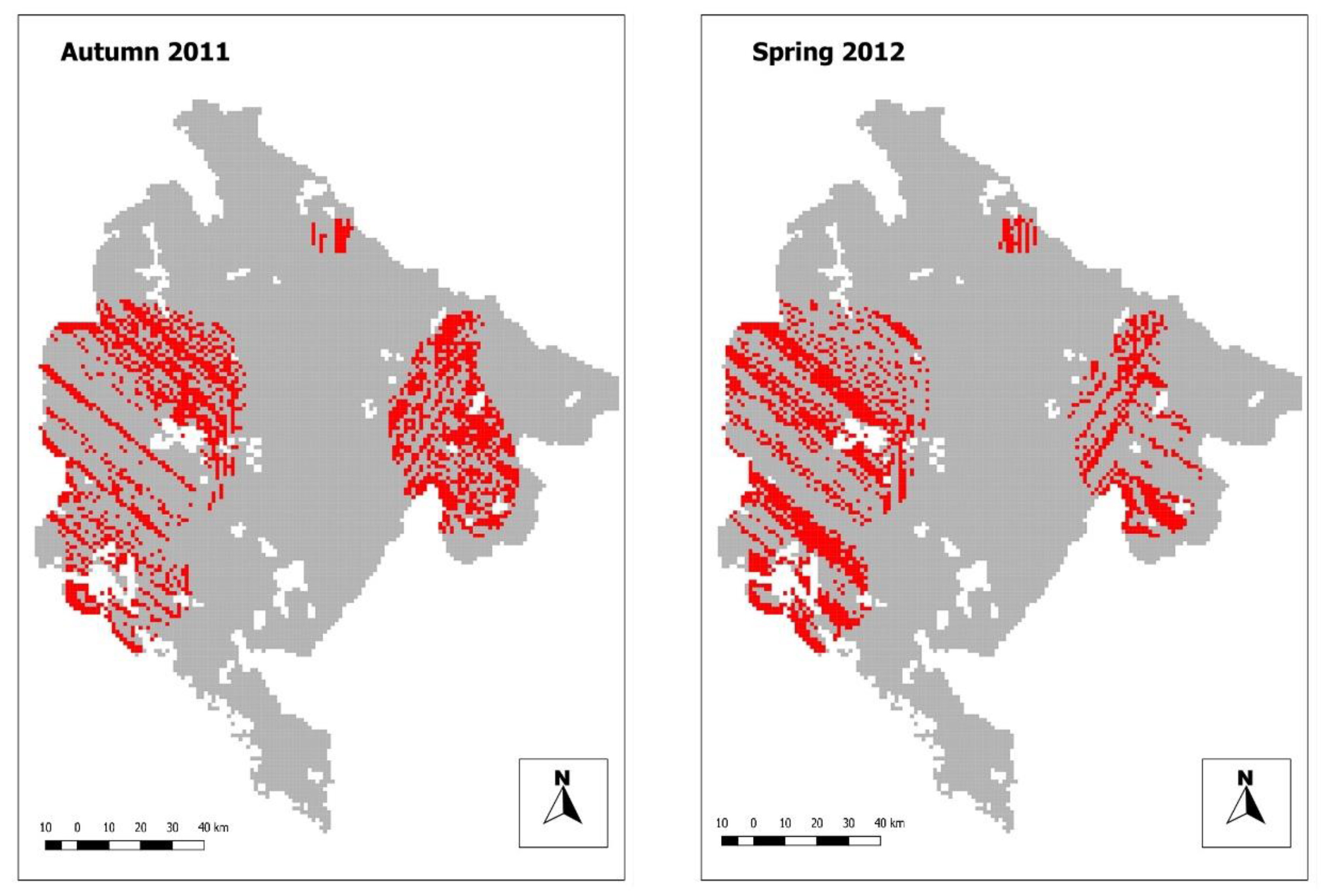
| Bait Density | Autumn 2011 | Spring 2012 | Combined 2011 and 2012 |
|---|---|---|---|
| Mean (IC 95%) | 19.94 (19.78–20.10) | 20.04 (19.89–20.18) | 39.98 (39.74–40.21) |
| Coverage (lower than 20 baits/km2) | 47.3% | 45.02% | 6.03% |
| Coverage (lower than 15 baits/km2) | 20.3% | 17.2% | 4.8% |
| Coverage (lower than 10 baits/km2) | 10.4% | 7.3% | 3.9% |
| Coverage (lower than 5 baits/km2) | 5.9% | 4.8% | 3.3% |
| Density | Indirect Census | Direct Census | Overall from Both Methods |
|---|---|---|---|
| Minimum | 0 | 0 | 0 |
| Maximum | 14.30 | 11.20 | 14.30 |
| Mean | 3.57 | 3.77 | 3.65 |
| Median | 2.30 | 3.25 | 3.0 |
| SD | 2.87 | 2.58 | 2.75 |
| Fox Density | km2 | Percentage |
|---|---|---|
| 0 to 2 | 725 | 5.6 |
| 2 to 4 | 7502 | 58.4 |
| 4 to 6 | 3976 | 31.0 |
| More than 6 | 639 | 5.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tizzani, P.; Fanelli, A.; Potzsch, C.; Henning, J.; Šašić, S.; Viviani, P.; Hrapović, M. Wildlife and Bait Density Monitoring to Describe the Effectiveness of a Rabies Vaccination Program in Foxes. Trop. Med. Infect. Dis. 2020, 5, 32. https://doi.org/10.3390/tropicalmed5010032
Tizzani P, Fanelli A, Potzsch C, Henning J, Šašić S, Viviani P, Hrapović M. Wildlife and Bait Density Monitoring to Describe the Effectiveness of a Rabies Vaccination Program in Foxes. Tropical Medicine and Infectious Disease. 2020; 5(1):32. https://doi.org/10.3390/tropicalmed5010032
Chicago/Turabian StyleTizzani, Paolo, Angela Fanelli, Carsten Potzsch, Joerg Henning, Srdjan Šašić, Paolo Viviani, and Mevlida Hrapović. 2020. "Wildlife and Bait Density Monitoring to Describe the Effectiveness of a Rabies Vaccination Program in Foxes" Tropical Medicine and Infectious Disease 5, no. 1: 32. https://doi.org/10.3390/tropicalmed5010032
APA StyleTizzani, P., Fanelli, A., Potzsch, C., Henning, J., Šašić, S., Viviani, P., & Hrapović, M. (2020). Wildlife and Bait Density Monitoring to Describe the Effectiveness of a Rabies Vaccination Program in Foxes. Tropical Medicine and Infectious Disease, 5(1), 32. https://doi.org/10.3390/tropicalmed5010032






