Risk Factors for Infectious Diseases in Urban Environments of Sub-Saharan Africa: A Systematic Review and Critical Appraisal of Evidence
Abstract
:1. Introduction
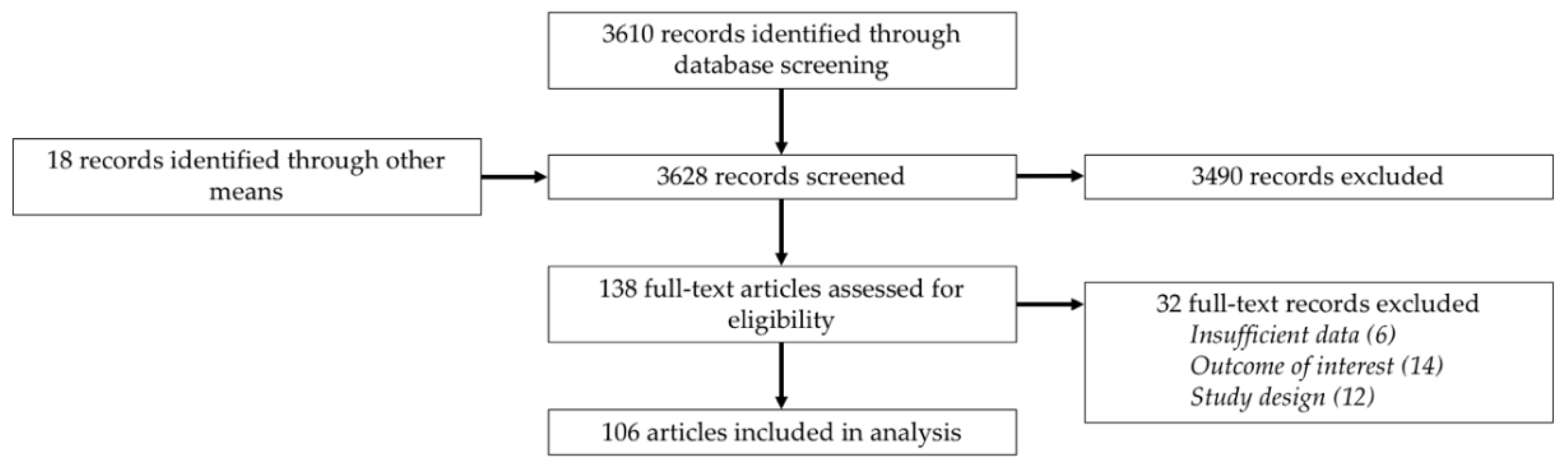
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Analysis
3. Results
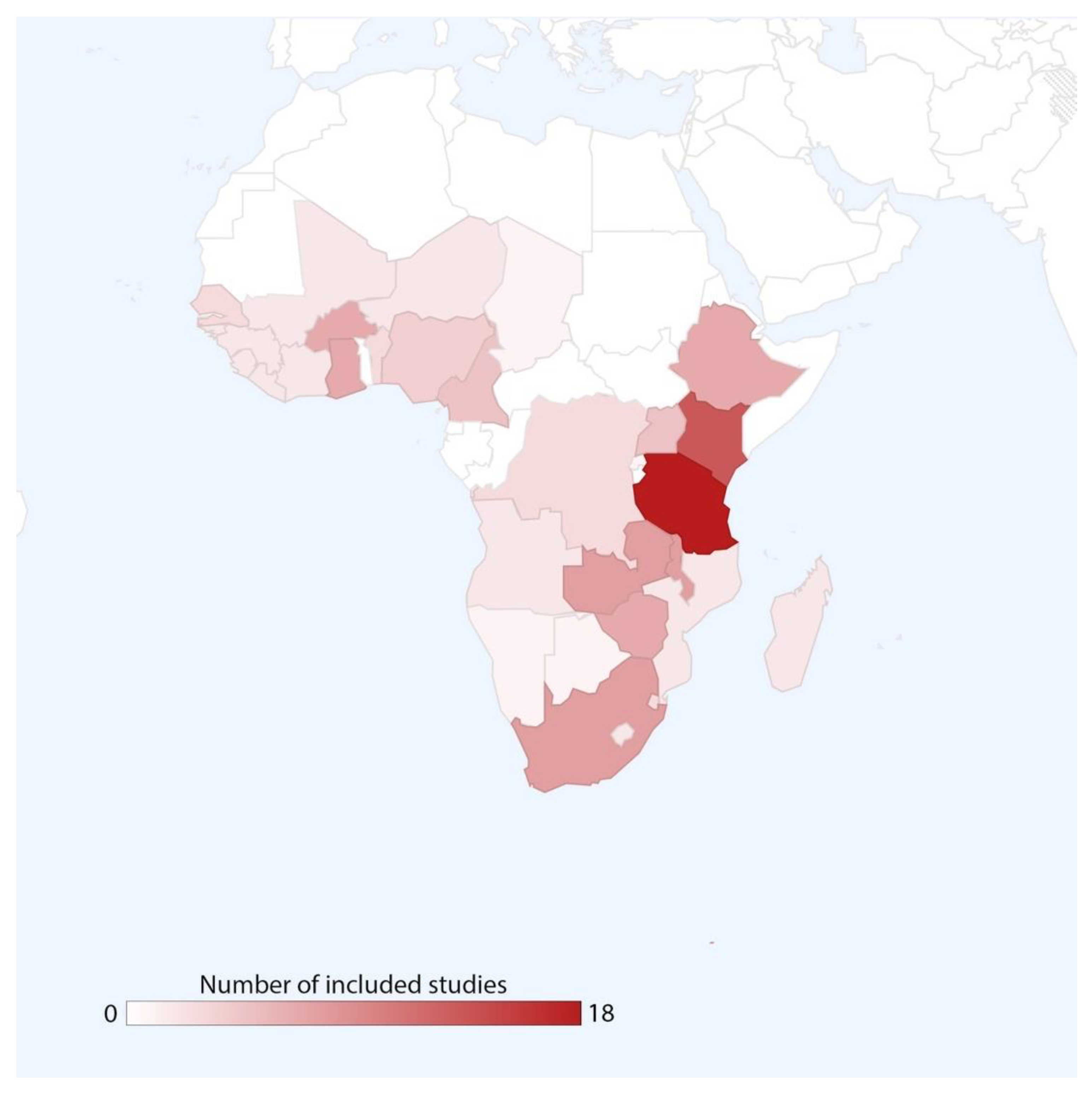
3.1. Study Characteristics
3.2. Urban Risk Factors for Infectious Diseases
3.2.1. Geographic Risk Factors
Population Density
The Built Environment
Municipal Services
Climate and Natural Environment
3.2.2. Behavioral Risk Factors
Hygiene and Sanitation Practices
Sexual Practices and Behaviors
Human Movement
Education and Employment
Socioeconomic Standing
3.2.3. Epidemiological Changes and Changing Disease Burdens
3.2.4. Control Programs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Hidden Cities: Unmasking and Overcoming Health Inequities in Urban Settings; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- United Nations. Revision of World Urbanization Prospects. 2018; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef] [PubMed]
- Förster, T.; Ammann, C. African Cities and the Development Conundrum. Int. Dev. Policy 2018, 10, 3–25. [Google Scholar] [CrossRef]
- Vearey, J.; Luginaah, I.; Francis Magitta, W.; Shilla, D.J.; Oni, T. Urban health in Africa: A critical global public health priority The importance of Africa for global public health. BMC Public Health 2019, 19, 340. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.; Sekajugo, J. Health transition in Africa: practical policy proposals for primary care. Bull. World Health Organ. 2010, 88, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N.; Alberti, K.G.M.M. Chronic non-communicable diseases. Ann. Trop. Med. Parasitol. 2006, 100, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife–Livestock–Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Potts, D. The slowing of sub-Saharan Africa’s urbanization: evidence and implications for urban livelihoods. Environ. Urban. 2009, 21, 253–259. [Google Scholar] [CrossRef]
- Potts, D. Viewpoint: What do we know about urbanisation in sub-Saharan Africa and does it matter? Int. Dev. Plan. Rev. 2012, 34, v–xxii. [Google Scholar] [CrossRef]
- WHO People living in informal settlements. Available online: http://www.who.int/docstore/peh/archives/EHIndicators.pdf (accessed on 20 May 2019).
- Mberu, B.U.; Haregu, T.N.; Kyobutungi, C.; Ezeh, A.C. Health and health-related indicators in slum, rural, and urban communities: A comparative analysis. Glob. Health Action 2016, 9, 33163. [Google Scholar] [CrossRef]
- WHO Urban health: Major opportunities for improving global health outcomes, despite persistent health inequities. Available online: https://www.who.int/news-room/detail/31-03-2016-urban-health-major-opportunities-for-improving-global-health-outcomes-despite-persistent-health-inequities (accessed on 20 May 2019).
- Magalhães, R.J.S.; Langa, A.; Sousa-Figueiredo, J.C.; Clements, A.C.; Nery, S.V. Finding malaria hot-spots in northern Angola: the role of individual, household and environmental factors within a meso-endemic area. Malar. J. 2012, 11, 385. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Faria, N.R.; Reiner, R.C.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.R.W.; et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015-16: a modelling study. Lancet. Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef]
- Orroth, K.K.; Freeman, E.E.; Bakker, R.; Buvé, A.; Glynn, J.R.; Boily, M.-C.; White, R.G.; Habbema, J.D.F.; Hayes, R.J. Understanding the differences between contrasting HIV epidemics in east and west Africa: Results from a simulation model of the Four Cities Study. Sex. Transm. Infect. 2007, 83 (Suppl. 1), i5–i16. [Google Scholar] [CrossRef]
- Wang, S.J.; Lengeler, C.; Smith, T.A.; Vounatsou, P.; Akogbeto, M.; Tanner, M. Rapid Urban Malaria Appraisal (RUMA) IV: Epidemiology of urban malaria in Cotonou (Benin). Malar. J. 2006, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Benbow, M.E.; Brenden, T.O.; Qi, J.; Johnson, R.C. Buruli ulcer disease prevalence in Benin, West Africa: Associations with land use/cover and the identification of disease clusters. Int J Health Geogr. 2008, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kandala, N.B.; Campbell, E.K.; Rakgoasi, S.D.; Madi-Segwagwe, B.C.; Fako, T.T. The geography of HIV/AIDS prevalence rates in Botswana. HIV AIDS (Auckl). 2012, 4, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragatti, M.; Fournet, F.; Henry, M.C.; Assi, S.; Ouedraogo, H.; Rogier, C.; Salem, G. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar. J. 2009, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Fournet, F.; Rican, S.; Vaillant, Z.; Roudot, A.; Meunier-Nikiema, A.; Kassié, D.; Dabiré, R.K.; Salem, G. The influence of urbanization modes on the spatial circulation of flaviviruses within Ouagadougou (Burkina Faso). Int. J. Environ. Res. Public Health 2016, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Kafando, B.; Windinpsidi Savadogo, P.; Millogo, T.; Sana, A.; Kouanda, S.; Sondo, B. Pollution de l’air intérieur et prévalence des infections respiratoires aiguës chez les enfants à Ouagadougou. Sante Publique (Paris). 2018, 30, 575. [Google Scholar] [CrossRef]
- Kirakoya-Samadoulougou, F.; Nagot, N.; Defer, M.C.; Yaro, S.; Fao, P.; Ilboudo, F.; Langani, Y.; Meda, N.; Robert, A. Epidemiology of Herpes Simplex Virus Type 2 Infection in Rural and Urban Burkina Faso. Sex. Transm. Dis. 2011, 38, 117–123. [Google Scholar] [CrossRef]
- Lagarde, E.; Congo, Z.; Meda, N.; Baya, B.; Yaro, S.; Sangli, G.; Traoré, Y.; Van Renthergem, H.; Caraël, M. Study Group on HIV Dynamic Among Young Adults in Burkina Faso Epidemiology of HIV infection in urban Burkina Faso. Int. J. STD AIDS 2004, 15, 395–402. [Google Scholar] [CrossRef]
- Magadi, M.A. The Disproportionate High Risk of HIV Infection Among the Urban Poor in Sub-Saharan Africa. AIDS Behav. 2013, 17, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Lengeler, C.; Smith, T.A.; Vounatsou, P.; Diadie, D.A.; Pritroipa, X.; Convelbo, N.; Kientga, M.; Tanner, M. Rapid urban malaria appraisal (RUMA) I: Epidemiology of urban malaria in Ouagadougou. Malar. J. 2005, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Apinjoh, T.O.; Anchang-Kimbi, J.K.; Mugri, R.N.; Tangoh, D.A.; Nyingchu, R.V.; Chi, H.F.; Tata, R.B.; Njumkeng, C.; Njua-Yafi, C.; Achidi, E.A. The Effect of Insecticide Treated Nets (ITNs) on Plasmodium falciparum Infection in Rural and Semi-Urban Communities in the South West Region of Cameroon. PLoS ONE 2015, 10, e0116300. [Google Scholar] [CrossRef] [PubMed]
- Essomba, E.N.; Kollo, B.; Kouoh Ngambi, M.; Owona Manga, L.J.; Mbunya, S.; Bita Fouda, A.; Dissongo, J.I.; Mikendeffo, D.; Lehman, L. Risky Sexual Behavior And Prevalence Of HIV In Sex Workers In Douala In 2011. Mali Med 2013, 28, 30–36. [Google Scholar] [PubMed]
- Lydié, N.; Robinson, N.J.; Ferry, B.; Akam, E.; De Loenzien, M.; Abega, S. Study Group on Heterogeneity of HIV Epidemics in African Cities Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. J. Acquir. Immune Defic. Syndr. 2004, 35, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Lenglet, A.; Beversluis, D.; de Jong, M.; Vernier, L.; Spencer, C.; Andayi, F.; Kamau, C.; Vollmer, S.; Hogema, B.; et al. A large outbreak of Hepatitis E virus genotype 1 infection in an urban setting in Chad likely linked to household level transmission factors, 2016–2017. PLoS ONE 2017, 12, e0188240. [Google Scholar] [CrossRef] [PubMed]
- Yapi, R.B.; Hürlimann, E.; Houngbedji, C.A.; Ndri, P.B.; Silué, K.D.; Soro, G.; Kouamé, F.N.; Vounatsou, P.; Fürst, T.; N’Goran, E.K.; et al. Infection and Co-infection with Helminths and Plasmodium among School Children in Côte d’Ivoire: Results from a National Cross-Sectional Survey. PLoS Negl. Trop. Dis. 2014, 8, e2913. [Google Scholar] [CrossRef]
- Ferrari, G.; Ntuku, H.M.T.; Ross, A.; Schmidlin, S.; Kalemwa, D.M.; Tshefu, A.K.; Lengeler, C. Identifying risk factors for Plasmodium infection and anaemia in Kinshasa, Democratic Republic of Congo. Malar. J. 2016, 15, 362. [Google Scholar] [CrossRef] [Green Version]
- Asiedu, C.; Asiedu, E.; Owusu, F. The Socio-Economic Determinants of HIV/AIDS Infection Rates in Lesotho, Malawi, Swaziland and Zimbabwe. Dev. Policy Rev. 2012, 30, 305–326. [Google Scholar] [CrossRef]
- Tejedor-Garavito, N.; Dlamini, N.; Pindolia, D.; Soble, A.; Ruktanonchai, N.W.; Alegana, V.; Le Menach, A.; Ntshalintshali, N.; Dlamini, B.; Smith, D.L.; et al. Travel patterns and demographic characteristics of malaria cases in Swaziland, 2010–2014. Malar. J. 2017, 16, 359. [Google Scholar] [CrossRef]
- Bugssa, G.; Dessalegn, B.; Dimtsu, B.; Berhane, Y. Prevalence and factors associated with HIV and hepatitis B virus infections among female commercial sex workers in Mekelle, Ethiopia: Cross sectional study. IJPSR 2015, 6, 135–146. [Google Scholar]
- Degife, L.H.; Worku, Y.; Belay, D.; Bekele, A.; Hailemariam, Z. Factors associated with dengue fever outbreak in Dire Dawa administration city, October, 2015, Ethiopia—Case control study. BMC Public Health 2019, 19, 650. [Google Scholar] [CrossRef] [PubMed]
- Peterson, I.; Borrell, L.N.; El-Sadr, W.; Teklehaimanot, A. A Temporal-Spatial Analysis of Malaria Transmission in Adama, Ethiopia. Am. J. Trop. Med. Hyg 2009, 81, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimeles, E.; Enquselassie, F.; Aseffa, A.; Tilahun, M.; Mekonen, A.; Wondimagegn, G.; Hailu, T. Risk factors for tuberculosis: A case–control study in Addis Ababa, Ethiopia. PLoS ONE 2019, 14, e0214235. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, Y.; Mulu, W.; Yimer, M.; Abera, B. Sero-prevalence and risk factors of hepatitis B virus and human immunodeficiency virus infection among pregnant women in Bahir Dar city, Northwest Ethiopia: A cross sectional study. BMC Infect. Dis. 2014, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yewhalaw, D.; Lo, E.; Zhong, D.; Wang, X.; Degefa, T.; Zemene, E.; Lee, M.; Kebede, E.; Tushune, K.; et al. Analysis of asymptomatic and clinical malaria in urban and suburban settings of southwestern Ethiopia in the context of sustaining malaria control and approaching elimination. Malar. J. 2016, 15, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda, R.B.; Darko, R.; Adanu, R.M.; Seffah, J.; Anarfi, J.K.; Gautam, S.; Hill, A.G. HIV prevalence and risk factors in women of Accra, Ghana: Results from the women’s health study of Accra. Am. J. Trop. Med. Hyg. 2005, 73, 63–66. [Google Scholar] [CrossRef]
- Fobil, J.N.; Levers, C.; Lakes, T.; Loag, W.; Kraemer, A.; May, J. Mapping urban malaria and diarrhea mortality in Accra, Ghana: Evidence of vulnerabilities and implications for urban health policy. J. Urban Health 2012, 89, 977–991. [Google Scholar] [CrossRef]
- Frank, C.; Krumkamp, R.; Sarpong, N.; Sothmann, P.; Fobil, J.N.; Foli, G.; Jaeger, A.; Ehlkes, L.; Owusu-Dabo, E.; Adu-Sarkodie, Y.; et al. Spatial heterogeneity of malaria in Ghana: A cross-sectional study on the association between urbanicity and the acquisition of immunity. Malar. J. 2016, 15, 84. [Google Scholar] [CrossRef]
- Klinkenberg, E.; McCall, P.J.; Hastings, I.M.; Wilson, M.D.; Amerasinghe, F.P.; Donnelly, M.J. Malaria and irrigated crops, Accra, Ghana. Emerg. Infect. Dis. 2005, 11, 1290–1293. [Google Scholar] [CrossRef]
- Sothmann, P.; Krumkamp, R.; Kreuels, B.; Sarpong, N.; Frank, C.; Ehlkes, L.; Fobil, J.; Gyau, K.; Jaeger, A.; Bosu, B.; et al. Urbanicity and Paediatric Bacteraemia in Ghana-A Case-Control Study within a Rural-Urban Transition Zone. PLoS ONE 2015, 10, e0139433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moe, C.L.; Null, C.; Raj, S.J.; Baker, K.K.; Robb, K.A.; Yakubu, H.; Ampofo, J.A.; Wellington, N.; Freeman, M.C.; et al. Multipathway Quantitative Assessment of Exposure to Fecal Contamination for Young Children in Low-Income Urban Environments in Accra, Ghana: The SaniPath Analytical Approach. Am. J. Trop. Med. Hyg. 2017, 97, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Odoi, A. Exploratory investigation of region level risk factors of Ebola Virus Disease in West Africa. PeerJ 2018, 6, e5888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balé, C.; Garly, M.L.; Martins, C.; Nielsen, J.; Whittle, H.; Aaby, P. Risk factors for measles in young infants in an urban African area with high measles vaccination coverage. Pediatr. Infect. Dis. J. 2011, 30, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, P.; Gomes, V.F.; Vieira, C.S.; Rabna, P.; Seng, R.; Johansson, P.; Sandström, A.; Norberg, R.; Lisse, I.; Samb, B.; et al. Tuberculosis in Bissau: incidence and risk factors in an urban community in sub-Saharan Africa. Int. J. Epidemiol. 2004, 33, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokhna, C.; Mboup, B.M.; Sow, P.G.; Camara, G.; Dieng, M.; Sylla, M.; Gueye, L.; Sow, D.; Diallo, A.; Parola, P.; et al. Communicable and non-communicable disease risks at the Grand Magal of Touba: The largest mass gathering in Senegal. Travel Med. Infect. Dis. 2017, 19, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, A.; Eagle, N.; Tatem, A.J.; Smith, D.L.; Noor, A.M.; Snow, R.W.; Buckee, C.O. Quantifying the impact of human mobility on malaria. Science 2012, 338, 267–270. [Google Scholar] [CrossRef]
- Wong, J.M.; Cosmas, L.; Nyachieo, D.; Williamson, J.M.; Olack, B.; Okoth, G.; Njuguna, H.; Feikin, D.R.; Burke, H.; Montgomery, J.M.; et al. Increased Rates of Respiratory and Diarrheal Illnesses in HIV-Negative Persons Living With HIV-Infected Individuals in a Densely Populated Urban Slum in Kenya. J. Infect. Dis. 2015, 212, 745–753. [Google Scholar] [CrossRef]
- Worrell, C.M.; Wiegand, R.E.; Davis, S.M.; Odero, K.O.; Blackstock, A.; Cuéllar, V.M.; Njenga, S.M.; Montgomery, J.M.; Roy, S.L.; Fox, L.M. A Cross-Sectional Study of Water, Sanitation, and Hygiene-Related Risk Factors for Soil-Transmitted Helminth Infection in Urban School- and Preschool-Aged Children in Kibera, Nairobi. PLoS ONE 2016, 11, e0150744. [Google Scholar] [CrossRef]
- Akullian, A.; Ng’eno, E.; Matheson, A.I.; Cosmas, L.; Macharia, D.; Fields, B.; Bigogo, G.; Mugoh, M.; John-Stewart, G.; Walson, J.L.; et al. Environmental Transmission of Typhoid Fever in an Urban Slum. PLoS Negl. Trop. Dis. 2015, 9, e0004212. [Google Scholar] [CrossRef]
- Breiman, R.F.; Olack, B.; Shultz, A.; Roder, S.; Kimani, K.; Feikin, D.R.; Burke, H. Healthcare-use for major infectious disease syndromes in an informal settlement in Nairobi, Kenya. J. Health. Popul. Nutr. 2011, 29, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hallett, T.B.; Aberle-Grasse, J.; Bello, G.; Boulos, L.-M.; Cayemittes, M.P.A.; Cheluget, B.; Chipeta, J.; Dorrington, R.; Dube, S.; Ekra, A.K.; et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex. Transm. Infect. 2006, 82 (Suppl. 1), i1–i8. [Google Scholar] [CrossRef]
- Keating, J.; Macintyre, K.; Mbogo, C.M.; Githure, J.I.; Beier, J.C. Self-reported malaria and mosquito avoidance in relation to household risk factors in a Kenyan coastal city. J. Biosoc. Sci. 2005, 37, 761–771. [Google Scholar] [CrossRef]
- Kimani, J.K.; Ettarh, R.; Ziraba, A.K.; Yatich, N. Marital status and risk of HIV infection in slum settlements of Nairobi, Kenya: results from a cross-sectional survey. Afr. J. Reprod. Health 2013, 17, 103–113. [Google Scholar] [PubMed]
- Madise, N.J.; Ziraba, A.K.; Inungu, J.; Khamadi, S.A.; Ezeh, A.; Zulu, E.M.; Kebaso, J.; Okoth, V.; Mwau, M. Are slum dwellers at heightened risk of HIV infection than other urban residents? Evidence from population-based HIV prevalence surveys in Kenya. Health Place 2012, 18, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omumbo, J.A.; Guerra, C.A.; Hay, S.I.; Snow, R.W. The influence of urbanisation on measures of Plasmodium falciparum infection prevalence in East Africa. Acta. Trop. 2005, 93, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domarle, O.; Razakandrainibe, R.; Rakotomalala, E.; Jolivet, L.; Randremanana, R.V.; Rakotomanana, F.; Ramarokoto, C.E.; Soares, J.-L.; Ariey, F. Malaria Journal Seroprevalence of malaria in inhabitants of the urban zone of Antananarivo, Madagascar. Malar. J. 2006, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanana, F.; Ratovonjato, J.; Randremanana, R. V; Randrianasolo, L.; Raherinjafy, R.; Rudant, J.-P.; Richard, V. Geographical and environmental approaches to urban malaria in Antananarivo (Madagascar). BMC Infect. Dis. 2010, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Amuquandoh, A.; Escamilla, V.; Mofolo, I.; Rosenberg, N.E. Exploring the spatial relationship between primary road distance to antenatal clinics and HIV prevalence in pregnant females of Lilongwe, Malawi. Int. J. STD AIDS 2019, 30, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Simwaka, B.; Ndhlovu, T.; Salaniponi, F.; Hallett, T.B. Evidence for changes in behaviour leading to reductions in HIV prevalence in urban Malawi. Sex. Transm. Infect. 2011, 87, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Jary, H.R.; Aston, S.; Ho, A.; Giorgi, E.; Kalata, N.; Nyirenda, M.; Mallewa, J.; Peterson, I.; Gordon, S.B.; Mortimer, K.; et al. Household air pollution, chronic respiratory disease and pneumonia in Malawian adults: A case-control study. Wellcome Open Res. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Kazembe, L.N.; Mathanga, D.P. Estimating risk factors of urban malaria in Blantyre, Malawi: A spatial regression analysis. Asian Pac. J. Trop. Biomed. 2016, 6, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Mathanga, D.P.; Tembo, A.K.; Mzilahowa, T.; Bauleni, A.; Mtimaukenena, K.; Taylor, T.E.; Valim, C.; Walker, E.D.; Wilson, M.L. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar. J. 2016, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Augusto, Â.; Young, P.W.; Horth, R.Z.; Inguane, C.; Sathane, I.; Ngale, K.; Benedetti, M.; Cummings, B.; Francisco, C.; Botão, S.; et al. High burden of HIV infection and risk behaviors among female sex workers in three main urban areas of Mozambique HHS Public Access. AIDS Behav. 2016, 20, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Sathane, I.; Horth, R.; Young, P.; Inguane, C.; Nalá, R.; Miranda, A.E.; Lane, T.; Raymond, H.F.; Cummings, B.; McFarland, W. Risk Factors Associated with HIV Among Men Who Have Sex Only with Men and Men Who Have Sex with Both Men and Women in Three Urban Areas in Mozambique. AIDS Behav. 2016, 20, 2296–2308. [Google Scholar] [CrossRef]
- Aulagnier, M.; Janssens, W.; De Beer, I.; van Rooy, G.; Gaeb, E.; Hesp, C.; van der Gaag, J.; Rinke de Wit, T.F. Incidence of HIV in Windhoek, Namibia: Demographic and socio-economic associations. PLoS ONE 2011, 6, e25860. [Google Scholar] [CrossRef]
- Bharti, N.; Djibo, A.; Ferrari, M.J.; Grais, R.F.; Tatem, A.J.; Cabe, C.; Bjornstad, O.N.; Grenfell, D.B.T. Measles hotspots and epidemiological connectivity. Epidemiol. Infect. 2010, 138, 1308–1316. [Google Scholar] [CrossRef] [Green Version]
- Awoh, A.B.; Plugge, E. Immunisation coverage in rural-urban migrant children in low and middle-income countries (LMICs): A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 305–311. [Google Scholar] [CrossRef]
- Fana, S.A.; Danladi Abubakar Bunza, M.; Anka, S.A.; Imam, A.U.; Nataala, S.U. Prevalence and risk factors associated with malaria infection among pregnant women in a semi-urban community of north-western Nigeria. Infect. Dis. Poverty 2015, 4, 24. [Google Scholar] [CrossRef]
- Shehu, N.Y.; Gomerep, S.S.; Isa, S.E.; Iraoyah, K.O.; Mafuka, J.; Bitrus, N.; Dachom, M.C.; Ogwuche, J.E.; Onukak, A.E.; Onyedibe, K.I.; et al. Lassa Fever 2016 Outbreak in Plateau State, Nigeria-The Changing Epidemiology and Clinical Presentation. Front. Public Heal. 2018, 6, 232. [Google Scholar] [CrossRef]
- Wagbatsoma, V.A.; Aimiuwu, U. Sanitary provision and helminthiasis among school children in Benin City, Nigeria. Niger. Postgrad. Med. J. 2008, 15, 105–111. [Google Scholar] [PubMed]
- Dos Santos, S.; Rautu, I.; Diop, M.; Abdou Illou, M.M.; Ndonky, A.; Le Hesran, J.-Y.; Lalou, R. The influence of environmental factors on childhood fever during the rainy season in an African city: A multilevel approach in Dakar, Senegal. Popul. Environ. 2015, 36, 429–451. [Google Scholar] [CrossRef]
- Baral, S.; Burrell, E.; Scheibe, A.; Brown, B.; Beyrer, C.; Bekker, L.-G. HIV Risk and Associations of HIV Infection among men who have sex with men in Peri-Urban Cape Town, South Africa. BMC Public Health 2011, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Burrell, E.; Mark, D.; Grant, R.; Wood, R.; Bekker, L.-G. Sexual risk behaviours and HIV-1 prevalence among urban men who have sex with men in Cape Town, South Africa. Sex. Health 2010, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, K.L.; Jewkes, R.K.; Brown, H.C.; Gray, G.E.; McIntryre, J.A.; Harlow, S.D. Transactional sex among women in Soweto, South Africa: prevalence, risk factors and association with HIV infection. Soc. Sci. Med. 2004, 59, 1581–1592. [Google Scholar] [CrossRef]
- Hussain, A.; Moodley, D.; Naidoo, S.; Esterhuizen, T.M. Pregnant women’s access to PMTCT and ART services in South Africa and implications for universal antiretroviral treatment. PLoS ONE 2011, 6, e27907. [Google Scholar] [CrossRef]
- Lohrmann, G.M.; Botha, B.; Violari, A.; Gray, G.E. HIV and the urban homeless in Johannesburg. South. Afr. J. HIV Med. 2012, 13, 174. [Google Scholar] [CrossRef]
- Otwombe, K.N.; Petzold, M.; Modisenyane, T.; Martinson, N.A.; Chirwa, T. Factors associated with mortality in HIV-infected people in rural and urban South Africa. Glob. Health Action 2014, 7, 25488. [Google Scholar] [CrossRef] [Green Version]
- Rispel, L.C.; Metcalf, C.A.; Cloete, A.; Reddy, V.; Lombard, C. HIV Prevalence and Risk Practices Among Men Who Have Sex With Men in Two South African Cities. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 57, 69–76. [Google Scholar] [CrossRef]
- Steenkamp, L.; Venter, D.; Walsh, C.; Dana, P. Socio-economic and demographic factors related to HIV status in urban informal settlements in the Eastern Cape, South Africa. African J. AIDS Res. 2014, 13, 271–279. [Google Scholar] [CrossRef]
- Maheu-Giroux, M.; Castro, M.C. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar. J. 2014, 13, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbizvo, E.M.; Msuya, S.; Hussain, A.; Chirenje, M.; Mbizvo, M.; Sam, N.; Stray-Pedersen, B. HIV and sexually transmitted infections among women presenting at urban primary health care clinics in two cities of sub-Saharan Africa. Afr. J. Reprod. Health 2005, 9, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mgode, G.F.; Mbugi, H.A.; Mhamphi, G.G.; Ndanga, D.; Nkwama, E.L. Seroprevalence of Leptospira infection in bats roosting in human settlements in Morogoro municipality in Tanzania. Tanzan. J. Health Res. 2014, 16. [Google Scholar] [CrossRef]
- Msamanga, G.; Fawzi, W.; Hertzmark, E.; McGrath, N.; Kapiga, S.; Kagoma, C.; Spiegelman, D.; Hunter, D. Socio-economic and demographic factors associated with prevalence of HIV infection among pregnant women in Dar es Salaam, Tanzania. East Afr. Med. J. 2006, 83, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Msuya, S.E.; Mbizvo, E.; Hussain, A.; Uriyo, J.; Sam, N.E.; Stray-Pedersen, B. HIV among pregnant women in Moshi Tanzania: The role of sexual behavior, male partner characteristics and sexually transmitted infections. AIDS Res. Ther. 2006, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Mwakitalu, M.E.; Malecela, M.N.; Pedersen, E.M.; Mosha, F.W.; Simonsen, P.E. Urban lymphatic filariasis in the metropolis of Dar es Salaam, Tanzania. Parasit. Vectors 2013, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Said, K.; Hella, J.; Knopp, S.; Nassoro, T.; Shija, N.; Aziz, F.; Mhimbira, F.; Schindler, C.; Mwingira, U.; Mandalakas, A.M.; et al. Schistosoma, other helminth infections, and associated risk factors in preschool-aged children in urban Tanzania. PLoS Negl. Trop. Dis. 2017, 11, e0006017. [Google Scholar] [CrossRef]
- Singh, R.K.; Patra, S. What Factors are Responsible for Higher Prevalence of HIV Infection among Urban Women than Rural Women in Tanzania? Ethiop. J. Health Sci. 2015, 25, 321–328. [Google Scholar] [CrossRef]
- Wang, S.J.; Lengeler, C.; Smith, T.A.; Vounatsou, P.; Cissé, G.; Tanner, M.; Tanner, M. Rapid Urban Malaria Appraisal (RUMA) II: epidemiology of urban malaria in Dar es Salaam (Tanzania). Malar. J. 2006, 5, 29. [Google Scholar] [CrossRef]
- Arroyo, M.A.; Hoelscher, M.; Sateren, W.; Samky, E.; Maboko, L.; Hoffmann, O.; Kijak, G.; Robb, M.; Birx, D.L.; McCutchan, F.E. HIV-1 diversity and prevalence differ between urban and rural areas in the Mbeya region of Tanzania. AIDS 2005, 19, 1517–1524. [Google Scholar] [CrossRef]
- Castro, M.C.; Tsuruta, A.; Kanamori, S.; Kannady, K.; Mkude, S. Community-based environmental management for malaria control: evidence from a small-scale intervention in Dar es Salaam, Tanzania. Malar. J. 2009, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Geissbühler, Y.; Kannady, K.; Chaki, P.P.; Emidi, B.; Govella, N.J.; Mayagaya, V.; Kiama, M.; Mtasiwa, D.; Mshinda, H.; Lindsay, S.W.; et al. Microbial Larvicide Application by a Large-Scale, Community-Based Program Reduces Malaria Infection Prevalence in Urban Dar Es Salaam, Tanzania. PLoS ONE 2009, 4, e5107. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, C.W.; Molteni, F.; Mandike, R.; Chacky, F.; Noor, A.M.; Snow, R.W.; Linard, C. Mapping intra-urban malaria risk using high resolution satellite imagery: A case study of Dar es Salaam. Int. J. Health Geogr. 2016, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, C.W.; Gilbert, M.; Noor, A.M.; Snow, R.W.; Linard, C. The impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar. J. 2017, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, G.F.; Govella, N.J.; Mlacha, Y.P.; Chaki, P.P. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. Lancet. Planet. Heal. 2019, 3, e132–e143. [Google Scholar] [CrossRef] [Green Version]
- Klinger, E.V.; Kapiga, S.H.; Sam, N.E.; Aboud, S.; Chen, C.-Y.; Ballard, R.C.; Larsen, U. A Community-Based Study of Risk Factors for Trichomonas vaginalis Infection Among Women and Their Male Partners in Moshi Urban District, Northern Tanzania. Sex. Transm. Dis. 2006, 33, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Bousema, T.; Zelman, B.; Gesase, S.; Hashim, R.; Maxwell, C.; Chandramohan, D.; Gosling, R. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS ONE 2014, 9, e87358. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.D.; Greenhouse, B.; Njama-Meya, D.; Nzarubara, B.; Maiteki-Sebuguzi, C.; Staedke, S.G.; Seto, E.; Kamya, M.R.; Rosenthal, P.J.; Dorsey, G. Factors Determining the Heterogeneity of Malaria Incidence in Children in Kampala, Uganda. J. Infect. Dis. 2008, 198, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Kizza, F.N.; List, J.; Nkwata, A.K.; Okwera, A.; Ezeamama, A.E.; Whalen, C.C.; Sekandi, J.N. Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect. Dis. 2015, 15, 165. [Google Scholar] [CrossRef]
- Lule, S.A.; Mawa, P.A.; Nkurunungi, G.; Nampijja, M.; Kizito, D.; Akello, F.; Muhangi, L.; Elliott, A.M.; Webb, E.L. Factors associated with tuberculosis infection, and with anti-mycobacterial immune responses, among five year olds BCG-immunised at birth in Entebbe, Uganda. Vaccine 2015, 33, 796–804. [Google Scholar] [CrossRef]
- Ssempiira, J.; Nambuusi, B.; Kissa, J.; Agaba, B.; Makumbi, F.; Kasasa, S.; Vounatsou, P. The contribution of malaria control interventions on spatio-temporal changes of parasitaemia risk in Uganda during 2009–2014. Parasit. Vectors 2017, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.L.; Jones, D.L.; Chitalu, N.; Weiss, S. Chlamydia and Gonorrhea Infections in HIV-positive Women in Urban Lusaka, Zambia. J. Glob. Infect. Dis. 2012, 4, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gabrysch, S.; Edwards, T.; Glynn, J.R. Study Group on Heterogeneity of HIV Epidemics in African Cities The role of context: neighbourhood characteristics strongly influence HIV risk in young women in Ndola, Zambia. Trop. Med. Int. Heal. 2008, 13, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Chiluba, C.; Phiri, C.; Lisulo, M.M.; Chomba, M.; Hill, P.C.; Ijaz, S.; Kelly, P. Seroepidemiology of Hepatitis E Virus Infection in an Urban Population in Zambia: Strong Association With HIV and Environmental Enteropathy. J Infect Dis 2013, 209, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kapina, M.; Reid, C.; Roman, K.; Cyrus-Cameron, E.; Kwiecien, A.; Weiss, S.; Vermund, S.H. HIV incidence rates and risk factors for urban women in Zambia: Preparing for a microbicide clinical trial. Sex. Transm. Dis. 2009, 36, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Suzuki, H.; Igarashi, K.; Tambatamba, B.; Mulenga, P. Spatial Analysis of Risk Factor of Cholera Outbreak for 2003–2004 in a Peri-urban Area of Lusaka, Zambia. Am. J. Trop. Med. Hyg. 2008, 79, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Suzuki, H.; Fujino, Y.; Kimura, Y.; Cheelo, M. Impact of Drainage Networks on Cholera Outbreaks in Lusaka, Zambia. Am. J. Public Health 2009, 99, 1982. [Google Scholar] [CrossRef]
- Chadambuka, A.; Chimusoro, A.; Maradzika, J.C.; Tshimanga, M.; Gombe, N.T.; Shambira, G. Factors associated with contracting sexually transmitted infections among patients in Zvishavane urban, Zimbabwe; 2007. Afr. Health Sci. 2011, 11, 535–542. [Google Scholar]
- Luque Fernandez, M.A.; Schomaker, M.; Mason, P.R.; Fesselet, J.F.; Baudot, Y.; Boulle, A.; Maes, P. Elevation and cholera: an epidemiological spatial analysis of the cholera epidemic in Harare, Zimbabwe, 2008–2009. BMC Public Health 2012, 12, 442. [Google Scholar] [CrossRef]
- Manyangadze, T.; Chimbari, M.J.; Macherera, M.; Mukaratirwa, S. Micro-spatial distribution of malaria cases and control strategies at ward level in Gwanda district, Matabeleland South, Zimbabwe. Malar. J. 2017, 16, 476. [Google Scholar] [CrossRef]
- Bekker, L.; Wood, R. The Changing Natural History of Tuberculosis and HIV Coinfection in an Urban Area of Hyperendemicity. Clin. Infect. Dis. 2010, 50, S208–S214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Snow, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Siri, J.G.; Wilson, M.L.; Murray, S.; Rosen, D.H.; Vulule, J.M.; Slutsker, L.; Lindblade, K.A. Significance of travel to rural areas as a risk factor for malarial anemia in an urban setting. Am. J. Trop. Med. Hyg. 2010, 82, 391–397. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.M.; Marshall, J.M. Factors Contributing to Urban Malaria Transmission in Sub-Saharan Africa: A Systematic Review. J. Trop. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J.; De Castro, M.C.; Smith, T.A.; Tanner, M.; Singer, B.H. Urbanization in Sub-Saharan Africa and Implication for Malaria Control. Am. J. Trop. Med. Hyg. 2004, 71, 118–127. [Google Scholar] [CrossRef]
- Voeten, H.A.C.M.; Vissers, D.C.J.; Gregson, S.; Zaba, B.; White, R.G.; de Vlas, S.J.; Habbema, J.D.F. Strong association between in-migration and HIV prevalence in urban sub-Saharan Africa. Sex. Transm. Dis. 2010, 37, 240–243. [Google Scholar] [CrossRef]
- Alirol, E.; Getaz, L.; Chappuis, F.; Loutan, L.; Alirol, E.; Getaz, L.; Stoll, B.; Chappuis, F. Urbanisation and infectious diseases in a globalised world. Lancet 2011, 11, 131–141. [Google Scholar] [CrossRef]
- Saghir, J.; Santoro, J. Urbanization in Sub-Saharan Africa: Meeting Challenges by Bridging Stakeholders; Center for Strategic and International Studies: Washington, DC, USA, 2018. [Google Scholar]
- World Health Organization Ten threats to global health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 30 July 2019).
- GardaWorld Madagascar: Measles Outbreak Continues in Antananarivo /Update 1. Available online: https://www.garda.com/crisis24/news-alerts/181701/madagascar-measles-outbreak-continues-in-antananarivo-update-1 (accessed on 29 May 2019).
- Sartorius, B.; Cohen, C.; Chirwa, T.; Ntshoe, G.; Puren, A.; Hofman, K. Identifying high-risk areas for sporadic measles outbreaks: lessons from South Africa. Bull. World Health Organ. 2013, 91, 174–183. [Google Scholar] [CrossRef]
- Rees, D.; Murray, J.; Nelson, G.; Sonnenberg, P. Oscillating Migration and the Epidemics of Silicosis, Tuberculosis, and HIV Infection in South African Gold Miners. Am. J. Ind. Med. 2010, 53, 398–404. [Google Scholar] [CrossRef]
- Dabo, A.; Diarra, A.Z.; Machault, V.; Touré, O.; Niambélé, D.S.; Kanté, A.; Ongoiba, A.; Doumbo, O. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect. Dis. Poverty 2015, 4, 4. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [PubMed]
- Rydin, Y.; Bleahu, A.; Davies, M.; Dávila, J.D.; Friel, S.; De Grandis, G.; Groce, N.; Hallal, P.C.; Hamilton, I.; Howden-Chapman, P.; et al. Shaping cities for health: Complexity and the planning of urban environments in the 21st century. Lancet (London, England) 2012, 379, 2079–2108. [Google Scholar] [CrossRef]
- Pinter-Wollman, N.; Jelić, A.; Wells, N.M. The impact of the built environment on health behaviours and disease transmission in social systems. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170245. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.E.; Katz, R. The International Flow of Risk: The Governance of Health in an Urbanizing World. Glob. Heal. Gov. 2011, 4, 1–17. [Google Scholar]
- WHO Cities: Urban planning and health. Available online: http://www.euro.who.int/__data/assets/pdf_file/0020/341129/Fact-Sheet-2-Cities-Urban-planning-and-health.pdf?ua=1 (accessed on 29 May 2019).
- Turok, I.; Mcgranahan, G. Urbanization and economic growth: the arguments and evidence for Africa and Asia. Environ. Urban. 2013, 25, 465–482. [Google Scholar] [CrossRef]
| Topic | Location(s) a | No. Citations |
|---|---|---|
| Enteric disease | Chad, Ghana, Kenya, Nigeria, Senegal, Zambia, Zimbabwe | 13 |
| HIV | Benin, Botswana, Burkina Faso, Cameroon, Côte d’Ivoire, DRC, Eswatini, Ethiopia, Ghana, Guinea, Kenya, Lesotho, Liberia, Malawi, Mali, Mozambique, Namibia, Rwanda, Senegal, Sierra Leone, South Africa, South Africa, Tanzania, Zambia, Zimbabwe | 34 |
| Malaria | Angola, Benin, Burkina Faso, Cameroon, Côte d’Ivoire, DRC, Eswatini, Ethiopia, Ghana, Kenya, Madagascar, Malawi, Nigeria, Senegal, Tanzania, Uganda, Zimbabwe | 38 |
| Respiratory | Burkina Faso, Ethiopia, Guinea-Bissau, Kenya, Malawi, Niger, Nigeria, Senegal, Uganda | 12 |
| Viral hemorrhagic fever | Angola, Burkina Faso, DRC, Ethiopia, Guinea, Liberia, Nigeria, Sierra Leone | 5 |
| Other diseases b | Benin, Burkina Faso, Côte d’Ivoire, Ethiopia, Kenya, Nigeria, Tanzania, Zambia, Zimbabwe | 16 |
| Risk Factor Category | Risk Factor | Disease or Disease Group | |||||
|---|---|---|---|---|---|---|---|
| Enteric Diseases | HIV | Malaria | Respiratory Diseases | Viral Hemorrhagic Fever | Other Diseases | ||
| Geographic | Population density | ||||||
| Built environment | |||||||
| Municipal services | |||||||
| Natural environment | |||||||
| Behavioral | Hygiene and sanitation | ||||||
| Education and employment | |||||||
| Sexual behaviors | |||||||
| Human movement | |||||||
| Socioeconomic standing | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyce, M.R.; Katz, R.; Standley, C.J. Risk Factors for Infectious Diseases in Urban Environments of Sub-Saharan Africa: A Systematic Review and Critical Appraisal of Evidence. Trop. Med. Infect. Dis. 2019, 4, 123. https://doi.org/10.3390/tropicalmed4040123
Boyce MR, Katz R, Standley CJ. Risk Factors for Infectious Diseases in Urban Environments of Sub-Saharan Africa: A Systematic Review and Critical Appraisal of Evidence. Tropical Medicine and Infectious Disease. 2019; 4(4):123. https://doi.org/10.3390/tropicalmed4040123
Chicago/Turabian StyleBoyce, Matthew R., Rebecca Katz, and Claire J. Standley. 2019. "Risk Factors for Infectious Diseases in Urban Environments of Sub-Saharan Africa: A Systematic Review and Critical Appraisal of Evidence" Tropical Medicine and Infectious Disease 4, no. 4: 123. https://doi.org/10.3390/tropicalmed4040123






