Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Mosquito Collection and Identification
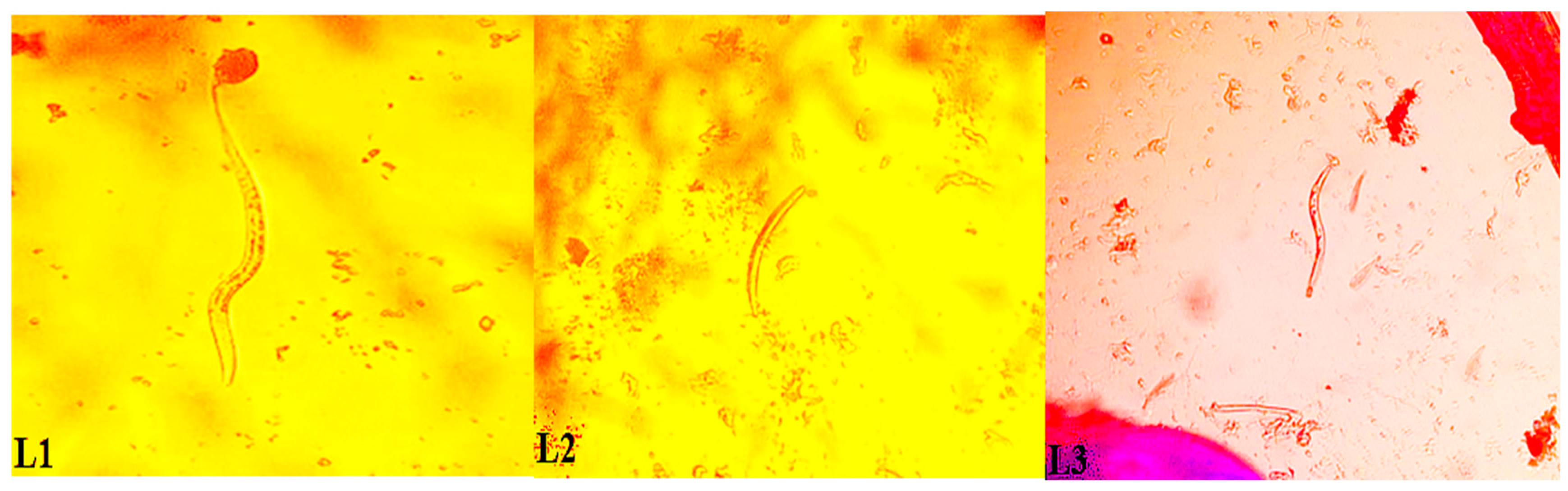
2.3. Mosquito Dissection
2.4. Extraction and Detection of W. bancrofti in Dissected Mosquitoes
2.5. Extraction of Nucleic Acids from Pooled Mosquitoes with a TRIzol Reagent
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Mosquito Abundance and Composition
3.2. Molecular Identification of An. gambiae and W. bancrofti
3.3. Transmission Indices of An. gambiae Complex from Ahanta West District
3.4. Detection of W. bancrofti Using Molecular Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Monitoring and Epidemiological Assessment of Mass Drug Administration in Global Programme to Eliminate Lymphatic Filariasis: A Manual for National Elimination Programmes; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Jones, C.; Ngasala, B.; Derua, Y.A.; Tarimo, D.; Reimer, L.; Bockarie, M.; Malecela, M.N. Lymphatic filariasis transmission in Rufiji district, southeastern Tanzania: infection status of the human population and mosquito vectors after twelve rounds of mass drug administration. Parasit. Vectors 2018, 11, 588. [Google Scholar] [CrossRef]
- Okorie, P.N.; de Souza, D.K. Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa. Trans. R. Soc.Trop. Med. Hyg. 2016, 110, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Biritwum, N.K.; de Souza, D.K.; Marfo, B.; Odoom, S.; Alomatu, B.; Asiedu, O.; Yeboah, A.; Hervie, T.E.; Mensah, E.O.; Yikpotey, P.; et al. Fifteen years of programme implementation for the elimination of lymphatic filariasis in Ghana: impact of MDA on immunoparasitological indicators. PLoS Negl. Trop. Dis. 2017, 11, e0005280. [Google Scholar] [CrossRef]
- Kanamitie, J.N.; Ahorlu, C.S.; Otchere, J.; Aboagye-Antwi, F.; Kwansa-Bentum, B.; Boakye, D.A.; Biritwum, N.-K.; Wilson, M.D.; de Souza, D.K. Twelve-month longitudinal parasitological assessment of lymphatic filariasis-positive individuals: Impact of a biannual treatment with ivermectin and albendazole. Trop. Med. Int. Health 2017, 22, 1451–1456. [Google Scholar] [CrossRef]
- Dorkenoo, M.A.; de Souza, D.K.; Apetogbo, Y.; Oboussoumi, K.; Yehadji, D.; Tchalim, M.; Etassoli, S.; Koudou, B.; Ketoh, G.K.; Sodahlon, Y.; et al. Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: No evidence for active transmission. Parasit. Vectors 2018, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.S.; Orelus, J.N.; Roberts, J.M.; Lammie, J.; Streit, T.G. PCR and mosquito dissection as tools to monitor filarial infection levels following mass treatment. Filaria J. 2003, 2, 11. [Google Scholar] [CrossRef][Green Version]
- Plichart, C.; Sechan, Y.; Davies, N.; Legrand, A.M. PCR and dissection as tools to monitor filarial infection of Aedes polynesiensis mosquitoes in French Polynesia. Filaria J. 2006, 5, 2. [Google Scholar] [CrossRef]
- Owusu, I.O.; de Souza, D.K.; Anto, F.; Wilson, M.D.; Boakye, D.A.; Bockarie, M.J.; Gyapong, J.O. Evaluation of human and mosquito based diagnostic tools for defining endpoints for elimination of Anopheles transmitted lymphatic filariasis in Ghana. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Bockarie, M.J. Molecular xenomonitoring of lymphatic filariasis. Am. J. Trop. Med. Hyg. 2007, 77, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, B.L.; de Souza, D.K.; Goepogui, A.; Narh, C.A.; King, S.A.; Mamadou, B.S.; Diakité, L.; Dadzie, S.K.; Boakye, D.A.; Utzinger, J.; et al. Assessing the presence of Wuchereria bancrofti in vector and human populations from urban communities in Conakry, Guinea. Parasit. Vectors 2015, 8, 492. [Google Scholar] [CrossRef]
- de Souza, D.; Otchere, J.; Ahorlu, C.; Adu-Amankwah, S.; Larbi, I.; Dumashie, E.; McCarthy, F.; King, S.; Otoo, S.; Osabutey, D.; et al. Low microfilaremia levels in three districts in coastal Ghana with at least 16 years of mass drug administration and persistent transmission of lymphatic filariasis. Trop. Med. Infect. Dis. 2018, 3, 105. [Google Scholar] [CrossRef] [PubMed]
- Pi-Bansa, S.; Osei, J.H.N.; Frempong, K.K.; Elhassan, E.; Akuoko, O.K.; Agyemang, D.; Ahorlu, C.; Appawu, M.A.; Koudou, B.G.; Wilson, M.D.; et al. Potential factors influencing lymphatic filariasis transmission in “hotspot” and “control” areas in Ghana: The importance of vectors. Infect. Dis. Poverty 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Pi-Bansa, S.; Osei, J.H.N.; Joannides, J.; Woode, M.E.; Agyemang, D.; Elhassan, E.; Dadzie, S.K.; Appawu, M.A.; Wilson, M.D.; Koudou, B.G.; et al. Implementing a community vector collection strategy using xenomonitoring for the endgame of lymphatic filariasis elimination. Parasit. Vectors 2018, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Publ. S. Afr. Inst. Med. Res. 1968, 54, 1–343. [Google Scholar]
- Gillies, M.T.; Coetzee, M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). Publ. S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fanello, C.; Santolamazza, F.; Della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 2002, 16, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Hunt, R.H.; Wilkerson, R.; Torre, A.D.; Coulibaly, M.B.; Besansky, N.J. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 2013, 3619, 246–274. [Google Scholar] [CrossRef]
- Naing, L.; Winn, T.; Rusli, B.N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- WHO. Lymphatic Filariasis: A Handbook of Practical Entomology for National Elimination Programmes; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- de Souza, D.K.; Sesay, S.; Moore, M.G.; Ansumana, R.; Narh, C.A.; Kollie, K.; Rebollo, M.P.; Koudou, B.G.; Koroma, J.B.; Bolay, F.K.; et al. No evidence for lymphatic filariasis transmission in big cities affected by conflict related rural-urban migration in Sierra Leone and Liberia. PLoS. Negl. Trop. Dis. 2014, 8, e2700. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, R.M.R.; Farid, H.A.; Kamal, I.H.; Ibrahim, G.H.; Morsy, Z.S.; Faris, R.; Weil, G.J.; Williams, S.A.; Gad, A.M. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans. R. Soc.Trop. Med. Hyg. 1997, 91, 156–160. [Google Scholar] [CrossRef]
- Rao, R.U.; Atkinson, L.J.; Ramzy, R.M.R.; Helmy, H.; Farid, H.A.; Bockarie, M.J.; Susapu, M.; Laney, S.J.; Williams, S.A.; Weil, G.J. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am. J. Trop. Med. Hyg. 2006, 74, 826–832. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Role of Polymerase Chain Reaction Techniques for Assessing Lymphatic Filariasis Transmission; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Boakye, D.A.; Baidoo, H.A.; Glah, E.; Brown, C.; Appawu, M.; Wilson, M.D. Monitoring lymphatic filariasis interventions: adult mosquito sampling, and improved PCR-based pool screening method for Wuchereria bancrofti infection in Anopheles mosquitoes. Filaria J. 2007, 6, 13. [Google Scholar] [CrossRef]
- Laney, S.J.; Ramzy, R.M.R.; Helmy, H.H.; Farid, H.A.; Ashour, A.A.; Weil, G.J.; Williams, S.A. Detection of Wuchereria bancrofti L3 larvae in mosquitoes: A reverse transcriptase PCR assay evaluating infection and infectivity. PLoS. Negl. Trop. Dis. 2010, 4, e602. [Google Scholar] [CrossRef] [PubMed]
- Katholi, C.R.; Toe, L.; Merriweather, A.; Unnasch, T.R. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J. Infect. Dis. 1995, 172, 1414–1417. [Google Scholar] [CrossRef]
- Appawu, M.A.; Dadzie, S.K.; Baffoe Wilmot, A.; Wilson, M.D. Lymphatic filariasis in Ghana: entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East region of Ghana. Trop. Med. Int. Health 2001, 6, 511–516. [Google Scholar] [CrossRef]
- Coulibaly, Y.; Dembele, B.; Diallo, A.; Kristensen, S.; Konate, S.; Dolo, H.; Dicko, I.; Sangare, M.; Keita, F.; Boatin, B.A.; et al. Wuchereria bancrofti transmission pattern in southern Mali prior to and following the institution of mass drug administration. Parasit. Vectors 2013, 6, 247. [Google Scholar] [CrossRef]
- Opoku, M.; Minetti, C.; Kartey-Attipoe, W.D.; Otoo, S.; Otchere, J.; Gomes, B.; de Souza, D.K.; Reimer, L.J. An assessment of mosquito collection techniques for xenomonitoring of anopheline-transmitted lymphatic filariasis in Ghana. Parasitology 2018, 145, 1783–1791. [Google Scholar] [CrossRef]
- Govella, N.J.; Chaki, P.P.; Geissbühler, Y.; Kannady, K.; Okumu, F.; Charlwood, J.D.; Anderson, R.A.; Killeen, G.F. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009, 8, 157. [Google Scholar] [CrossRef]
- Wong, J.; Bayoh, N.; Olang, G.; Killeen, G.F.; Hamel, M.J.; Vulule, J.M.; Gimnig, J.E. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013, 12, 143. [Google Scholar] [CrossRef]
- Pam, D.D.; de Souza, D.K.; D’Souza, S.; Opoku, M.; Sanda, S.; Nazaradden, I.; Anagbogu, I.N.; Okoronkwo, C.; Davies, E.; Elhassan, E.; et al. Is mass drug administration against lymphatic filariasis required in urban settings? The experience in Kano, Nigeria. PLoS. Negl. Trop. Dis. 2017, 11, e0006004. [Google Scholar] [CrossRef] [PubMed]
- WHO. Training Module on Malaria Control: Malaria Entomology and Vector Control. Guide for Tutors; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Briët, O.J.T.; Huho, B.J.; Gimnig, J.E.; Bayoh, N.; Seyoum, A.; Sikaala, C.H.; Govella, N.; Diallo, D.A.; Abdullah, S.; Smith, T.A.; et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: A pooled-analysis of 13 comparisons with human landing catches. Malar J. 2015, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Boakye, D.A.; Wilson, M.D.; Appawu, M.A.; Gyapong, J. Vector competence, for Wuchereria bancrofti, of the Anopheles populations in the Bongo district of Ghana. Ann. Trop. Med. Parasitol. 2004, 98, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Gyapong, J.O.; Webber, R.H.; Morris, J.; Bennett, S. Prevalence of hydrocele as a rapid diagnostic index for lymphatic filariasis. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 40–43. [Google Scholar] [CrossRef]
- de Souza, D.; Kelly-Hope, L.; Lawson, B.; Wilson, M.; Boakye, D. Environmental factors associated with the distribution of Anopheles gambiae s.s. in Ghana; an important vector of lymphatic filariasis and malaria. PLoS ONE 2010, 5, e9927. [Google Scholar] [CrossRef] [PubMed]
- Dunyo, S.K.; Appawu, M.; Nkrumah, F.K.; Baffoe-Wilmot, A.; Pedersen, E.M.; Simonsen, P.E. Lymphatic filariasis on the coast of Ghana. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 634–638. [Google Scholar] [CrossRef]
- Southgate, B.A.; Bryan, J.H. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 523–530. [Google Scholar] [CrossRef]
- Amuzu, H.; Wilson, M.D.; Boakye, D.A. Studies of Anopheles gambiae s.l. (Diptera: Culicidae) exhibiting different vectorial capacities in lymphatic filariasis transmission in the Gomoa district, Ghana. Parasit. Vectors 2010, 3, 85. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.K.; Koudou, B.; Kelly-Hope, L.A.; Wilson, M.D.; Bockarie, M.J.; Boakye, D.A. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit. Vectors 2012, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Southgate, B.A. The significance of low density microfilaraemia in the transmission of lymphatic filarial parasites. J. Trop. Med. Hyg. 1992, 95, 79–86. [Google Scholar] [PubMed]
- Bockarie, M.J.; Pedersen, E.M.; White, G.B.; Michael, E. Role of vector control in the Global Program to Eliminate Lymphatic Filariasis. Annu. Rev. Entomol. 2009, 54, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Hope, L.A.; Molyneux, D.H.; Bockarie, M.J. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasit. Vectors 2013, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Ughasi, J.; Bekard, H.; Coulibaly, M.; Adabie-Gomez, D.; Gyapong, J.; Appawu, M.; Wilson, M.; Boakye, D. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit. Vectors 2012, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Anosike, J.C.; Nwoke, B.E.; Ajayi, E.G.; Onwuliri, C.O.; Okoro, O.U.; Oku, E.E.; Asor, J.E.; Amajuoyi, O.U.; Ikpeama, C.A.; Ogbusu, F.I.; et al. Lymphatic filariasis among the Ezza people of Ebonyi state, eastern Nigeria. Ann. Agric. Environ. Med. 2005, 12, 181–186. [Google Scholar] [PubMed]
- Agi, P.; Ebenezer, A. Observations on filarial infection in Amassoma community in the Niger Delta, Nigeria. J. Appl. Sci. Environ. Manag. 2010, 13, 15–19. [Google Scholar] [CrossRef]
| District | Community | Number of MDA Rounds | Microfilariae Prevalence in 2000 (%) | Microfilariae Prevalence in 2014 (%) | Number of An. gambiae Dissected |
|---|---|---|---|---|---|
| Ahanta West | Asemkow Antseambua | 16 | 19.5 | 2.7 | 320 |
| Mpohor | Obrayebona Ampeasem | 11 | 0.0 | 0.0 | 368 |
| Kassena Nankana West | Navio Central Badunu | 15 | 29.4 | 1.3 | 217 |
| Bongo | Atampiisi Bongo Balungu Nabiisi | 13 | 16.7 | 0.0 | 211 |
| Method | Community | District | An. gambiae | Culex species | Mansonia species | Aedes species | An. pharoensis | An. coustani | Total Collected |
|---|---|---|---|---|---|---|---|---|---|
| Human landing catches | Asemkow Antseambua | Ahanta West | 18,213 | 1200 | 2386 | 8 | 0 | 4 | |
| Obrayebona Ampeasem | Mpohor | 4109 | 66 | 72 | 6 | 0 | 3 | ||
| Badunu Navio Central | Kassena Nankana West | 426 | 489 | 11 | 42 | 2 | 4 | ||
| Atampiisi Bongo Balungu Nabiisi | Bongo | 354 | 301 | 5 | 36 | 2 | 0 | ||
| Pyrethrum spray catches | Asemkow Antseambua | Ahanta West | 271 | 4 | 19 | 1 | 36 | 0 | |
| Obrayebona Ampeasem | Mpohor | 375 | 14 | 7 | 0 | 1 | 0 | ||
| Badunu Navio Central | Kassena Nankana West | 801 | 384 | 0 | 1 | 1 | 9 | ||
| Atampiisi Bongo Balungu Nabiisi | Bongo | 437 | 318 | 0 | 5 | 2 | 1 | ||
| Window exit trap | Asemkow Antseambua | Ahanta West | 396 | 17 | 29 | 0 | 0 | 0 | |
| Obrayebona Ampeasem | Mpohor | 119 | 1 | 1 | 1 | 9 | 0 | ||
| Badunu Navio Central | Kassena Nankana West | 12 | 6 | 1 | 1 | 1 | 0 | ||
| Atampiisi Bongo Balungu Nabiisi | Bongo | 35 | 7 | 0 | 1 | 0 | 1 | ||
| Total | 25,548 | 2807 | 2531 | 102 | 54 | 22 | 31,064 |
| District | Sibling Species of the Anopheles gambiae Complex | |||||||
|---|---|---|---|---|---|---|---|---|
| An. gambiae s. s. | An. arabiensis | An. melas | An. coluzzii | |||||
| n | % | n | % | n | % | n | % | |
| Ahanta West | 3 | 0.9 | 11 | 3.4 | 275 | 85.9 | 12 | 3.8 |
| Mpohor | 226 | 61.4 | 0 | 0 | 1 | 0.3 | 122 | 33.2 |
| Kassena Nankana West | 57 | 26.3 | 25 | 11.5 | 0 | 0 | 124 | 57.1 |
| Bongo | 54 | 25.6 | 0 | 0 | 0 | 0 | 142 | 67.3 |
| District | Average Number of An. gambiae Sampled per Month | Vector Biting Density (MBR) | Annual Biting Rate (ABR) | Average Number of An. gambiae Dissected per Month | Average Infection per Month | Average Infectivity per Month | Infection Rate (%) | Infectivity Rate (%) | Annual Infective Biting Rate (AIBR) | Average Worm Load per Month | Annual Transmission Potential (ATP) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahanta West | 1401 | 43.8 | 15,987 | 25 | 0.620 | 0.150 | 0.025 (2.5) | 0.006 (0.6) | 95.922 | 0.077 | 7.386 |
| Mpohor | 316 | 9.9 | 3614 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kassena Nankana West | 33 | 1.0 | 365 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bongo | 27 | 0.8 | 292 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Species | District | Number of Pools | Average Pool Size | Number of Mosquitoes Processed | Positive (Infection/Infectivity) | 95% CI |
|---|---|---|---|---|---|---|
| An. gambiae | Ahanta West | 97 | 20.6 | 2000 | 0 | 0–0.00095 |
| Mpohor | 91 | 22.0 | 2000 | 0 | 0–0.00095 | |
| Kassena Nankana West | 13 | 19.5 | 253 | 0 | 0–0.00756 | |
| Bongo | 13 | 17.3 | 225 | 0 | 0–0.00849 | |
| Mansonia species | Ahanta West | 83 | 21.1 | 1754 | 0 | 0–0.00109 |
| Mpohor | 2 | 25.0 | 50 | 0 | 0–0.03767 | |
| Kassena Nankana West | 1 | 14.0 | 14 | 0 | 0–0.12815 | |
| Bongo | 1 | 5.0 | 5 | 0 | 0–3.18868 | |
| Culex species | Ahanta West | 63 | 20.0 | 1261 | 0 | 0–0.00152 |
| Mpohor | 2 | 19.0 | 38 | 0 | 0–0.04927 | |
| Kassena Nankana West | 19 | 19.4 | 369 | 0 | 0–0.00518 | |
| Bongo | 8 | 16.3 | 133 | 0 | 0–0.01433 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi-Bansa, S.; Osei, J.H.N.; Kartey-Attipoe, W.D.; Elhassan, E.; Agyemang, D.; Otoo, S.; Dadzie, S.K.; Appawu, M.A.; Wilson, M.D.; Koudou, B.G.; et al. Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana. Trop. Med. Infect. Dis. 2019, 4, 49. https://doi.org/10.3390/tropicalmed4010049
Pi-Bansa S, Osei JHN, Kartey-Attipoe WD, Elhassan E, Agyemang D, Otoo S, Dadzie SK, Appawu MA, Wilson MD, Koudou BG, et al. Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana. Tropical Medicine and Infectious Disease. 2019; 4(1):49. https://doi.org/10.3390/tropicalmed4010049
Chicago/Turabian StylePi-Bansa, Sellase, Joseph H. N. Osei, Worlasi D. Kartey-Attipoe, Elizabeth Elhassan, David Agyemang, Sampson Otoo, Samuel K. Dadzie, Maxwell A. Appawu, Michael D. Wilson, Benjamin G. Koudou, and et al. 2019. "Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana" Tropical Medicine and Infectious Disease 4, no. 1: 49. https://doi.org/10.3390/tropicalmed4010049
APA StylePi-Bansa, S., Osei, J. H. N., Kartey-Attipoe, W. D., Elhassan, E., Agyemang, D., Otoo, S., Dadzie, S. K., Appawu, M. A., Wilson, M. D., Koudou, B. G., de Souza, D. K., Utzinger, J., & Boakye, D. A. (2019). Assessing the Presence of Wuchereria bancrofti Infections in Vectors Using Xenomonitoring in Lymphatic Filariasis Endemic Districts in Ghana. Tropical Medicine and Infectious Disease, 4(1), 49. https://doi.org/10.3390/tropicalmed4010049





