Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bait Constructs
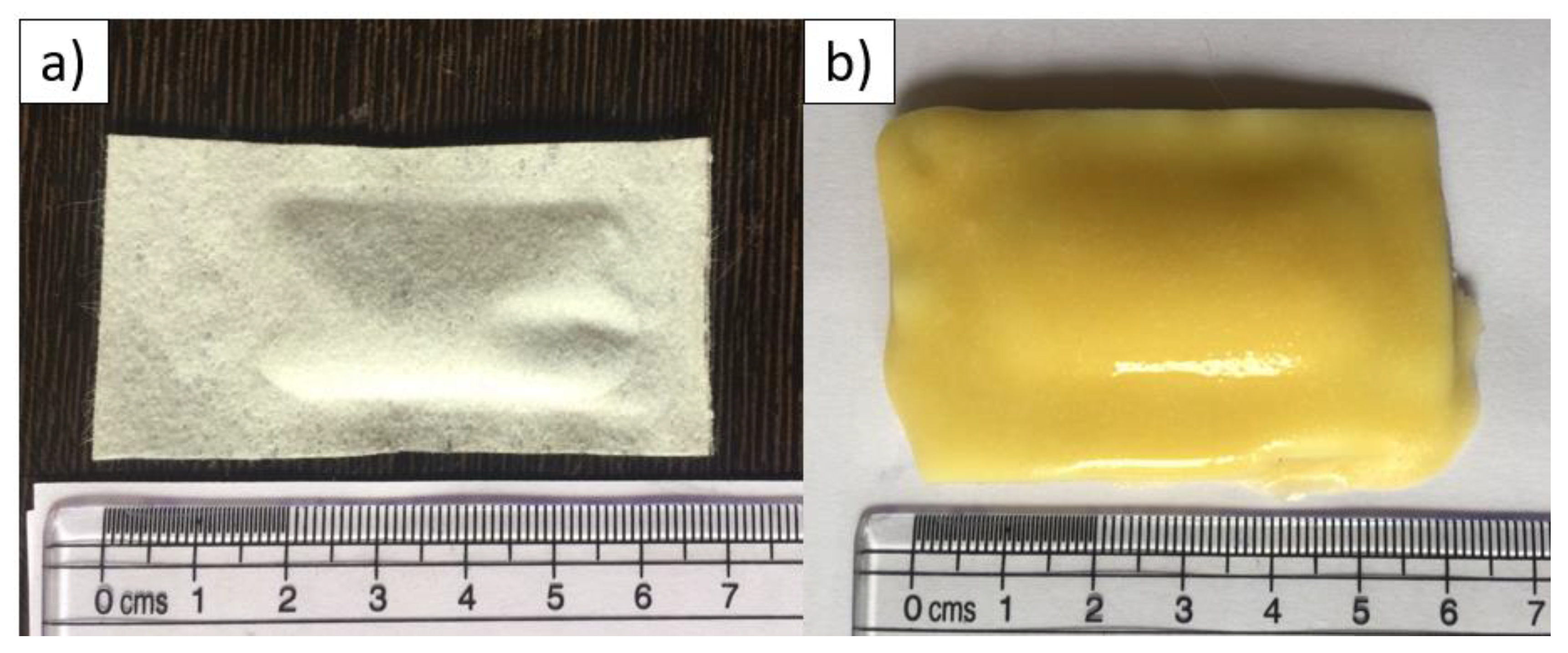
2.2.1. Capsule
2.2.2. Egg-Bait
2.2.3. Gravy-Bait
3. Study Design and Bait Distribution
4. Data Collection
5. Statistical Analysis
6. Ethical Statement
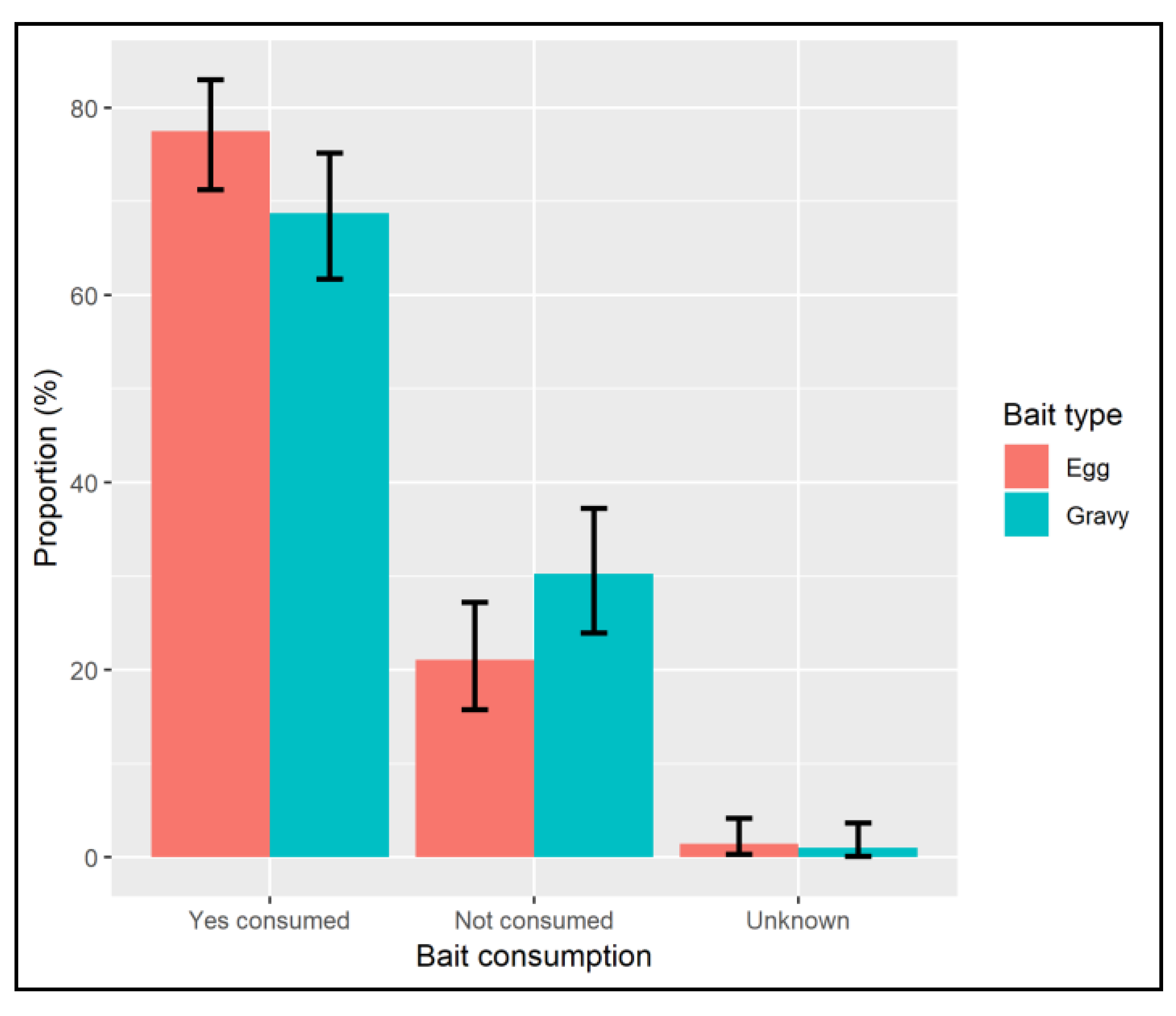
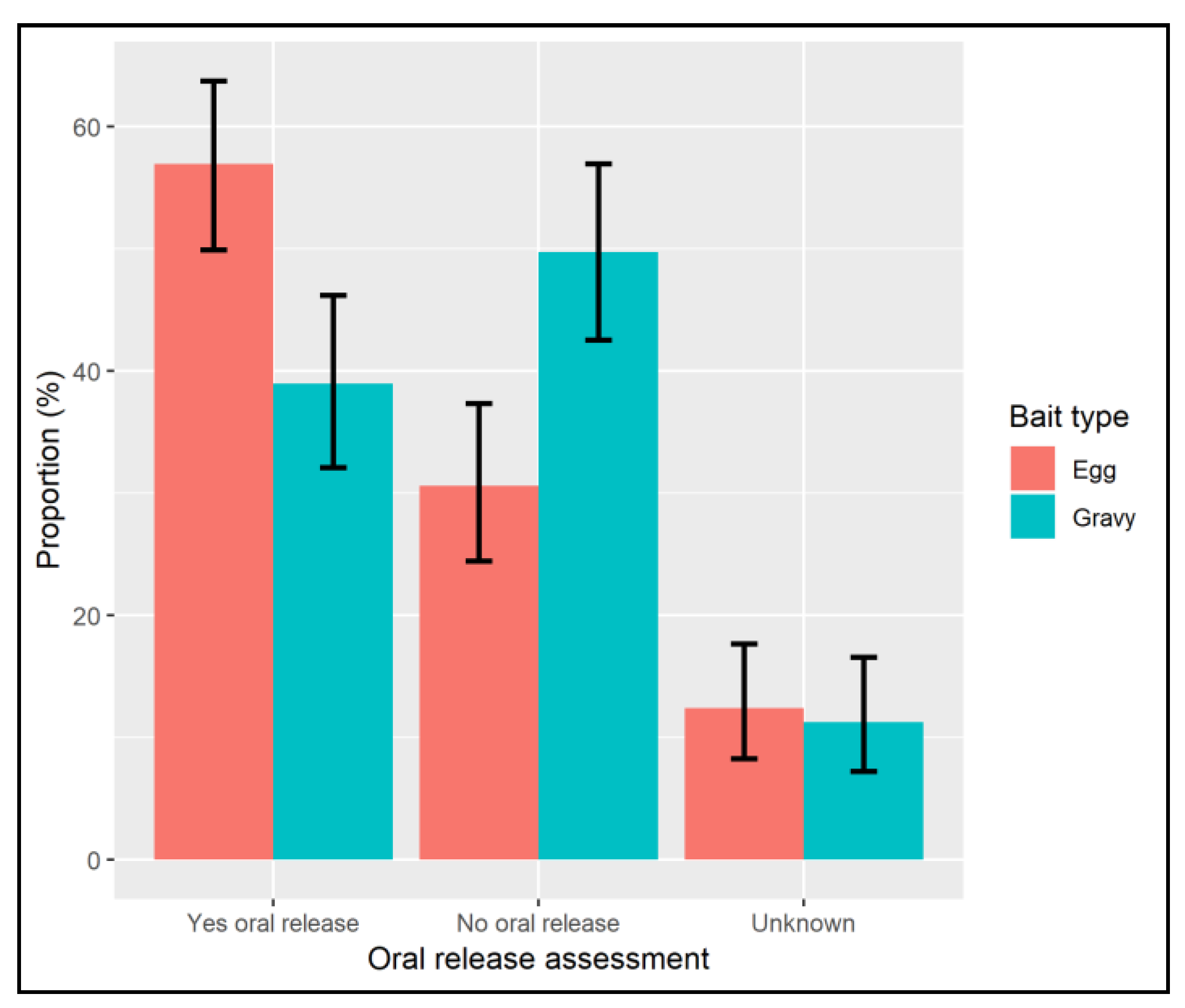
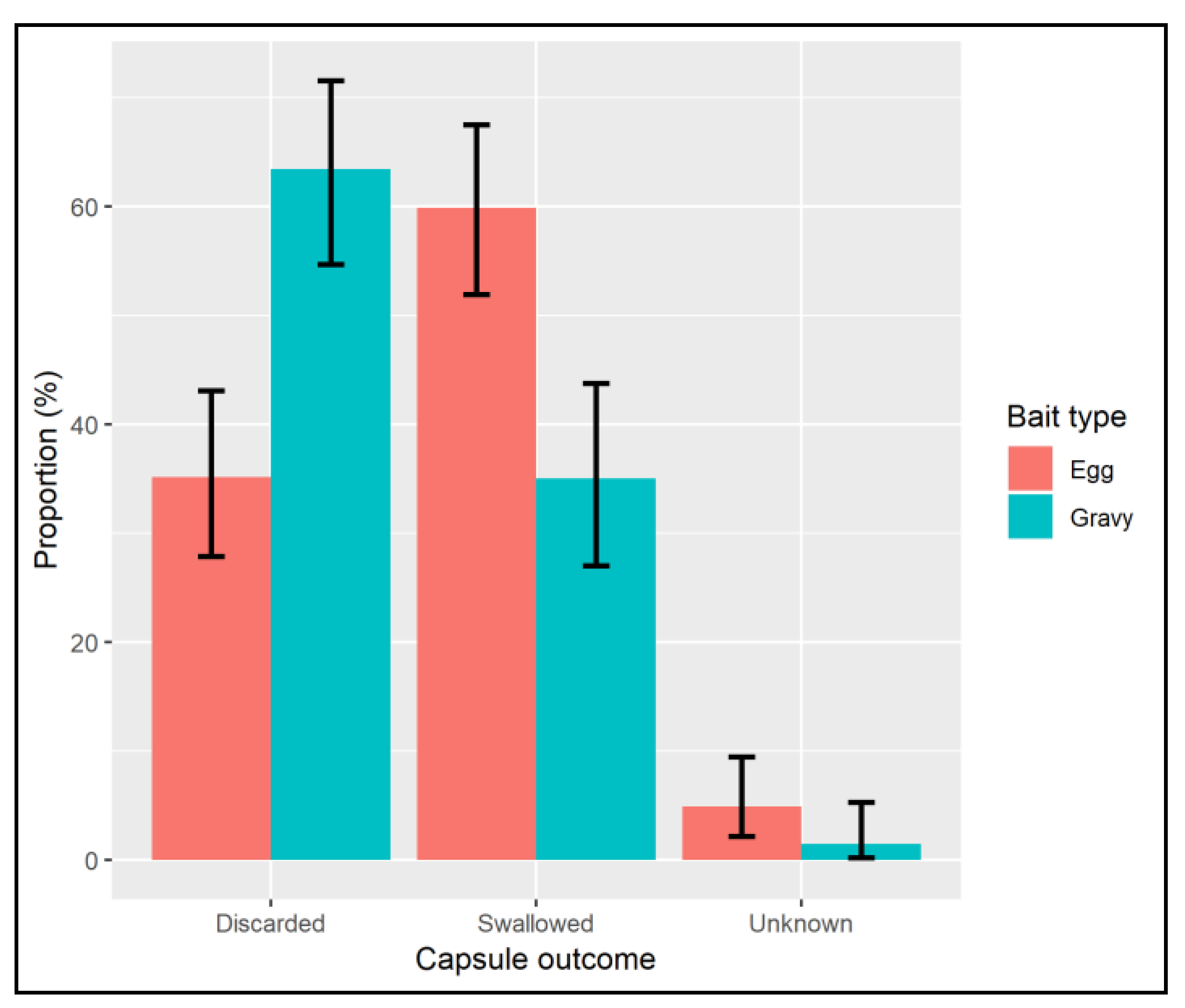
7. Results
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Abela-Ridder, B.; Knopf, L.; Martin, S.; Taylor, L.; Torres, G.; De Balogh, K. 2016, the Beginning of the End of Rabies? Lancet Glob. Health 2016, 4, e780–e781. [Google Scholar] [CrossRef]
- Fahrion, A.S.; Taylor, L.H.; Torres, G.; Müller, T.; Dürr, S.; Knopf, L.; De Balogh, K.; Nel, L.H.; Gordoncillo, M.J.; Abela-Ridder, B. The Road to Dog Rabies Control and Elimination—What Keeps Us from Moving Faster? Front. Public Health 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies, Third Report; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2018; ISSN 0512-3054. [Google Scholar]
- Borse, R.H.; Atkins, C.Y.; Gambhir, M.; Undurraga, E.A.; Blanton, J.D.; Kahn, E.B.; Dyer, J.L.; Rupprecht, C.E.; Meltzer, M.I. Cost-effectiveness of dog rabies vaccination programs in East Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006490. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.D.; Handel, I.G.; Shervell, K.; Roux, T.; Mayer, D.; Muyila, S.; Maruwo, G.B.; Nkhulungo, E.M.; Foster, R.A.; Chikungwa, P.; et al. The Vaccination of 35,000 Dogs in 20 Working Days Using Combined Static Point and Door-to-Door Methods in Blantyre, Malawi. PLoS Negl. Trop. Dis. 2016, 10, e0004824. [Google Scholar] [CrossRef] [PubMed]
- Léchenne, M.; Oussiguere, A.; Naissengar, K.; Mindekem, R.; Mosimann, L.; Rives, G.; Hattendorf, J.; Moto, D.D.; Alfaroukh, I.O.; Zinsstag, J. Operational performance and analysis of two rabies vaccination campaigns in N’Djamena, Chad. Vaccine 2016, 34, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Kaare, M.; Tiringa, P.; Mlengeya, T.; Barrat, J. A dog rabies vaccination campaign in rural Africa: Impact on the incidence of dog rabies and human dog-bite injuries. Vaccine 2003, 21, 1965–1973. [Google Scholar] [CrossRef]
- Reece, J.F.; Chawla, S.K. Control of rabies in Jaipur, India, by the sterilisation and vaccination of neighbourhood dogs. Vet. Rec. 2006, 159, 379–383. [Google Scholar] [CrossRef]
- Belsare, A.V.; Gompper, M.E. Assessing demographic and epidemiologic parameters of rural dog populations in India during mass vaccination campaigns. Prev. Vet. Med. 2013, 111, 139–146. [Google Scholar] [CrossRef]
- Widyastuti, M.D.; Bardosh, K.L.; Sunandar Basri, C.; Basuno, E.; Jatikusumah, A.; Arief, R.A.; Putra, A.A.; Rukmantara, A.; Estoepangestie, A.T.; Willyanto, I. On dogs, people, and a rabies epidemic: Results from a sociocultural study in Bali, Indonesia. Infect. Dis. Poverty 2015, 4, 30. [Google Scholar] [CrossRef]
- Gibson, A.D.; Ohal, P.; Shervell, K.; Handel, I.G.; Bronsvoort, B.M.; Mellanby, R.J.; Gamble, L. Vaccinate-assess-move method of mass canine rabies vaccination utilising mobile technology data collection in Ranchi, India. BMC Infect. Dis. 2015, 15, 589. [Google Scholar] [CrossRef]
- Vos, A.; Freuling, C.M.; Hundt, B.; Kaiser, C.; Nemitz, S.; Neubert, A.; Nolden, T.; Teifke, J.P.; te Kamp, V.; Ulrich, R.; et al. Oral vaccination of wildlife against rabies: Differences among host species in vaccine uptake efficiency. Vaccine 2017, 35, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Sidwa, T.J.; Wilson, P.J.; Moore, G.M.; Oertli, E.H.; Hicks, B.N.; Rohde, R.E.; Johnston, D.H. Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J. Am. Vet. Med. Assoc. 2005, 227, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yakobson, B.A.; King, R.; Amir, S.; Devers, N.; Sheichat, N.; Rutenberg, D.; Mildenberg, Z.; David, D. Rabies vaccination programme for red foxes (Vulpes vulpes) and golden jackals (Canis aureus) in Israel (1999–2004). Dev. Biol. 2005, 125, 133–140. [Google Scholar]
- Mueller, T.; Freuling, C.M.; Wysocki, P.; Roumiantzeff, M.; Freney, J.; Mettenleiter, T.C.; Vos, A. Terrestrial rabies control in the European Union: Historical achievements and challenges ahead. Vet. J. 2015, 203, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.A.; Chipman, R.B.; Slate, D.; Huyvaert, K.P.; VerCauteren, K.C.; Gilbert, A.T. Management and modeling approaches for controlling raccoon rabies: The road to elimination. PLoS Negl. Trop. Dis. 2017, 11, e0005249. [Google Scholar] [CrossRef]
- Report of the Scientific Committee on Animal Health and Animal Welfare. The Oral Vaccination of Foxes against Rabies; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- DG Health and Food Safety Overview Report-Rabies Eradication in the EU; European Commission: Brussels, Belgium, 2017. [CrossRef]
- Cliquet, F.; Guiot, A.L.; Aubert, M.; Robardet, E.; Rupprecht, C.E.; Meslin, F.X. Oral vaccination of dogs: A well-studied and undervalued tool for achieving human and dog rabies elimination. Vet. Res. BioMed 2018, 49, 1–11. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018; OIE: Paris, France, 2018; Chapter 3.1.17. [Google Scholar]
- Gibson, A.D.; Yale, G.; Vos, A.; Corfmat, J.; Airikkala-Otter, I.; King, A.; Wallace, R.M.; Gamble, L.; Handel, I.G.; Mellanby, R.J.; et al. Oral bait handout as a method to access roaming dogs for rabies vaccination in Goa, India: A proof of principle study. Vaccine X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Linhart, S.B.; Baer, G.M.; Balderastorres, J.M.; Engeman, A.M.; Wlodkowskp, J.C. Acceptance of candidate baits by domestic dogs for delivery of oral rabies vaccines. J. Vet. Res. 1997, 124, 115–124. [Google Scholar]
- Frontini, M.G.; Fishbein, D.B.; Ramos, J.G.; Collins, E.F.; Torres, J.M.; Huerta, G.Q.; Rodriguez, J.D.; Belotto, A.J.; Dobbins, J.G.; Linhart, S.B.; et al. A field evaluation in Mexico of four baits for oral rabies vaccination of dogs. Am. J. Trop. Med. Hyg. 1992, 47, 310–316. [Google Scholar] [CrossRef]
- Schuster, P.; Gulsen, N.; Neubert, A.; Vos, A. Field Trials Evaluating Bait Uptake by an Urban Dog Population in Turkey. J. Etlik Vet. Microbiol. 1998, 9, 73–81. [Google Scholar]
- Corn, J.L.; Méndez, J.R.; Catalán, E.E. Evaluation of baits for delivery of oral rabies vaccine to dogs in Guatemala. Am. J. Trop. Med. Hyg. 2003, 69, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.C.; Kharmachi, H.; Haddad, N.; Youssef, S.B.; Sghaier, C.; Khelifa, R.B.; Jemli, J.; Mrabet, L.D.; Meslin, F.X.; Wandeler, A.I. Test of three bait types for oral immunization of dogs against rabies in Tunisia. Am. J. Trop. Med. Hyg. 1995, 52, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Vos, A.; De Leon, R.; Mueller, T. Field trial with oral vaccination of dogs against rabies in the Philippines. BMC Infect. Dis. 2001, 1, 2010–2012. [Google Scholar] [CrossRef]
- Estrada, R.; Vos, A.; De Leon, R. Acceptability of local made baits for oral vaccination of dogs against rabies in the Philippines. BMC Infect. Dis. 2001, 1. [Google Scholar] [CrossRef]
- Bender, S.; Bergman, D.; Vos, A.; Martin, A.; Chipman, R. Field Studies Evaluating Bait Acceptance and Handling by Dogs in Navajo Nation, USA. Trop. Med. Infect. Dis. 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Kasemsuwan, S.; Chanachai, K.; Pinyopummintr, T. Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Millien, M.; Vos, A.; Fracciterne, F.A.; Crowdis, K.; Chirodea, C.; Medley, A.; Chipman, R.; Qin, Y.; Blanton, J.; et al. Evaluation of immune responses in dogs to oral rabies vaccine under field conditions. Vaccine 2017, 37, 4743–4749. [Google Scholar] [CrossRef]
- Aylan, O.; Vos, A. Efficacy of oral rabies vaccine baits in indigenous Turkish dogs. Rev. Infect. Dis. 2000, 2, 74–77. [Google Scholar]
- Bonne, K.; Verbeke, W. Religious values informing halal meat production and the control and delivery of halal credence quality. Agric. Hum. Values 2008, 25, 35–47. [Google Scholar] [CrossRef]
- Gamble, L.; Gibson, A.; Mazeri, S.; de CBronsvoort, B.M.; Handel, I.; Mellanby, R.J. Development of non-governmental organisation-academic partnership to tackle rabies in Africa and Asia. J. Small Anim. Pract. 2019, 60, 18–20. [Google Scholar] [CrossRef]
- Nokireki, T.; Nevalainen, M.; Sihvonen, L.; Gadd, T. Adverse reactions from consumption of oral rabies vaccine baits in dogs in Finland. Acta Vet. Scand. BioMed 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.D.; Mazeri, S.; Lohr, F.; Mayer, D.; Bailey, J.L.; Wallace, R.M.; Handel, I.G.; Shervell, K.; Barend, M.; Mellanby, R.J.; et al. One million dog vaccinations recorded on mHealth innovation used to direct teams in numerous rabies control campaigns. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org (accessed on 8 August 2018).
- Bartoń, K. MuMIn: Multi-Model Inference. Available online: https://cran.r-project.org/package=MuMIn (accessed on 8 August 2018).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: http:%0A//ggplot2 (accessed on 8 August 2018).
- OIE. Stray dog population control. In Terrestrial Animal Health Code; OIE: Paris, France, 2018; pp. 1–12. [Google Scholar]
- Tiwari, H.K.; Robertson, I.D.; O‘Dea, M.; Vanak, A.T. Knowledge, attitudes and practices (KAP) towards rabies and free roaming dogs (FRD) in Panchkula district of north India: A cross-sectional study of urban residents. PLoS Negl. Trop. Dis. 2019, 13, e0007384. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Gilbert, A.; Vos, A.; Freuling, C.M.; Ellis, C.; Kliemt, J.; Müller, T. Rabies Virus Antibodies from Oral Vaccination as a Correlate of Protection against Lethal Infection in Wildlife. Trop. Med. Infect. Dis. 2017, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.; Aylan, O.; Estrada, R. Oral vaccination campaigns of dogs against rabies. In Proceedings of the Seventh South East African Rabies Group/World Health Organization Meeting, Ezulwini, Swaziland, 12–15 May 2003; pp. 125–130. [Google Scholar]
- Head, J.R.; Vos, A.; Blanton, J.; Müller, T.; Chipman, R.; Pieracci, E.G.; Cleaton, J.; Wallace, R. Environmental distribution of certain modified live-virus vaccines with a high safety profile presents a low-risk, high-reward to control zoonotic diseases. Sci. Rep. 2019, 9, 6783. [Google Scholar] [CrossRef] [PubMed]
- Arief, R.A.; Hampson, K.; Jatikusumah, A.; Widyastuti, M.D.; Basri, C.; Putra, A.A.; Willyanto, I.; Estoepangestie, A.T.; Mardiana, I.W.; Kesuma, I.K.; et al. Determinants of Vaccination Coverage and Consequences for Rabies Control in Bali, Indonesia. Front. Vet. Sci. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wera, E.; Mourits, M.C.M.; Hogeveen, H. Uptake of Rabies Control Measures by Dog Owners in Flores Island, Indonesia. PLoS Negl. Trop. Dis. 2015, 9, e0003589. [Google Scholar] [CrossRef]
| Not Consumed | Bait Consumed | ||||||
|---|---|---|---|---|---|---|---|
| Bait Type | Not Interested | Interested, Not Consumed | Not Perforated | Perforation Unconfirmed * | Perforation Seen, Oral Contact Unconfirmed * | Perforation Seen, Oral Contact Confirmed | Total |
| Egg | 37 (17.7%) | 10 (4.8%) | 13 (6.2%) | 16 (7.7%) | 14 (6.7%) | 119 (56.9%) | 209 |
| Gravy | 36 (18.5%) | 25 (12.8%) | 34 (17.4%) | 11 (5.6%) | 13 (6.7%) | 76 (39.0%) | 195 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, A.D.; Mazeri, S.; Yale, G.; Desai, S.; Naik, V.; Corfmat, J.; Ortmann, S.; King, A.; Müller, T.; Handel, I.; et al. Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India. Trop. Med. Infect. Dis. 2019, 4, 118. https://doi.org/10.3390/tropicalmed4030118
Gibson AD, Mazeri S, Yale G, Desai S, Naik V, Corfmat J, Ortmann S, King A, Müller T, Handel I, et al. Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India. Tropical Medicine and Infectious Disease. 2019; 4(3):118. https://doi.org/10.3390/tropicalmed4030118
Chicago/Turabian StyleGibson, Andrew D., Stella Mazeri, Gowri Yale, Santosh Desai, Vilas Naik, Julie Corfmat, Steffen Ortmann, Alasdair King, Thomas Müller, Ian Handel, and et al. 2019. "Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India" Tropical Medicine and Infectious Disease 4, no. 3: 118. https://doi.org/10.3390/tropicalmed4030118
APA StyleGibson, A. D., Mazeri, S., Yale, G., Desai, S., Naik, V., Corfmat, J., Ortmann, S., King, A., Müller, T., Handel, I., Bronsvoort, B. M., Gamble, L., Mellanby, R. J., & Vos, A. (2019). Development of a Non-Meat-Based, Mass Producible and Effective Bait for Oral Vaccination of Dogs against Rabies in Goa State, India. Tropical Medicine and Infectious Disease, 4(3), 118. https://doi.org/10.3390/tropicalmed4030118






