The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Well-Equipped Clinical Centres and Laboratories
3.2. Peripheral Clinics and Laboratories
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mycetoma is Added to WHO List of ‘Neglected Tropical Diseases’. Available online: https://www.dndi.org/2016/media-centre/press-releases/mycetoma-who-ntd-list-response/ (accessed on 10 June 2019).
- Van de Sande, W.W.J. Global Burden of Human Mycetoma: A Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2550. [Google Scholar] [CrossRef] [PubMed]
- Fahal, A.H. Mycetoma: A global medical and socio-economic dilemma. PLoS Negl. Trop. Dis. 2017, 11, e0005509. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; e Silva, C.D.; Da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2016, 30, 233–276. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Galhardo, M.C.; Freitas, D.F.; do Valle, A.C.; Almeida-Paes, R.; de Oliveira, M.M.; Zancopé-Oliveira, R.M. Epidemiological aspects of Sporotrichosis epidemic in Brazil. Curr. Fungal Infect. Rep. 2015, 9, 238–245. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet 2017, 17, 367–377. [Google Scholar] [CrossRef]
- Arenas, R.; Sánchez-Cardenas, C.; Ramirez-Hobak, L.; Ruíz Arriaga, L.; Vega Memije, M. Sporotrichosis: From KOH to Molecular Biology. J. Fungi 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Crabol, Y.; Poiree, S.; Bougnoux, M.E.; the French Mycosis Study Group. Last generation triazoles for imported eumycetoma in eleven consecutive adults. PLoS Negl. Trop. Dis. 2014, 8, e3232. [Google Scholar] [CrossRef]
- Engelman, D.; Fuller, L.C.; Solomon, A.W.; McCarthy, J.S.; Hay, R.J.; Lammie, P.J.; Steer, A.C. Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol. 2016, 32, 843–854. [Google Scholar] [CrossRef]
- Hay, R. Skin NTDs: An opportunity for integrated care. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 679–680. [Google Scholar] [CrossRef]
- Mitja, O.; Marks, M.; Bertran, L.; Kollie, K.; Argaw, D.; Fahal, A.H.; Fitzpatrick, C.; Fuller, L.C.; Izquierdo, B.G.; Hay, R.; et al. Integrated Control and Management of Neglected Tropical Skin Diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005136. [Google Scholar] [CrossRef]
- WHO. Recognizing Neglected Tropical Diseases through Changes on the Skin A Training Guide for Front-Line Health Workers; WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/neglected_diseases/resources/9789241513531/en/ (accessed on 10 June 2019).
- Wilson, M.L.; Fleming, K.A.; Kuti, M.; Looi, L.M.; Lago, N.; Ru, K. Access to pathology and laboratory medicine services: A critical gap. Lancet 2018, 139, 1927–1938. [Google Scholar] [CrossRef]
- Van de Sande, W.; Fahal, A.; Ahmed, S.A.; Serrano, J.A.; Bonifaz, A.; Zijlstra, E. Closing the mycetoma knowledge gap. Med. Mycol. 2018, 56 (Suppl. 1), 153–164. [Google Scholar] [CrossRef]
- Ahmed, A.A.; van de Sande, W.; Fahal, A.H. Mycetoma laboratory diagnosis: Review article. PLoS Negl. Trop. Dis. 2017, 11, e0005638. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Borman, A.M.; Johnson, E.M. Rapid and Robust Identification of the Agents of Black-Grain Mycetoma by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2017, 55, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- El Badawi, H.S.; Mahgoub, E.; Mahmoud, N.; Fahal, A.H. Use of immunoblotting in testing Madurella mycetomatis specific antigen. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 312–316. [Google Scholar] [CrossRef] [PubMed]
- El Shamy, M.E.; Fahal, A.H.; Shakir, M.Y.; Homeida, M.M. New MRI grading system for the diagnosis and management of mycetoma. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Bakhiet, S.M.; Fahal, A.H.; Musa, A.M.; Omer, R.F.; Ahmed, E.S.; El Nour, M.; Manar El Sheikh, A.R.; Suliman, S.H.; El Mamoun, M.A.; El Amin, H.M. A holistic approach to the mycetoma management. PLoS Negl. Trop. Dis. 2018, 12, e0006391. [Google Scholar] [CrossRef]
- Reis, L.M.; Lima, B.Z.; Zillo, F.D.; Rezende, C.M.; Fabricio, L.H.; Pinto, C.A. Dermoscopy assisting the diagnosis of mycetoma: Case report and literature review. An. Bras. Dermatol. 2014, 89, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Litaiem, N.; Midassi, O.; Zeglaoui, F. Detecting subclinical mycetoma’s black grains using dermoscopy. Int. J. Dermatol. 2018, 58, 231–232. [Google Scholar] [CrossRef]
- Fransisca, C.; He, Y.; Chen, Z.; Liu, H.; Xi, L. Molecular identification of Chromoblastomycosis clinical isolates in Guangdong. Med. Mycol. 2017, 55, 851–858. [Google Scholar] [CrossRef]
- Gomes, R.R.; Vicente, V.A.; de Azevedo, C.M.; Salgado, C.G.; da Silva, M.B.; Queiroz-Telles, F.; Marques, S.G.; Santos, D.W.; de Andrade, T.S.; Takagi, E.H.; et al. Molecular Epidemiology of Agents of Human Chromoblastomycosis in Brazil with the Description of Two Novel Species. PLoS Negl. Trop. Dis. 2016, 10, e0005102. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.J.; Sun, J.; Vicente, V.A.; de Hoog, G.S. Rapid identification of fungal pathogens by rolling circle amplification using Fonsecaea as a model. Mycoses 2011, 54, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Santmyire, A. The Effectiveness of a Multifocal Training to Improve the Treatment of Chromoblastomycosis in Rural Madagascar. J. Health Care Poor Underserv. 2016, 27, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- Mugleston, B.J.; Usatine, R.P.; Rosen, T. Wide Morphologic Variability of Chromoblastomycosis in the Western Hemisphere. Skinmed 2016, 14, 423–427. [Google Scholar] [PubMed]
- Subhadarshani, S.; Yadav, D. Dermoscopy of Chromoblastomycosis. Dermatol. Pract. Concept. 2017, 7, 23–24. [Google Scholar] [CrossRef]
- Xavier, M.O.; Bittencourt, L.R.; Silva, C.M.; Vieira, R.S.; Pereira, H.C. Atypical presentation of Sporotrichosis: Report of three cases. Rev. Soc. Bras. Med. Trop. 2013, 46, 116–118. [Google Scholar] [CrossRef]
- Papaiordanou, F.; da Silveira, B.R.; Abulafia, L.A. Hypersensitivity reaction to Sporothrix schenckii: Erythema nodosum associated with Sporotrichosis. Rev. Soc. Bras. Med. Trop. 2015, 48, 504. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A. Sporotrichosis: Update on Diagnostic Techniques. Curr. Fungal Infect. Rep. 2017, 11, 134–140. [Google Scholar] [CrossRef]
- Suzuki, R.; Yikelamu, A.; Tanaka, R.; Igawa, K.; Yokozeki, H.; Yaguchi, T. Studies in Phylogeny, Development of Rapid Identification Methods, Antifungal Susceptibility, and Growth Rates of Clinical Strains of Sporothrix schenckii Complex in Japan. Med. Mycol. J. 2016, 57, E47–E57. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Fernandes, G.F.; Araujo, L.M.; Della Terra, P.P.; dos Santos, P.O.; Pereira, S.A.; Schubach, T.M.; Burger, E.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Proteomics-Based Characterization of the Humoral Immune Response in Sporotrichosis: Toward Discovery of Potential Diagnostic and Vaccine Antigens. PLoS Negl. Trop. Dis. 2015, 9, e0004016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardes-Engemann, A.R.; de Lima Barros, M.; Zeitune, T.; Russi, D.C.; Orofino-Costa, R.; Lopes-Bezerra, L.M. Validation of a serodiagnostic test for Sporotrichosis: A follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med. Mycol. 2015, 53, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Baca, E.; Hernández-Mendoza, G.; Cuéllar-Cruz, M.; Toriello, C.; López-Romero, E.; Gutiérrez-Sánchez, G. Detection of 2 immunoreactive antigens in the cell wall of Sporothrix brasiliensis and Sporothrix globosa. Diagn. Microbiol. Infect. Dis. 2014, 79, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Sánchez, A.; Bonifaz, A. Nodular Lymphangitis (Sporotrichoid Lymphocutaneous Infections). Clues to Differential Diagnosis. J. Fungi 2018, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Toriello, C.; Araiza, J.; Ramírez-Soto, M.C.; Tirado-Sánchez, A. Sporotrichin Skin Test for the Diagnosis of Sporotrichosis. J. Fungi 2018, 4, 55. [Google Scholar] [CrossRef]
- Dabas, G.; Kaur, H.; Vinay, K.; Kumaran, M.S.; Shivaprakash, M.R.; Saikia, U.N. Dermoscopy in disseminated Sporotrichosis. J. Eur. Acad. Derm. Venereol. 2018, 33, e33–e34. [Google Scholar] [CrossRef]
- Sławińska, M.; Hlebowicz, M.; Iżycka-Świeszewska, E.; Sikorska, M.; Sokołowska-Wojdyło, M.; Smiatacz, T.; Nowicki, R.; Sobjanek, M. Dermoscopic observations in disseminated cryptococcosis with cutaneous involvement. J. Eur. Acad. Derm. Venereol. 2018, 32, e223–e224. [Google Scholar] [CrossRef]
- Neglected Tropical Diseases. Available online: https://www.who.int/neglected_diseases/skin-ntds/en/ (accessed on 10 June 2019).
- World Health Organization Model List of Essential In Vitro Diagnostics. Available online: http://www.who.int/medical_devices/diagnostics/EDL_ExecutiveSummary_15may.pdf (accessed on 10 June 2019).
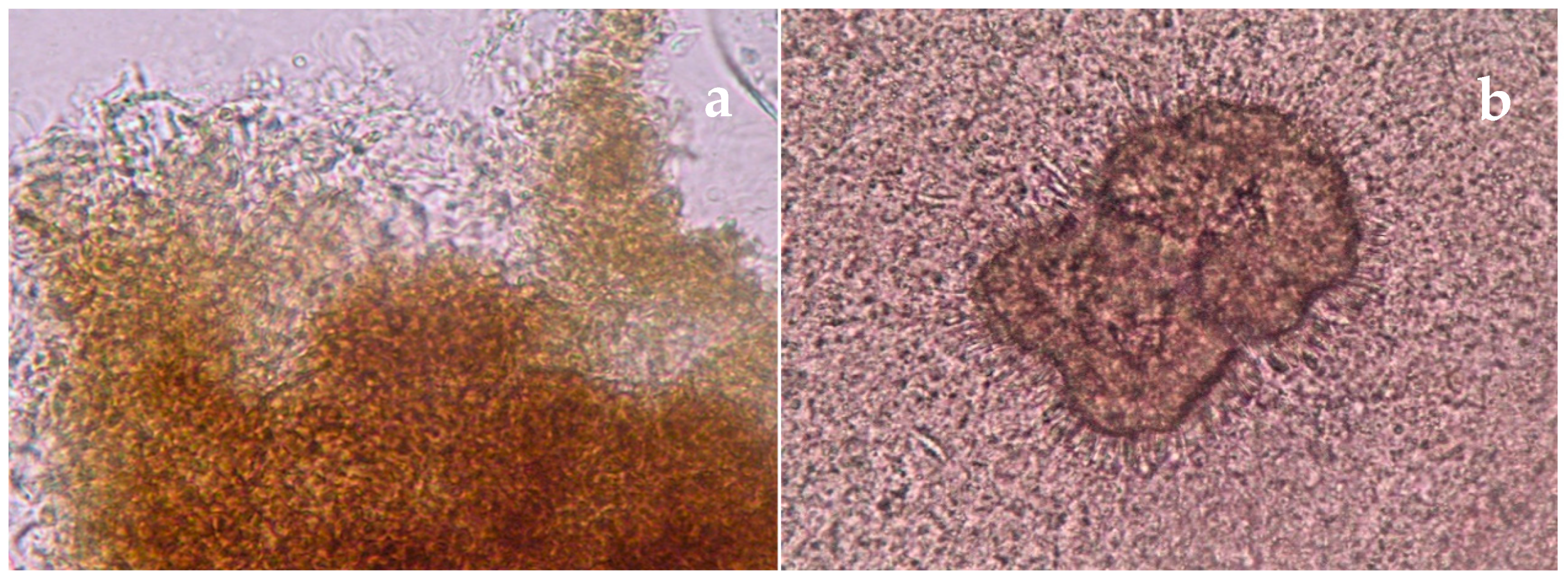
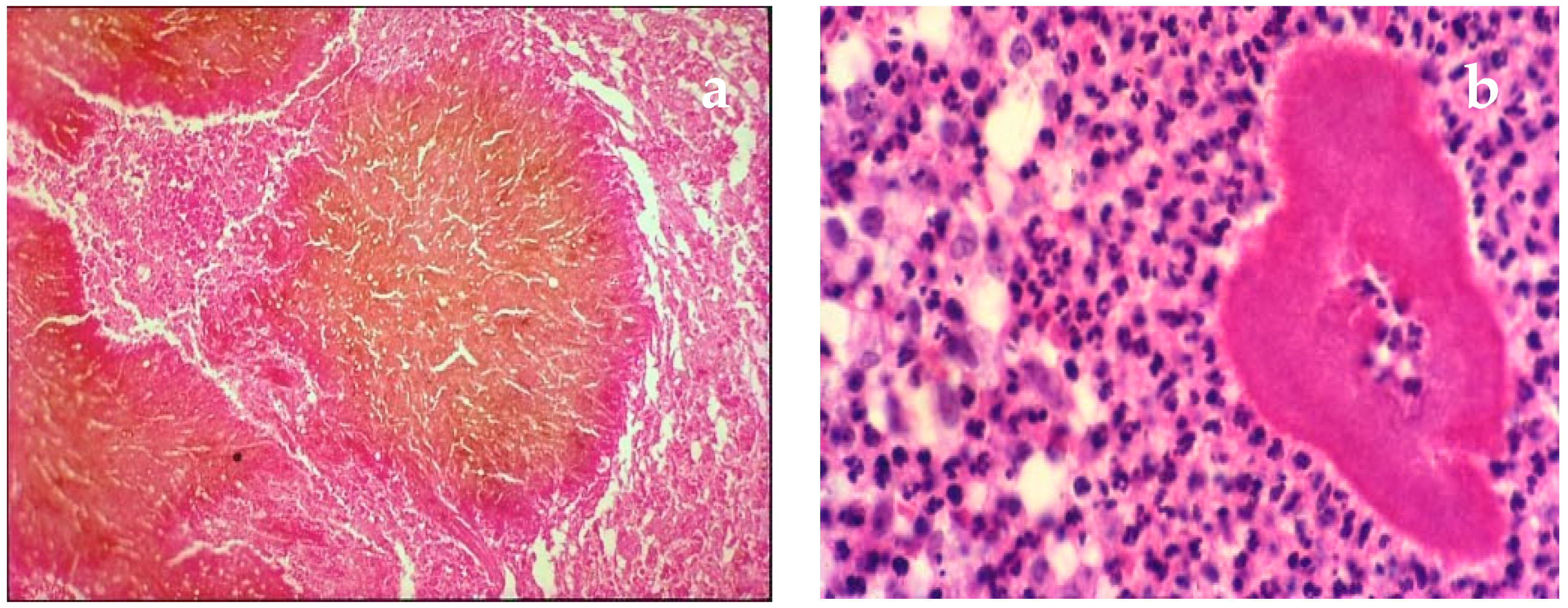
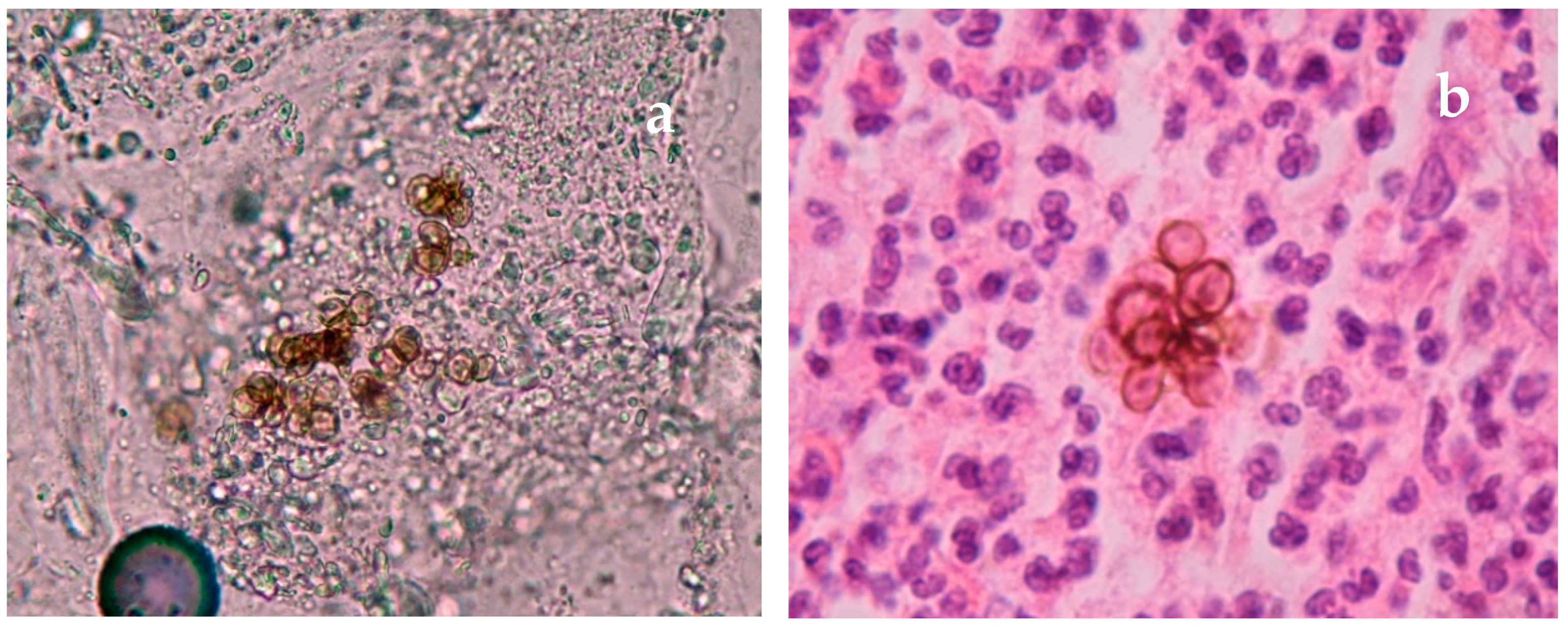
| Disease | Clinical Features | Direct Microscopy | Culture | Serology | Molecular Diagnosis | Histopathology | Other |
|---|---|---|---|---|---|---|---|
| Mycetoma | 92% | 88% | 96% | 8% | 71% | 88% | imaging, dermoscopy |
| Chromoblastomycosis | 88% | 92% | 96% | 8% | 50% | 92% | dermoscopy |
| Sporotrichosis | 83% | 25% 1 | 96% | 4% | 50% | 67% | intradermal test |
| Disease | Clinical Features | Direct Microscopy | Culture | Serology | Molecular Diagnosis | Histopathology | Other |
|---|---|---|---|---|---|---|---|
| Mycetoma | 96% | 88% | 33% | - | 13% | 43% | imaging |
| Chromoblastomycosis | 96% | 92% | 33% | 8% | 4% | 54% | |
| Sporotrichosis | 88% | 42% | 50% | 4% | 45 | 21% |
| Responders Choice | Swab from Broken Skin or Sinuses | Impression Smear | Skin Scraping from Broken Skin or Sinuses | Punch or Incision Biopsy or Curettage | Excision Biopsy |
|---|---|---|---|---|---|
| Mycetoma | 38% | 42% | 54% | 74% | 61% |
| Chromoblastomycosis | 8% | 29% | 67% | 79% | 42% |
| Sporotrichosis | 21% | 17% | 50% | 75% | 58% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hay, R.; Denning, D.W.; Bonifaz, A.; Queiroz-Telles, F.; Beer, K.; Bustamante, B.; Chakrabarti, A.; Chavez-Lopez, M.d.G.; Chiller, T.; Cornet, M.; et al. The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report. Trop. Med. Infect. Dis. 2019, 4, 122. https://doi.org/10.3390/tropicalmed4040122
Hay R, Denning DW, Bonifaz A, Queiroz-Telles F, Beer K, Bustamante B, Chakrabarti A, Chavez-Lopez MdG, Chiller T, Cornet M, et al. The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report. Tropical Medicine and Infectious Disease. 2019; 4(4):122. https://doi.org/10.3390/tropicalmed4040122
Chicago/Turabian StyleHay, Roderick, David W Denning, Alexandro Bonifaz, Flavio Queiroz-Telles, Karlyn Beer, Beatriz Bustamante, Arunaloke Chakrabarti, Maria de Guadalupe Chavez-Lopez, Tom Chiller, Muriel Cornet, and et al. 2019. "The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report" Tropical Medicine and Infectious Disease 4, no. 4: 122. https://doi.org/10.3390/tropicalmed4040122
APA StyleHay, R., Denning, D. W., Bonifaz, A., Queiroz-Telles, F., Beer, K., Bustamante, B., Chakrabarti, A., Chavez-Lopez, M. d. G., Chiller, T., Cornet, M., Estrada, R., Estrada-Chavez, G., Fahal, A., Gomez, B. L., Li, R., Mahabeer, Y., Mosam, A., Soavina Ramarozatovo, L., Rakoto Andrianarivelo, M., ... Zijlstra, E. E. (2019). The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report. Tropical Medicine and Infectious Disease, 4(4), 122. https://doi.org/10.3390/tropicalmed4040122







