Q Fever in the First Trimester: A Case Report from Northern Rural New South Wales
Abstract
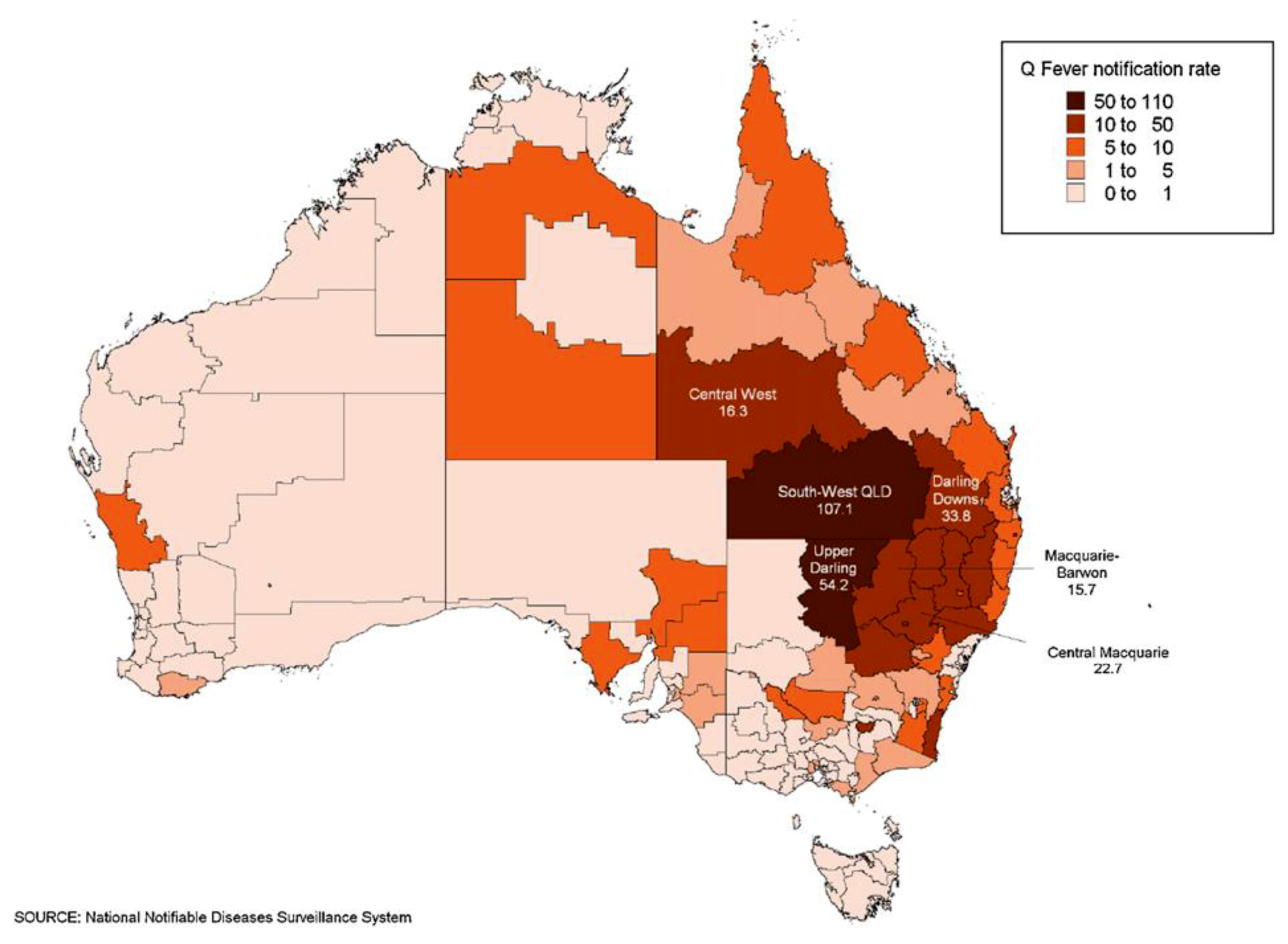
1. Introduction
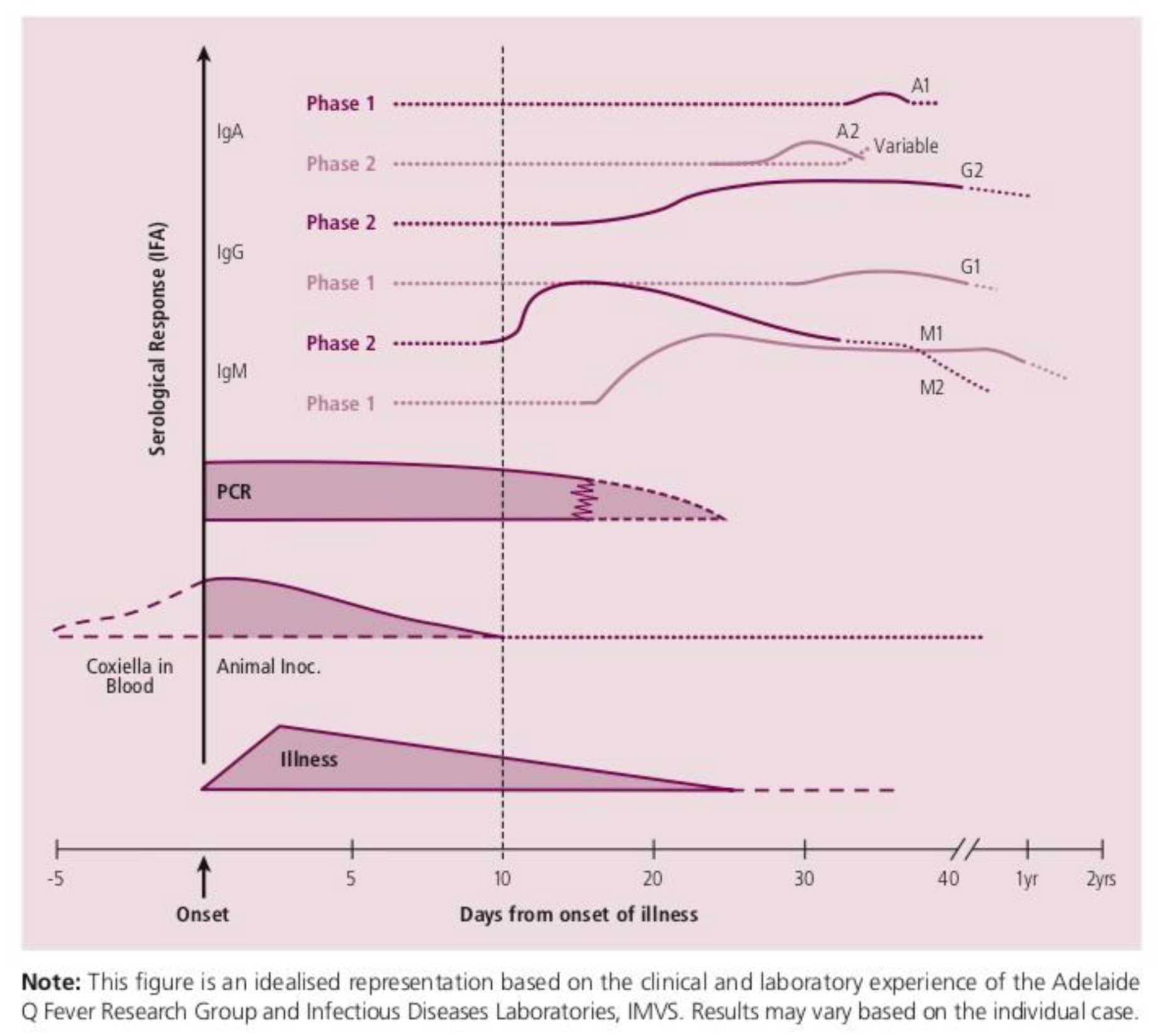
2. Case Discussion
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Permission
Appendix A
| IFA Serology | 9 Weeks Gestation | 36 Weeks Gestation | 3 Months Post-Partum | Reference |
|---|---|---|---|---|
| Phase II IgM | >400 | 50 | <25 | <25 |
| Phase II IgG | 3200 | 1600 | 100 | <25 |
| Phase I IgG | <100 | 800 | 1600 | <25 |
| Serum PCR | N/A | negative | negative |
| Test | Acute Infection | Persistent Infection | Notes |
|---|---|---|---|
| Immunofluorescence | Phase II IgG titre > 200 AND Phase II IgM titre > 50 OR Four-fold rise in phase II IgG (paired sera) | Phase I IgG titre > 800 OR Persistently elevated Phase I IgG/IgM | Gold standard |
| PCR | Maybe positive early in the infective sequence (prior to antibody response) | Maybe performed on placental tissues/products, joint aspirate, sera | Does not differentiate acute vs. chronic |
| Culture | Stains and immunofluorescence are not routinely performed | - | Highly infectious, requires PC3 facility |
References
- Eastwood, K.; Graves, S.R.; Massey, P.D.; Bosward, K.; Van den Berg, D.; Hutchinson, P. Q fever: A rural disease with potential urban consequences. Aust. J. Gen. Pract. 2018, 47, 112–116. Available online: https://www1.racgp.org.au/ajgp/2018/march/q-fever (accessed on 30 January 2019).
- Archer, B.N.; Hallahan, C.; Stanley, P.; Seward, K.; Lesjak, M.; Hope, K.; Brown, A. Atypical outbreak of Q fever affecting low-risk residents of a remote rural town in New South Wales. Commun. Dis. Intell. Q. Rep. 2017, 41, 125–133. [Google Scholar]
- Million, M.; Raould, D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J. Infect. 2015, 71, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Tissot-Dupont, H.; Vaillant, V.; Rey, S.; Raoult, D. Role of sex, age, previous valve lesions, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin. Infect. Dis. 2007, 44, 232–237. [Google Scholar] [CrossRef]
- Q Fever Laboratory Case Definition. The Public Health Laboratory Network 2017. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-qfever.htm (accessed on 10 March 2019).
- A Guide to Q Fever and Q Fever Vaccination—Sequiris Australia. Available online: https://www.sequiris.com.au/docs/461/380/Q_Fever_booklet,0.pdf (accessed on 3 May 2019).
- Million, M.; Roblot, F.; Carles, D.; D’Amato, F.; Protopopescu, C.; Carrieri, M.P.; Raoult, D. Reevaluation of the risk of fetal death and malformation after Q fever. Clin. Infect. Dis. 2014, 59, 256–260. [Google Scholar] [CrossRef]
- Stein, A.; Raoult, D. Q fever during pregnancy: A public health problem in Southern France. Clin. Infect. Dis. 1998, 27, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Fenollar, F.; Stein, A. Q fever during pregnancy: Diagnosis, treatment and follow up. Arch. Intern. Med. 2002, 162, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.M.; Malik, S.V.; Kaur, S.; Kumar, S.; Barbuddhe, S.B. Comparison of PCR, immunofluorescence assay, and pathogen isolation for diagnosis of Q fever in humans with spontaneous abortions. J. Clin. Microbiol. 2008, 46, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.Y.; Milbak, K.; Henriksen, T.B.; Krogfelt, K.A.; Larsen, C.S.; Villumsen, S. Adverse pregnancy outcomes and Coxiella urnetiid antibodies in pregnant women, Denmark. Emerg. Infect. Dis. 2014, 20, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.; Shubber, Z.; Jao, J.; Abrams, E.J.; Frigati, L.; Mofenson, L. Safety of cotrimoxazole in pregnancy: A systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2014, 66, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Carcopino, X.; Raoult, D.; Bretelle, F.; Boubli, L.; Stein, A. Managing Q fever during pregnancy: The benefits of long-term cotrimoxazole therapy. Clin. Infect. Dis. 2007, 45, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Munster, J.M.; Leenders, A.C.; Hamilton, C.J.; Meekelenkamp, J.C.; Schneeberger, P.M.; van der Hoek, W. Routine screening for Coxiella urnetiid infection during pregnancy: A clustered randomized controlled trial during an outbreak, the Netherlands, 2010. Euro Surveill. 2010, 18, 24. Available online: https://www.eurosurveillance.org/content/10.2807/ese.18.24.20504-en (accessed on 30 January 2019).
- Q Fever Laboratory Case Definition, Communicable Diseases Network Australia. Available online: www.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-qfever.htm (accessed on 3 May 2019).
- Gidding, H.F.; Wallace, C.; Lawrence, G.L.; McIntyre, P.B. Australia’s national Q fever vaccination program. Vaccine 2009, 27, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, S.; Olenski, M. Q Fever in the First Trimester: A Case Report from Northern Rural New South Wales. Trop. Med. Infect. Dis. 2019, 4, 90. https://doi.org/10.3390/tropicalmed4020090
Marks S, Olenski M. Q Fever in the First Trimester: A Case Report from Northern Rural New South Wales. Tropical Medicine and Infectious Disease. 2019; 4(2):90. https://doi.org/10.3390/tropicalmed4020090
Chicago/Turabian StyleMarks, Sarah, and Maxwell Olenski. 2019. "Q Fever in the First Trimester: A Case Report from Northern Rural New South Wales" Tropical Medicine and Infectious Disease 4, no. 2: 90. https://doi.org/10.3390/tropicalmed4020090
APA StyleMarks, S., & Olenski, M. (2019). Q Fever in the First Trimester: A Case Report from Northern Rural New South Wales. Tropical Medicine and Infectious Disease, 4(2), 90. https://doi.org/10.3390/tropicalmed4020090




