Strategic Planning for Tuberculosis Control in the Republic of Fiji
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Approach
2.2. Background Demographics
2.3. Model of TB Transmission and Progression
2.4. Simulation of the Health Care System
2.5. Automatic Calibration and Uncertainty
2.6. Interventions
2.7. Economic Analysis
3. Results
3.1. Calibration and Validation
3.2. Intervention Effectiveness
3.3. Impact of T2DM Burden on TB Epidemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO TB Country Profile of Fiji; WHO: Geneva, Switzerland; Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=FJ&LAN=EN&outtype=html (accessed on 19 June 2017).
- Fiji Ministry of Health and Medical Services. TB National Strategic Plan 2015–2019; Fiji Ministry of Health and Medical Services: Suva, Fiji, 2014.
- Lin, S.; Tukana, I.; Linhart, C.; Morrell, S.; Taylor, R.; Vatucawaqa, P.; Magliano, D.J.; Zimmet, P. Diabetes and obesity trends in Fiji over 30 years. J. Diabetes 2016, 8, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, 1091–1101. [Google Scholar]
- World Health Organization. Country Profiles on HIV. Available online: http://www.wpro.who.int/hiv/data/countries/fji/en/ (accessed on 19 June 2017).
- Alo, A.; Gounder, S.; Graham, S.M. Clinical characteristics and treatment outcomes of tuberculosis cases hospitalised in the intensive phase in Fiji. Public Heal. Action 2014, 4, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Trauer, J.M.; Ragonnet, R.; Doan, T.N.; McBryde, E.S. Modular programming for tuberculosis control, the ‘AuTuMN’ platform. BMC Infect. Dis. 2017, 17, 546. [Google Scholar] [CrossRef] [PubMed]
- Ragonnet, R.; Trauer, J.M.; Scott, N.; Meehan, M.T.; Denholm, J.T.; McBryde, E.S. Optimally capturing latency dynamics in models of tuberculosis transmission. Epidemics 2017, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Trauer, J.M.; Moyo, N.; Tay, E.-L.; Dale, K.; Ragonnet, R.; McBryde, E.S.; Denholm, J.T. Risk of Active Tuberculosis in the Five Years Following Infection... 15%? Chest 2016, 149, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Tiemersma, E.W.; van der Werf, M.J.; Borgdorff, M.W.; Williams, B.G.; Nagelkerke, N.J. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS ONE 2011, 6, e17601. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.K. Monte Carlo Sampling Methods Using Markov Chains and Their Applications. Biometrika 1970, 57, 97–109. [Google Scholar] [CrossRef]
- Thiam, S.; LeFevre, A.M.; Hane, F.; Ndiaye, A.; Ba, F.; Fielding, K.L.; Ndir, M.; Lienhardt, C. Effectiveness of a Strategy to Improve Adherence to Tuberculosis Treatment in a Resource-Poor Setting. JAMA 2007, 297, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef]
- Sollai, S.; Galli, L.; de Martino, M.; Chiappini, E. Systematic review and meta-analysis on the utility of Interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: A 2013 update. BMC Infect. Dis. 2014, 14, S6. [Google Scholar] [CrossRef] [PubMed]
- Smieja, M.J.; Marchetti, C.; Cook, D.J.; Smaill, F.M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Bandason, T.; Duong, T.; Dauya, E.; Makamure, B.; Churchyard, G.J.; Williams, B.G.; Munyati, S.S.; Butterworth, A.E.; Mason, P.R.; et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): A cluster-randomised trial. Lancet 2010, 376, 1244–1253. [Google Scholar] [CrossRef]
- Jaramillo, E. The impact of media-based health education on tuberculosis diagnosis in Cali, Colombia. Health Policy Plan. 2001, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Bank Data: Population Ages 0–14. Washington, DC, USA, 2017. Available online: https://data.worldbank.org/indicator/SP.POP.0014.TO.ZS?locations=FJ (accessed on 19 June 2017).
- Lönnroth, K.; Roglic, G.; Harries, A.D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014, 2, 730–739. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. PLoS MED 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Viney, K.; Brostrom, R.; Nasa, J.; Defang, R.; Kienene, T. Diabetes and tuberculosis in the Pacific Islands region. Lancet Diabetes Endocrinol 2014, 2, 932. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2016. WHO: Geneva, Switzerland, 2017. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 19 June 2017).
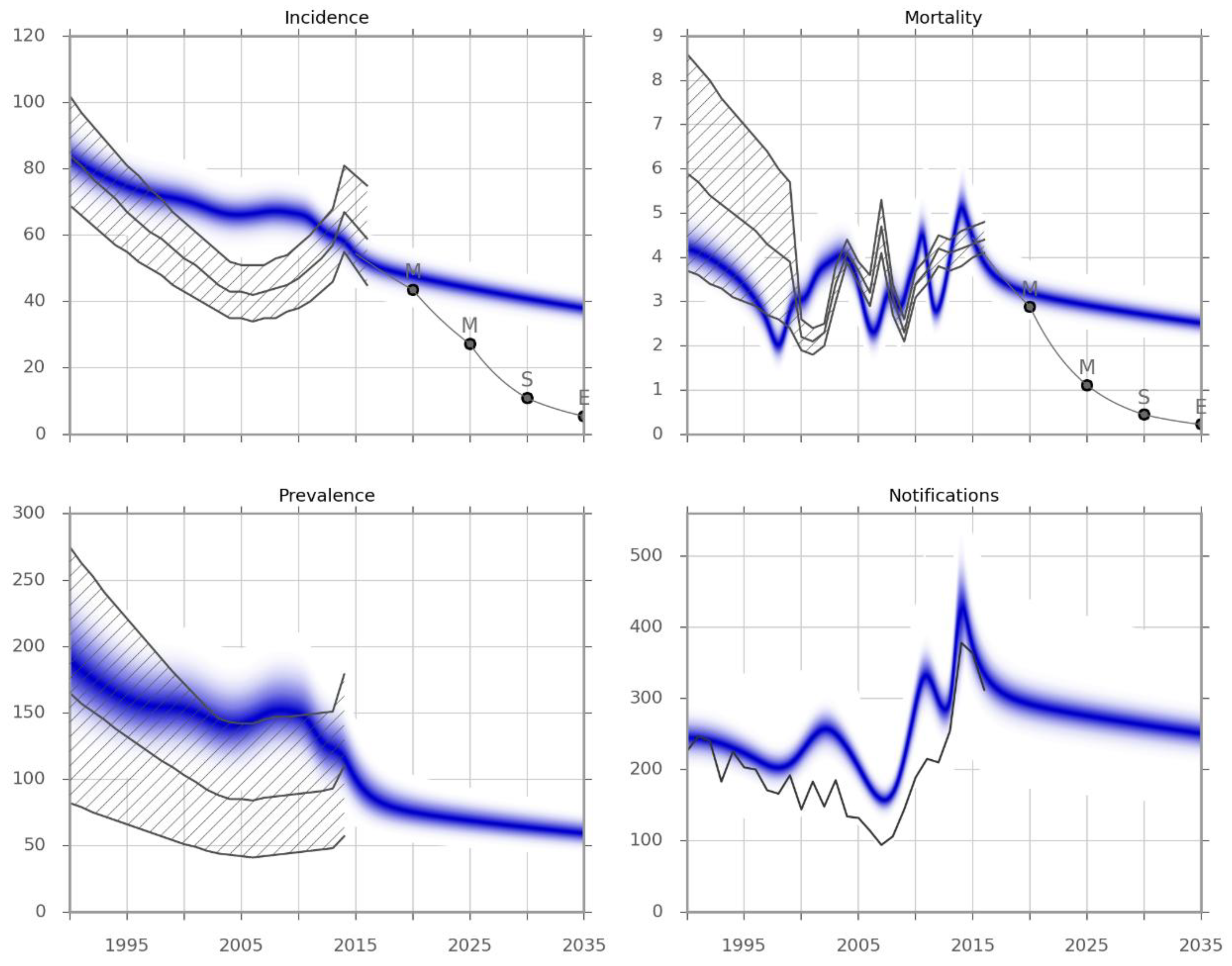

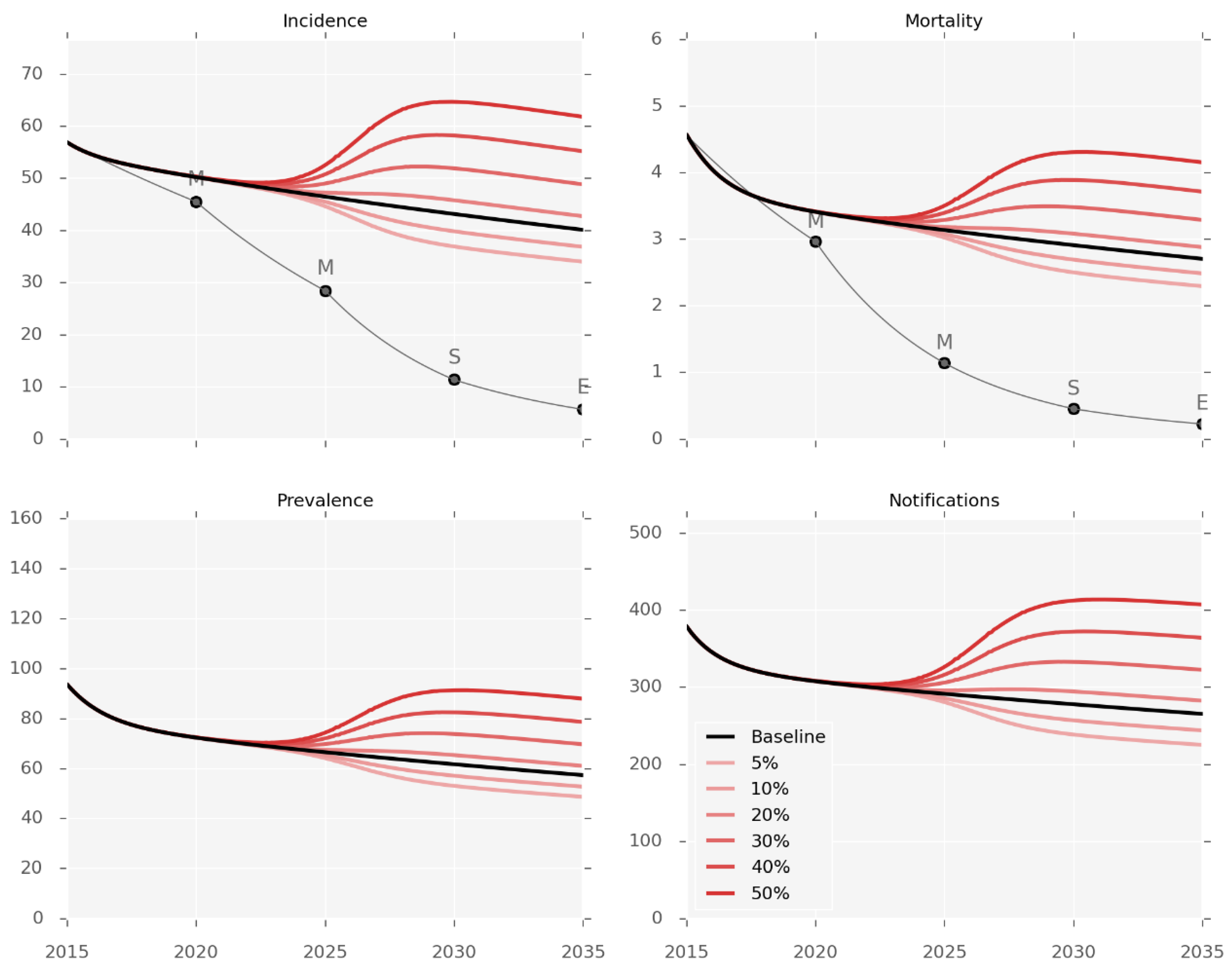
| Scenario | Description | Level of Evidence * | Applicability of Evidence | Primary Evidence Source | Coverage Achievable (by 2020) | Model Implementation |
|---|---|---|---|---|---|---|
| 1. Support for patients under treatment | Health worker visits patients upon their return home from the hospital, treatment adherence checks, regular clinic appointments | 2 | Programmatic | Thiam et al. 2007 [12] | 100% | Decrease in all treatment outcomes other than success (i.e., death and non-death unfavourable) by 43% (range 21% to 89%) |
| 2. Decentralisation of care | Transfer of diagnostic and treatment facilities to most remote communities to remove access barriers | 5 | Mechanism-based reasoning | N/A | 70% | Increase in case detection rate from baseline value to idealised value informed by those reported by the best-performing regional TB programs, increasing to 75% (range 65 to 90%) |
| 3. GeneXpert replaces smear | GeneXpert replaces smear microscopy as the primary diagnostic test for passive case detection across the health service | 1 | Programmatic | Boehme et al. 2011 [13] | 100% | Decrease in smear-negative cases missed by diagnostic algorithm by 76.9% (range 56.3% to 100%), decrease in time to treatment commencement to seven days (range one to 30 days) |
| 4. Isoniazid preventive therapy (IPT) | Expansion of coverage of IPT for contacts of pulmonary cases from existing coverage levels (23.6% of under 5 years old in 2014) to broad coverage of all cases under 15 years old | 1 | Clinical | Sollai et al. 2014, Smieja et al. 2000 [14,15] | 80% | Proportion of infection occurring in households is 60% (range 40% to 80%), sensitivity of testing for LTBI 70% (range 70% to 80%), efficacy of treatment is 60% (range 48% to 69%) |
| 5. Active case finding (ACF) | Van and ferry-based outreach to detect previously unrecognised cases | 2 | Programmatic | Corbett et al. 2010 [16] | 50% | GeneXpert test performed in individuals presenting any TB-related symptom (27% of individuals) Proportion of cases diagnosed during a single ACF round is 23% (range 10% to 30%), with one round of ACF conducted per year |
| 6. Awareness raising | Broad mass media campaign and community engagement to improve community knowledge, attitudes, and practices associated with TB | 3 | Programmatic | Jaramillo et al. [17] | 50% | Rate of presentation for care for undiagnosed increases 1.52-fold (range 1.34 to 1.92-fold) the baseline value |
| 7. Combination of scenarios 1–6 | All the interventions described above were implemented simultaneously | |||||
| Intervention * | Incidence in 2035 | True mortality in 2035 | Observed Mortality in 2035 | Additional Intervention Costs | |||
|---|---|---|---|---|---|---|---|
| Per 100,000 per Year | Relative Change (%) | Per 100,000 per Year | Relative Change (%) | Per 100,000 per Year | Relative Change (%) | (USD, per Year) | |
| Baseline projection | 39.1 | - | 7.1 | - | 2.6 | - | - |
| 1 (Treatment support) | 38.4 (38.2 to 38.7) | −1.7 (−2.3 to −1.0) | 5.5 (5.0 to 6.2) | −22.5 (−29.7 to −12.4) | 1.2 (0.8 to 1.9) | −53.2 (−70.2 to −29.3) | 441,096 |
| 2 (Decentralisation) | 38.4 (36.1 to 38.9) | −1.8 (−7.6 to −0.6) | 6.6 (5.3 to 6.9) | −7.3 (−25.7 to −3.2) | 2.8 (2.7 to 2.8) | 6.4 (3.8 to 7.2) | 532,825 |
| 3 (GeneXpert) | 38.3 (38.2 to 38.5) | −2.1 (−2.3 to −1.5) | 6.8 (6.7 to 6.9) | −5.0 (−5.6 to −3.7) | 2.7 (2.7 to 2.7) | 2.9 (2.2 to 3.2) | 2,046,850 |
| 4 (IPT) | 38.3 (38.2 to 38.5) | −2.0 (−2.1 to −1.9) | 6.8 (6.7 to 6.9) | −4.8 (−5.0 to −4.7) | 2.7 (2.7 to 2.7) | 2.8 (2.7 to 2.9) | 24,688 |
| 5 (ACF) | 38.3 (37.9 to 38.6) | −2.2 (−3.1 to −1.3) | 6.7 (6.5 to 6.9) | −6.5 (−9.0 to −3.7) | 2.6 (2.6 to 2.6) | −0.1 (−0.2 to −0.1) | 31,414,371 |
| 6 (Awareness) | 37.7 (37.3 to 38.3) | −3.5 (−4.6 to −2.2) | 6.3 (6.1 to 6.6) | −11.5 (−15.1 to −7.2) | 2.7 (2.6 to 2.7) | 0.7 (0.4 to 0.9) | 4,576,185** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragonnet, R.; Underwood, F.; Doan, T.; Rafai, E.; Trauer, J.; McBryde, E. Strategic Planning for Tuberculosis Control in the Republic of Fiji. Trop. Med. Infect. Dis. 2019, 4, 71. https://doi.org/10.3390/tropicalmed4020071
Ragonnet R, Underwood F, Doan T, Rafai E, Trauer J, McBryde E. Strategic Planning for Tuberculosis Control in the Republic of Fiji. Tropical Medicine and Infectious Disease. 2019; 4(2):71. https://doi.org/10.3390/tropicalmed4020071
Chicago/Turabian StyleRagonnet, Romain, Frank Underwood, Tan Doan, Eric Rafai, James Trauer, and Emma McBryde. 2019. "Strategic Planning for Tuberculosis Control in the Republic of Fiji" Tropical Medicine and Infectious Disease 4, no. 2: 71. https://doi.org/10.3390/tropicalmed4020071
APA StyleRagonnet, R., Underwood, F., Doan, T., Rafai, E., Trauer, J., & McBryde, E. (2019). Strategic Planning for Tuberculosis Control in the Republic of Fiji. Tropical Medicine and Infectious Disease, 4(2), 71. https://doi.org/10.3390/tropicalmed4020071






