Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole
Abstract
1. Introduction
2. A Strategy to Eliminate HAT
2.1. Global Alliances and Innovative Partnerships
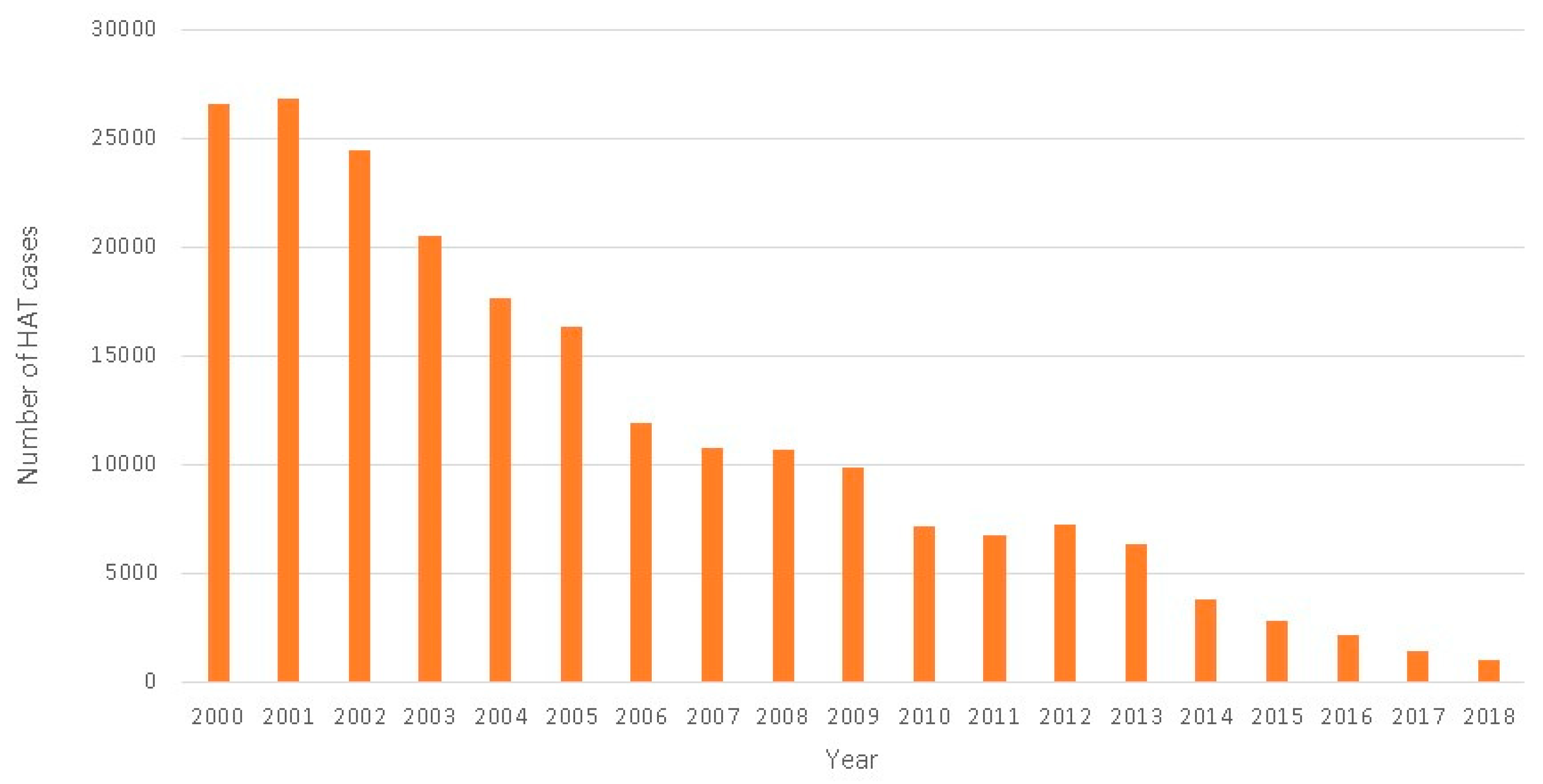
2.2. The Success of Collaborative Strategies for HAT Elimination
2.3. The Need for New Treatment Options
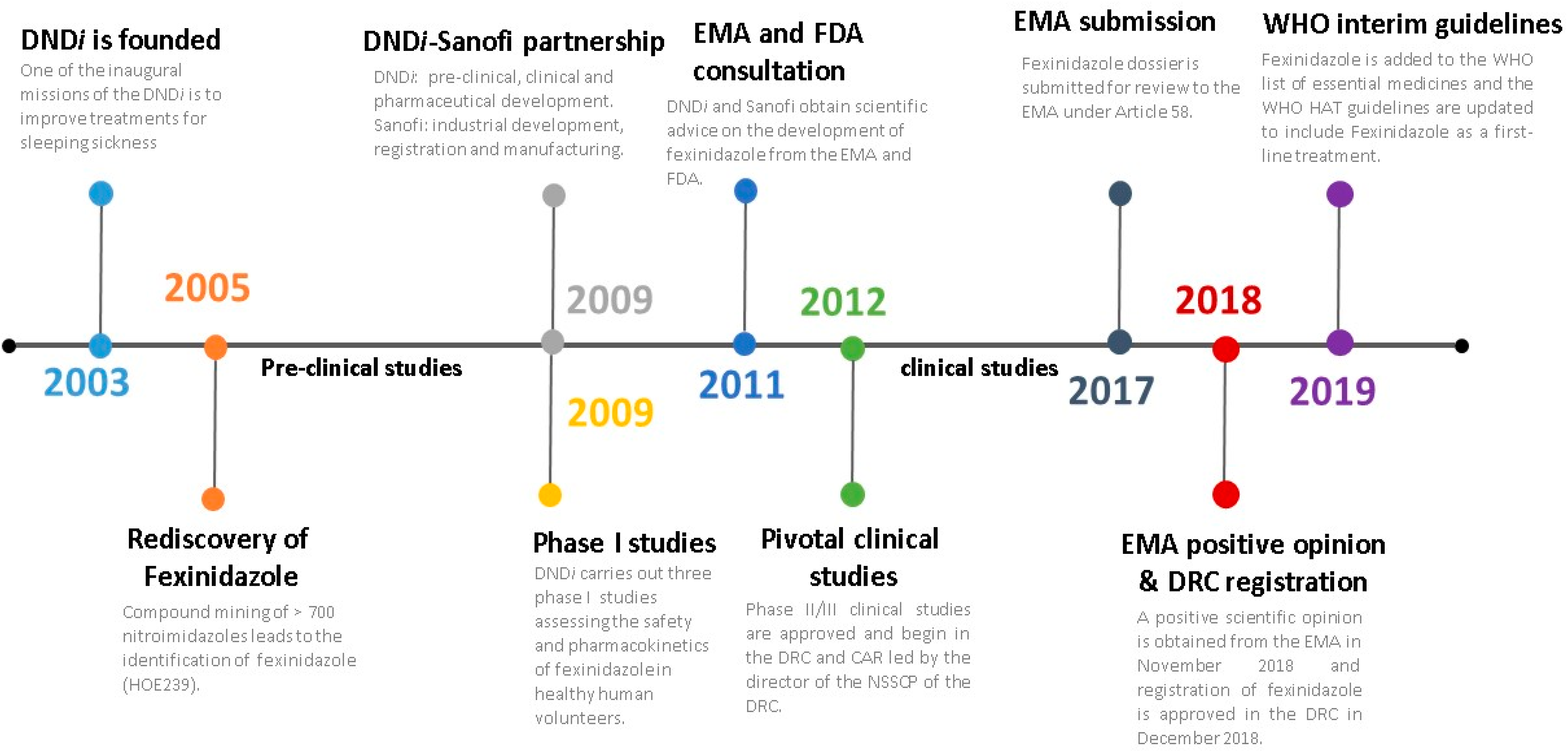
3. The Development of a New Oral Treatment for HAT: The Story of Fexinidazole
3.1. The Rediscovery of Fexinidazole
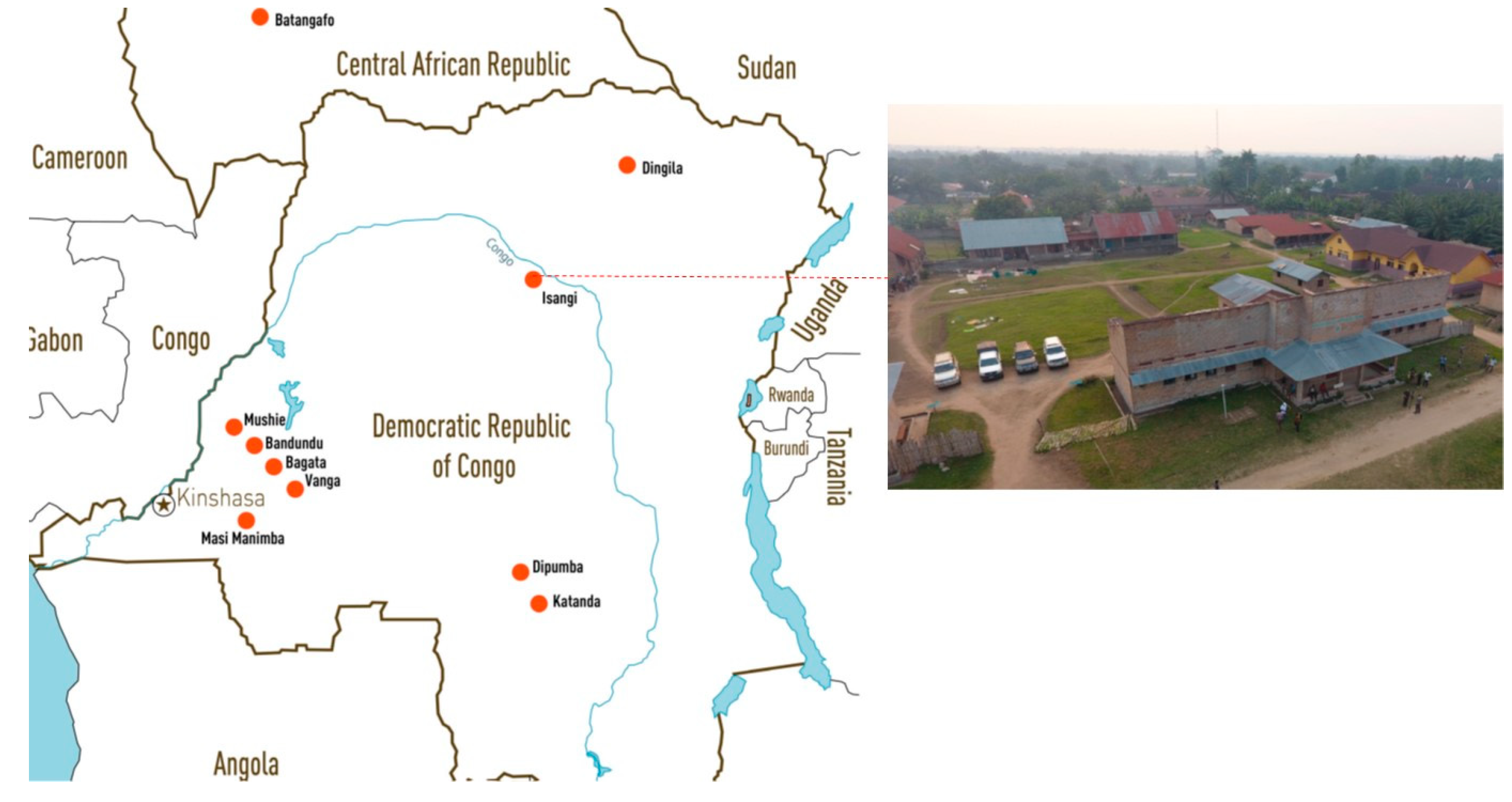
3.2. Clinical Trials
3.3. A Positive Opinion from the European Medicines Agency
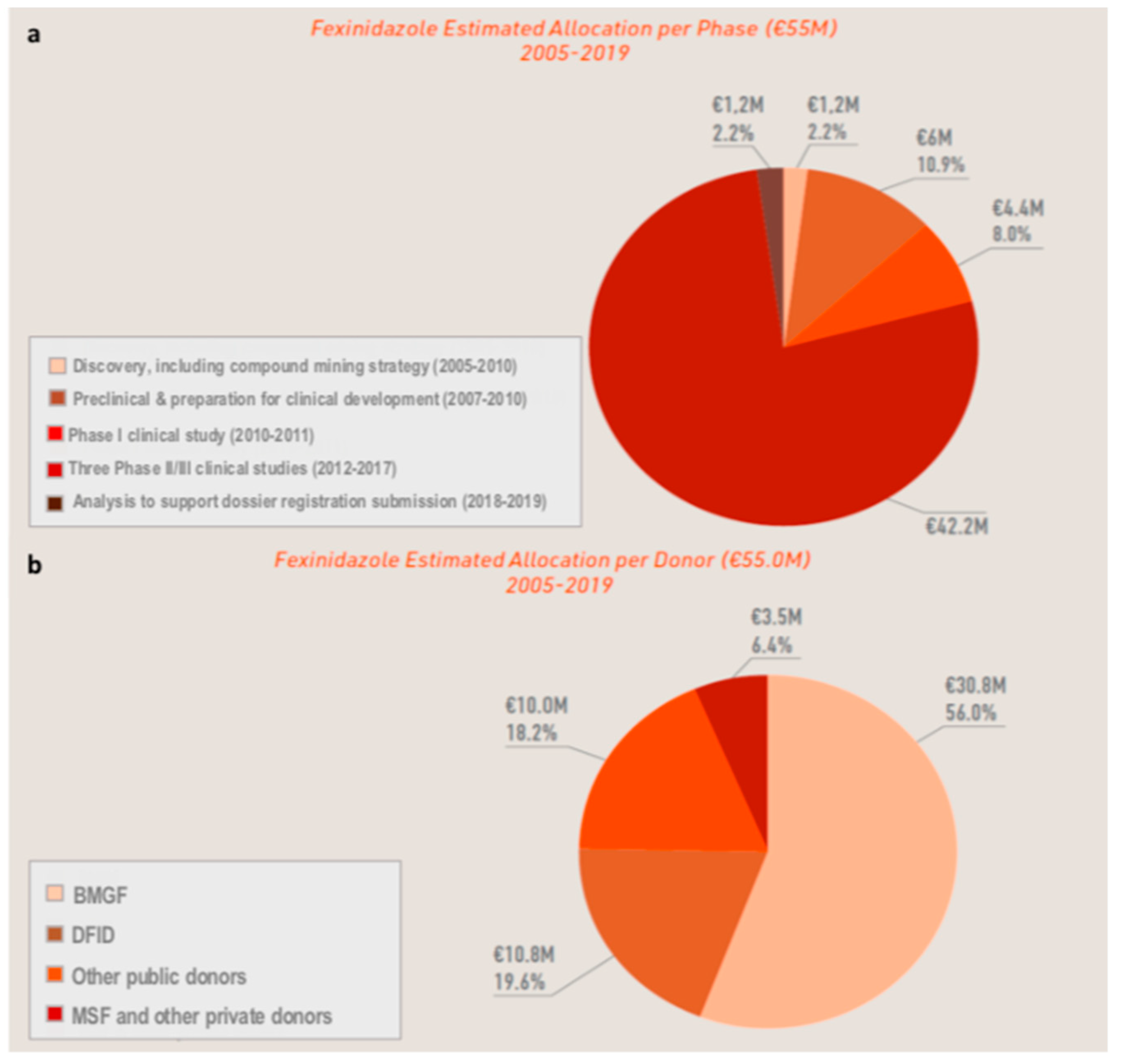
3.4. The Cost of Developing a New Chemical Entity
4. Conclusions
Main Partners
Author Contributions
Funding
Conflicts of Interest
References
- Drugs for Neglected Diseases Initiative. About Sleeping Sickness. Available online: https://www.dndi.org/diseases-projects/hat/ (accessed on 11 November 2019).
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Simarro, P.P.; Zhao, W.; Argaw, D. Monitoring the elimination of human African trypanosomiasis: Update to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006890. [Google Scholar] [CrossRef]
- Cecchi, G.; Paone, M.; Franco, J.R.; Fevre, E.M.; Diarra, A.; Ruiz, J.A.; Mattioli, R.C.; Simarro, P.P. Towards the Atlas of human African trypanosomiasis. Int. J. Health Geogr. 2009, 8, 15. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- World Health Organization. WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis. 2019. Available online: https://www.who.int/trypanosomiasis_african/resources/9789241550567/en/ (accessed on 17 December 2019).
- Checchi, F.; Funk, S.; Chandramohan, D.; Haydon, D.T.; Chappuis, F. Updated estimate of the duration of the meningo-encephalitic stage in gambiense human African trypanosomiasis. BMC Res. Notes 2015, 8, 292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHO. Expert Committee on the Control and Surveillance of African Trypanosomiasis & World Health Organization. In Control and Surveillance of African Trypanosomiasis: Report of A WHO Expert Committee; WHO technical report series; World Health Organization: Geneva, Switzerland, 1998; p. 881. [Google Scholar]
- World Health Organization. Resolutions and decisions, annexes. In Proceedings of the Fiftieth World Health Assembly, Geneva, Switzerland, 5–14 May 1997. Available online: https://apps.who.int/iris/handle/10665/179638 (accessed on 17 December 2019).
- World Health Organization. WHO Programme to Eliminate Sleeping Sickness: Building A Global Alliance 2002. Available online: https://www.who.int/trypanosomiasis_african/resources/who_cds_csr_eph_2002.13/en/ (accessed on 17 December 2019).
- World Health Organisation. WHO HAT Elimination Partners: Donors. Available online: https://www.who.int/trypanosomiasis_african/partners/partners_donors/en/ (accessed on 11 November 2019).
- World Health Organisation. London Declaration on Neglected Tropical Diseases. Available online: https://www.who.int/neglected_diseases/London_Declaration_NTDs.pdf (accessed on 11 November 2019).
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Mattioli, R.C.; Argaw, D. Monitoring the elimination of human African trypanosomiasis: Update to 2014. PLoS Negl. Trop. Dis. 2017, 11, e0005585. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.A.; Fevre, E.M.; Mattioli, R.C.; Jannin, J.G. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl. Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- SANOFI. Fighting Negleted Tropical Diseases Factsheet. Available online: https://www.sanofi.com/.../Fighting_Neglected_Tropical_Diseases_2018.pdf (accessed on 11 November 2019).
- World Health Organisation. Global Health Observatory Data Repository, Human African Trypanosomiasis. Available online: http://apps.who.int/gho/data/node.main.A1635 (accessed on 25 November 2019).
- World Health Organization. Control and Surveillance of Human African Trypanosomiasis: Report of A WHO Expert Committee; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2013; pp. 1–237. [Google Scholar]
- Barrett, M.P. The elimination of human African trypanosomiasis is in sight: Report from the third WHO stakeholders meeting on elimination of gambiense human African trypanosomiasis. PLoS Negl. Trop. Dis. 2018, 12, e0006925. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, F. Oral fexinidazole for human African trypanosomiasis. Lancet 2018, 391, 100–102. [Google Scholar] [CrossRef]
- Tong, J.; Valverde, O.; Mahoudeau, C.; Yun, O.; Chappuis, F. Challenges of controlling sleeping sickness in areas of violent conflict: Experience in the Democratic Republic of Congo. Confl. Health 2011, 5, 7. [Google Scholar] [CrossRef]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Gharashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtomox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374. [Google Scholar] [CrossRef]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Neglected Diseases Initiative. NECT Dossier. Available online: https://www.dndi.org/achievements/nect/ (accessed on 11 November 2019).
- Sanofi. Summary of Product Characteristics: Fexinidazole Winthrop 600 mg Tablets; Sanofi-Aventis Groupe: Paris, France, 2019. [Google Scholar]
- Drugs for Neglected Diseases Initiative. Target Product Profile—Sleeping Sickness. Available online: https://www.dndi.org/diseases-projects/hat/hat-target-product-profile/ (accessed on 11 November 2019).
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Fexinidazole: First Global Approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- Deeks, E.D.; Lyseng-Williamson, K.A. Fexinidazole in human African trypanosomiasis: A profile of its use. Drugs Ther. Perspect. 2019, 35, 529–535. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative. Human African Trypanosomiasis (HAT) Platform. Available online: https://www.dndi.org/strengthening-capacity/hat-platform/ (accessed on 11 November 2019).
- Raether, W.; Hanel, H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 2003, 90 (Suppl. S1), S19–S39. [Google Scholar] [CrossRef]
- Sanofi. Sleeping Sickness: Providing Hope for the Forgotten. Connectome 2011, 2, 5. [Google Scholar]
- Hänel, H. (Sanofi-Aventis Deutschland, Frankfurt am Main, Germany). Personal Communication, 2019.
- Jennings, F.W.; Urquhart, G.M. The use of the 2 substituted 5-nitroimidazole, fexinidazole (Hoe 239) in the treatment of chronic T. brucei infections in mice. Zeitschrift für Parasitenkunde 1983, 69, 577–581. [Google Scholar] [CrossRef]
- Raether, W.; Seidenath, H. The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 1983, 77, 13–26. [Google Scholar] [CrossRef]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazue, G.; Bray, M.A.; Pecoul, B. Fexinidazole—A new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative. European Medicines Agency Recommends Fexinidazole, the First All-Oral Treatment for Sleeping Sickness; [press release]; Drugs for Neglected Diseases Initiative: Geneva, Switzerland, 2018. [Google Scholar]
- Drugs for Neglected Diseases Initiative. Clinical Trials. Available online: https://www.dndi.org/category/clinical-trials/ (accessed on 11 November 2019).
- European Medicines Agency Assessment Report: Fexinidazole Winthrop 2018; European Medicines Agency: London, UK, 2018.
- Mesu, V.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.F.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative. Achievements: Fexinidazole. Available online: https://www.dndi.org/achievements/fexinidazole/ (accessed on 11 November 2019).
- Pelfrene, E.; Harvey Allchurch, M.; Ntamabyaliro, N.; Nambasa, V.; Ventura, F.V.; Nagercoil, N.; Cavaleri, M. The European Medicines Agency’s scientific opinion on oral fexinidazole for human African trypanosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007381. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Neglected Diseases Initiative. Fexinidazole, the First All-Oral Treatment for Sleeping Sickness, Approved in Democratic Republic of Congo; [press release]; Drugs for Neglected Diseases Initiative: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. WHO Model Lists of Essential Medicines, 21st List 2019. Available online: https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 17 December 2019).
- Drugs for Neglected Diseases Initiative. Acoziborole. Available online: https://www.dndi.org/diseases-projects/portfolio/acoziborole/ (accessed on 18 December 2019).

| HAT Stage | Drug Name (Marketing Date) | Route, Dose | Comments |
|---|---|---|---|
| Stage 1 | Pentamidine (1940) | Single IM dose of 4 mg/kg/day for 7 days (preferred) or IV injections | Skilled health workers, adverse events (metabolic disorders, pancreatitis, local abscesses), heavy treatment burden (for patients and families), parasite resistance. Effective in stage-1 g-HAT only |
| Suramin (1920s) | Test dose then weekly IV injections for 5 weeks | Primarily for stage-1 r-HAT rarely used for g-HAT, adverse events (anaphylactic reactions, renal toxicity), heavy treatment burden (for patients and families) | |
| Stage 2 | NECT (2009) | Nifurtimox: 5 mg/kg PO every 8 h (15 mg/kg/day) for 10 days. Eflornithine: 200 mg/kg/day IV infusion every 12 h (400 mg/kg/day) for 7 days | Systematic hospitalization, skilled health workers, logistical concerns. First-line treatment for stage 2 g-HAT |
| Eflornithine or DFMO (1990) | 100 mg/kg/day IV infusion every 6 h (400 mg/kg/day) for 14 days | Systematic hospitalization, skilled health workers, long therapy, heavy treatment burden (for patients and families). Mainly used as a second-line treatment for stage-2 g-HAT | |
| Melarsoprol (1949) | 2.2 mg/kg/day slow IV injection for 10 days | Highly toxic, painful, parasite resistance, skilled health workers. Restricted to cases refractory to NECT for stage-2 g-HAT. Remains the only drug effective in stage-2 r-HAT |
| Study Name, Designation, Phase and Duration | Study Description | Patients | Key Results |
|---|---|---|---|
| DNDiFEX004 NCT01685827 (II/III: 2012–2015) | Efficacy and safety of fexinidazole compared to NECT in patients (aged ≥ 15) with late stage 2 g-HAT: a pivotal, non-inferiority, multicenter, randomized, open-label study | 394 (264 fexinidazole; 130 NECT) | Study confirms the efficacy of fexinidazole in late stage 2 g-HAT patients: success rate at 18 months post-treatment of 91.2% for fexinidazole, versus 97.6% for NECT within the margin of acceptable difference (97.06% CI −11.2 to −1.6; p = 0.0029). [18,39] |
| DNDiFEX005 NCT02169557 (II/III: 2014–2017) | Efficacy and safety of fexinidazole in patients (aged ≥ 15) with stage 1 or early stage 2 g-HAT: a prospective, multicenter, open-label and single arm cohort study, plug-in to the pivotal study | 230 | Studies confirm the efficacy of fexinidazole in stage-1 and early-stage-2 g-HAT patients: success rates in adults of 98.7% (95% CI [96.2%–99.7%]) and in children of 97.6% (95% CI [93.1%–99.5%] at 12 months post-treatment. [24] |
| DNDiFEX006 NCT02184689 (II/III: 2014–2017) | Efficacy and safety of fexinidazole in children (≥6 years and <15; ≥20 kg) with g-HAT of any stage: a prospective, multicenter, open-label and single arm cohort study, plug-in to the pivotal study | 125 | |
| DNDiFEX09HAT NCT03025789\(IIIb:2016–ongoing) | An open-label study assessing effectiveness, safety and compliance with fexinidazole in patients with g-HAT at any stage | 174 | Follow up ongoing [37] |
| Infrastructure |
|
| Supply of Equipment |
|
| Training of Staff |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neau, P.; Hänel, H.; Lameyre, V.; Strub-Wourgaft, N.; Kuykens, L. Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole. Trop. Med. Infect. Dis. 2020, 5, 17. https://doi.org/10.3390/tropicalmed5010017
Neau P, Hänel H, Lameyre V, Strub-Wourgaft N, Kuykens L. Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole. Tropical Medicine and Infectious Disease. 2020; 5(1):17. https://doi.org/10.3390/tropicalmed5010017
Chicago/Turabian StyleNeau, Philippe, Heinz Hänel, Valérie Lameyre, Nathalie Strub-Wourgaft, and Luc Kuykens. 2020. "Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole" Tropical Medicine and Infectious Disease 5, no. 1: 17. https://doi.org/10.3390/tropicalmed5010017
APA StyleNeau, P., Hänel, H., Lameyre, V., Strub-Wourgaft, N., & Kuykens, L. (2020). Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole. Tropical Medicine and Infectious Disease, 5(1), 17. https://doi.org/10.3390/tropicalmed5010017





