High Endemicity of Soil-Transmitted Helminths in a Population Frequently Exposed to Albendazole but No Evidence of Antiparasitic Resistance
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. STH Prevalence in Studied Population
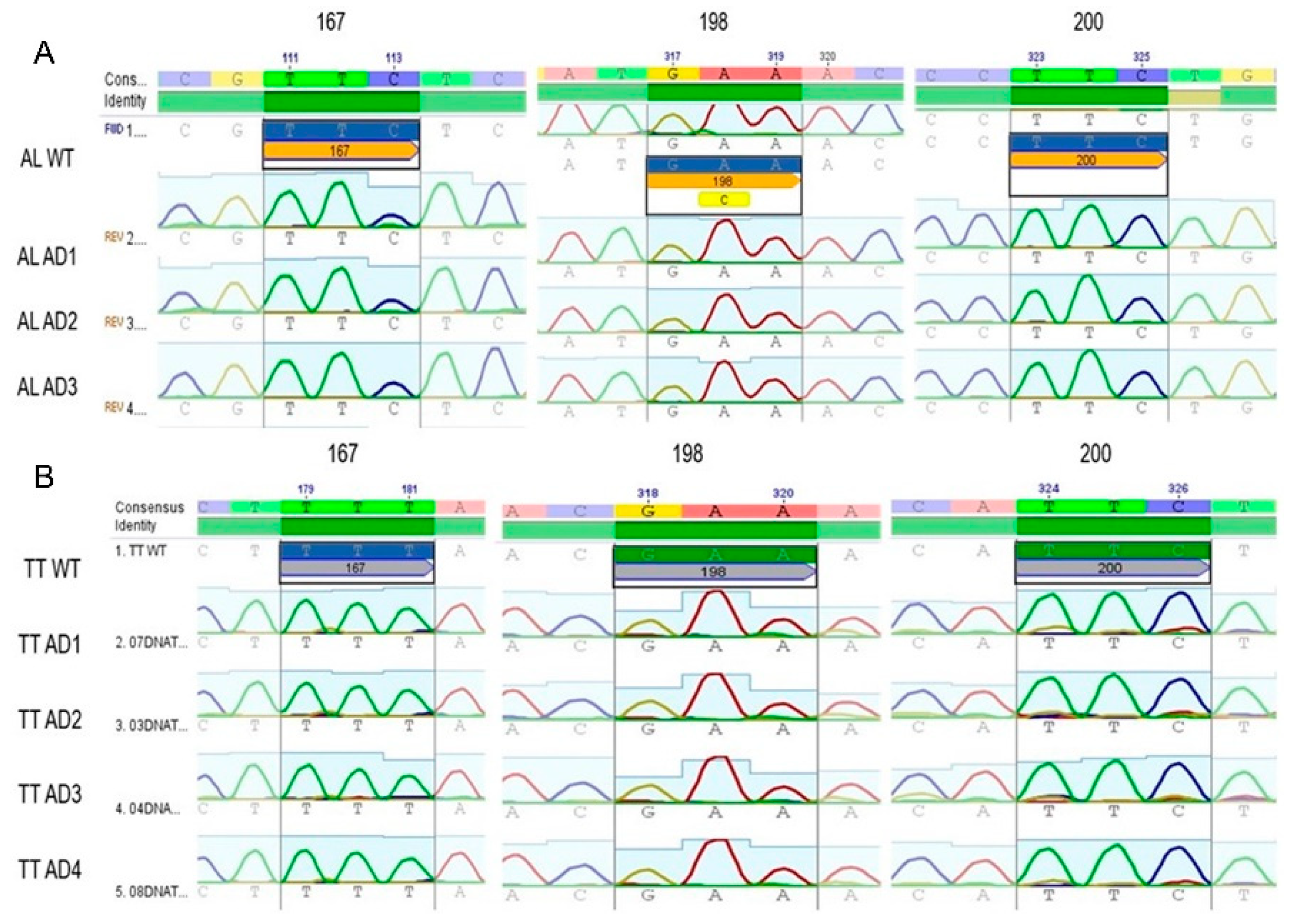
3.2. Genotyping of Codons 200, 198, and 167 from the β-Tubulin Gene in T. trichiura and A. lumbricoides
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Bottazzi, M.E.; Franco-Paredes, C.; Ault, S.K.; Periago, M.R. The neglected tropical diseases of Latin America and the Caribbean: A review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2008, 2, e300. [Google Scholar] [CrossRef]
- WHO. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Preventive chemotherapy for helminth diseases: Progress report, 2014. Wkly. Epidemiol. Rec. 2016, 8, 93–104. [Google Scholar]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Lacey, E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 1988, 18, 885–936. [Google Scholar] [CrossRef]
- Clarke, N.E.; Clements, A.C.; Doi, S.A.; Wang, D.; Campbell, S.J.; Gray, D.; Nery, S.V. Differential effect of mass deworming and targeted deworming for soil-transmitted helminth control in children: A systematic review and meta-analysis. Lancet 2017, 389, 287–297. [Google Scholar] [CrossRef]
- Hansen, T.V.; Thamsborg, S.M.; Olsen, A.; Prichard, R.K.; Nejsum, P. Genetic variations in the beta-tubulin gene and the internal transcribed spacer 2 region of Trichuris species from man and baboons. Parasit. Vectors 2013, 6, 236. [Google Scholar] [CrossRef]
- Vercruysse, J.; Behnke, J.M.; Albonico, M.; Ame, S.M.; Angebault, C.; Bethony, J.M.; Engels, D.; Guillard, B.; Nguyen, T.V.; Kang, G.; et al. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2011, 5, e948. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Mgeni, A.F.; Khamis, I.S.; Steinmann, P.; Stothard, J.R.; Rollinson, D.; Marti, H.; Utzinger, J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: Effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl. Trop. Dis. 2008, 2, e331. [Google Scholar] [CrossRef]
- Albonico, M. Methods to sustain drug efficacy in helminth control programmes. Acta Trop. 2003, 86, 233–242. [Google Scholar] [CrossRef]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef]
- Sanchez, A.L.; Gabrie, J.A.; Rueda, M.M.; Mejia, R.E.; Bottazzi, M.E.; Canales, M. A scoping review and prevalence analysis of soil-transmitted helminth infections in Honduras. PLoS Negl. Trop. Dis. 2014, 8, e2653. [Google Scholar] [CrossRef]
- Sanchez, A.L.; Gabrie, J.A.; Canales, M.; Rueda, M.M.; Fontecha, G.A.; Mason, P.W.; Bearman, G.; Stevens, M.P. Soil-Transmitted Helminths, Poverty, and Malnutrition in Honduran Children Living in Remote Rural Communities. Hum. Parasit. Dis. 2016, 8, 27–35. [Google Scholar]
- Jaeger, L.H.; Carvalho-Costa, F.A. Status of benzimidazole resistance in intestinal nematode populations of livestock in Brazil: A systematic review. BMC Vet. Res. 2017, 13, 358. [Google Scholar] [CrossRef]
- Ali, Q.; Rashid, I.; Shabbir, M.Z.; Aziz Ul, R.; Shahzad, K.; Ashraf, K.; Sargison, N.D.; Chaudhry, U. Emergence and the spread of the F200Y benzimidazole resistance mutation in Haemonchus contortus and Haemonchus placei from buffalo and cattle. Vet. Parasitol. 2019, 265, 48–54. [Google Scholar] [CrossRef]
- Milhes, M.; Guillerm, M.; Robin, M.; Eichstadt, M.; Roy, C.; Grisez, C.; Prevot, F.; Lienard, E.; Bouhsira, E.; Franc, M.; et al. A real-time PCR approach to identify anthelmintic-resistant nematodes in sheep farms. Parasitol. Res. 2017, 116, 909–920. [Google Scholar] [CrossRef]
- Zongze, Z.; Xin, Y.; Awais, A.A.; Weiqiang, L.; Chunqun, W.; Di, W.; Yanqin, Z.; Junlong, Z.; Rui, F.; Min, H. Development of a tetra-primer ARMS-PCR for detecting the E198A SNP in the isotype-1 beta-tubulin gene of Haemonchus contortus populations in China. Vet. Parasitol. 2018, 252, 127–130. [Google Scholar] [CrossRef]
- Barrere, V.; Alvarez, L.; Suarez, G.; Ceballos, L.; Moreno, L.; Lanusse, C.; Prichard, R.K. Relationship between increased albendazole systemic exposure and changes in single nucleotide polymorphisms on the beta-tubulin isotype 1 encoding gene in Haemonchus contortus. Vet. Parasitol. 2012, 186, 344–349. [Google Scholar] [CrossRef]
- Ghisi, M.; Kaminsky, R.; Maser, P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet. Parasitol. 2007, 144, 313–320. [Google Scholar] [CrossRef]
- Diawara, A.; Drake, L.J.; Suswillo, R.R.; Kihara, J.; Bundy, D.A.; Scott, M.E.; Halpenny, C.; Stothard, J.R.; Prichard, R.K. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl. Trop. Dis. 2009, 3, e397. [Google Scholar] [CrossRef]
- Diawara, A.; Schwenkenbecher, J.M.; Kaplan, R.M.; Prichard, R.K. Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2013, 88, 1052–1061. [Google Scholar] [CrossRef]
- Rashwan, N.; Bourguinat, C.; Keller, K.; Gunawardena, N.K.; de Silva, N.; Prichard, R. Isothermal Diagnostic Assays for Monitoring Single Nucleotide Polymorphisms in Necator americanus Associated with Benzimidazole Drug Resistance. PLoS Negl. Trop. Dis. 2016, 10, e0005113. [Google Scholar] [CrossRef]
- Avramenko, R.W.; Redman, E.M.; Melville, L.; Bartley, Y.; Wit, J.; Queiroz, C.; Bartley, D.J.; Gilleard, J.S. Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. Int. J. Parasitol. 2019, 49, 13–26. [Google Scholar] [CrossRef]
- Hansen, T.V.; Nejsum, P.; Olsen, A.; Thamsborg, S.M. Genetic variation in codons 167, 198 and 200 of the beta-tubulin gene in whipworms (Trichuris spp.) from a range of domestic animals and wildlife. Vet. Parasitol. 2013, 193, 141–149. [Google Scholar] [CrossRef]
- Diawara, A.; Halpenny, C.M.; Churcher, T.S.; Mwandawiro, C.; Kihara, J.; Kaplan, R.M.; Streit, T.G.; Idaghdour, Y.; Scott, M.E.; Basanez, M.G.; et al. Association between response to albendazole treatment and beta-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl. Trop. Dis. 2013, 7, e2247. [Google Scholar] [CrossRef]
- Zuccherato, L.W.; Furtado, L.F.; Medeiros, C.D.S.; Pinheiro, C.D.S.; Rabelo, E.M. PCR-RFLP screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006766. [Google Scholar] [CrossRef]
- Keegan, J.D.; Good, B.; de Waal, T.; Fanning, J.; Keane, O.M. Genetic basis of benzimidazole resistance in Teladorsagia circumcincta in Ireland. Ir. Vet. J. 2017, 70, 8. [Google Scholar] [CrossRef]
- Ramunke, S.; Melville, L.; Rinaldi, L.; Hertzberg, H.; de Waal, T.; von Samson-Himmelstjerna, G.; Cringoli, G.; Mavrot, F.; Skuce, P.; Krucken, J.; et al. Benzimidazole resistance survey for Haemonchus, Teladorsagia and Trichostrongylus in three European countries using pyrosequencing including the development of new assays for Trichostrongylus. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 230–240. [Google Scholar] [CrossRef]
- Redman, E.; Whitelaw, F.; Tait, A.; Burgess, C.; Bartley, Y.; Skuce, P.J.; Jackson, F.; Gilleard, J.S. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterised by multiple independent hard and soft selective sweeps. PLoS Negl. Trop. Dis. 2015, 9, e0003494. [Google Scholar] [CrossRef]
- Prichard, R.K. Anthelmintic resistance in nematodes: Extent, recent understanding and future directions for control and research. Int. J. Parasitol. 1990, 20, 515–523. [Google Scholar] [CrossRef]
- Vercruysse, J.; Albonico, M.; Behnke, J.M.; Kotze, A.C.; Prichard, R.K.; McCarthy, J.S.; Montresor, A.; Levecke, B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011, 1, 14–27. [Google Scholar] [CrossRef]
- Gabrie, J.A.; Rueda, M.M.; Rodriguez, C.A.; Canales, M.; Sanchez, A.L. Immune Profile of Honduran Schoolchildren with Intestinal Parasites: The Skewed Response against Geohelminths. J. Parasitol. Res. 2016, 2016, 1769585. [Google Scholar] [CrossRef]
- Sanchez, A.L.; Mahoney, D.L.; Gabrie, J.A. Interleukin-10 and soil-transmitted helminth infections in Honduran children. BMC Res. Notes 2015, 8, 55. [Google Scholar] [CrossRef]
- Sanchez, A.L.; Gabrie, J.A.; Usuanlele, M.T.; Rueda, M.M.; Canales, M.; Gyorkos, T.W. Soil-transmitted helminth infections and nutritional status in school-age children from rural communities in Honduras. PLoS Negl. Trop. Dis. 2013, 7, e2378. [Google Scholar] [CrossRef]
- WHO. Eliminating Soil-Transmitted Helminthiases As A Public Health Problem In Children: Progress Report 2001–2010 And Strategic Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Brooker, S.; Pullan, R. Ascaris lumbricoides and Ascariasis: Estimating Numbers Infected and Burden of Disease. In Ascaris: The Neglected Parasite; Holland, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mejia Torres, R.E.; Franco Garcia, D.N.; Fontecha Sandoval, G.A.; Hernandez Santana, A.; Singh, P.; Mancero Bucheli, S.T.; Saboya, M.; Paz, M.Y. Prevalence and intensity of soil-transmitted helminthiasis, prevalence of malaria and nutritional status of school going children in honduras. PLoS Negl. Trop. Dis. 2014, 8, e3248. [Google Scholar] [CrossRef]
- Matamoros, G.; Schultz, J.; Fontecha Sandoval, G.A.; Sanchez, A.L. Persistent prevalence of infections by soil-transmitted helminths in Honduras: poverty, low efficacy of treatment and potential emergence of parasitic resistance. Investig. Clín. 2017, 58, 393–405. [Google Scholar]
- Prichard, R. Anthelmintic resistance. Vet. Parasitol. 1994, 54, 259–268. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Fairweather, I.; Prichard, R.; von Samson-Himmelstjerna, G.; Sangster, N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004, 20, 469–476. [Google Scholar] [CrossRef]
- Tchuem Tchuente, L.A. Control of soil-transmitted helminths in sub-Saharan Africa: diagnosis, drug efficacy concerns and challenges. Acta Trop. 2011, 120 (Suppl. 1), S4–11. [Google Scholar] [CrossRef]
- Furtado, L.F.V.; de Aguiar, P.H.N.; Zuccherato, L.W.; Teixeira, T.T.G.; Alves, W.P.; da Silva, V.J.; Gasser, R.B.; Rabelo, E.M.L. Albendazole resistance induced in Ancylostoma ceylanicum is not due to single-nucleotide polymorphisms (SNPs) at codons 167, 198, or 200 of the beta-tubulin gene, indicating another resistance mechanism. Parasitol. Res. 2019, 118, 837–849. [Google Scholar] [CrossRef]
| Infection | Treatment per Day | |||
|---|---|---|---|---|
| DAY 1 | DAY 2 | DAY 3 | DAY 4 | |
| Trichuris trichiura | Pyrantel-oxantel A | Pyrantel-oxantel | Pyrantel-oxantel | ABZ |
| Ascaris lumbricoides | Piperazine B | ABZ C | ABZ | ABZ |
| Mixed | Pyrantel-oxantel and piperazine | Pyrantel-oxantel | Pyrantel-oxantel | ABZ |
| Infection | Pre-SAC | SAC | TOTAL | |||
|---|---|---|---|---|---|---|
| (0–6 Years) | 7–13 Years | |||||
| n | %/106 | n | %/106 | N | %/106 | |
| T. trichiura only | 28 | 26.4% | 18 | 17% | 46 | 43.4% |
| T. trichiura + A. lumbricoides | 5 | 4.7% | 7 | 6.6% | 12 | 11.3% |
| A. lumbricoides only | 2 | 1.9% | 3 | 2.8% | 5 | 4.7% |
| Hookworm only | 0 | 0% | 0 | 0% | 0 | 0% |
| Hookworm + T. trichiura | 1 | 1% | 0 | 0 | 1 | 1% |
| Hookworm + A. lumbricoides + T. trichiura | 1 | 1% | 0 | 0 | 1 | 1% |
| No helminths observed | 23 | 21.7% | 18 | 17% | 41 | 38.7% |
| Total | 60 | 56% | 46 | 43.4% | 106 | 100% |
| Infection | Cases of Intensity of Infection * | ||
|---|---|---|---|
| Light | Moderate | Heavy | |
| T. trichiura | 48/60 (80%) | 11/60 (18%) | 1/60 (2%) |
| A. lumbricoides | 12/18 (67%) | 6/18 (33%) | 0/18 (0%) |
| Hookworm | 2/2 (100%) | 0/0 (0%) | 0/0 (0%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matamoros, G.; Rueda, M.M.; Rodríguez, C.; Gabrie, J.A.; Canales, M.; Fontecha, G.; Sanchez, A. High Endemicity of Soil-Transmitted Helminths in a Population Frequently Exposed to Albendazole but No Evidence of Antiparasitic Resistance. Trop. Med. Infect. Dis. 2019, 4, 73. https://doi.org/10.3390/tropicalmed4020073
Matamoros G, Rueda MM, Rodríguez C, Gabrie JA, Canales M, Fontecha G, Sanchez A. High Endemicity of Soil-Transmitted Helminths in a Population Frequently Exposed to Albendazole but No Evidence of Antiparasitic Resistance. Tropical Medicine and Infectious Disease. 2019; 4(2):73. https://doi.org/10.3390/tropicalmed4020073
Chicago/Turabian StyleMatamoros, Gabriela, María Mercedes Rueda, Carol Rodríguez, Jose A. Gabrie, Maritza Canales, Gustavo Fontecha, and Ana Sanchez. 2019. "High Endemicity of Soil-Transmitted Helminths in a Population Frequently Exposed to Albendazole but No Evidence of Antiparasitic Resistance" Tropical Medicine and Infectious Disease 4, no. 2: 73. https://doi.org/10.3390/tropicalmed4020073
APA StyleMatamoros, G., Rueda, M. M., Rodríguez, C., Gabrie, J. A., Canales, M., Fontecha, G., & Sanchez, A. (2019). High Endemicity of Soil-Transmitted Helminths in a Population Frequently Exposed to Albendazole but No Evidence of Antiparasitic Resistance. Tropical Medicine and Infectious Disease, 4(2), 73. https://doi.org/10.3390/tropicalmed4020073








