Investigation of a Scabies Outbreak in Drought-Affected Areas in Ethiopia
Abstract
:1. Background
1.1. Drought
1.2. Government Priorities/Political Setting
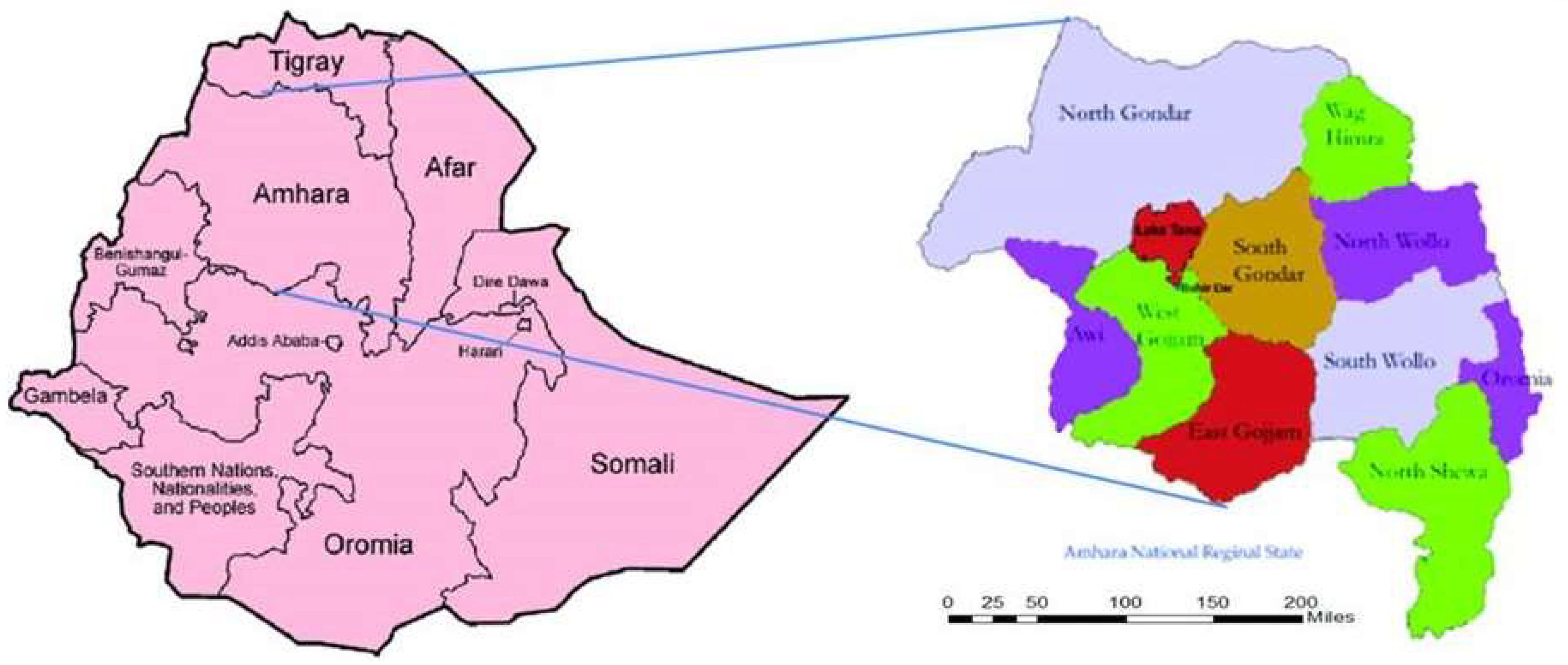
2. Methods
2.1. Phase 1
2.2. Phase 2
3. Results
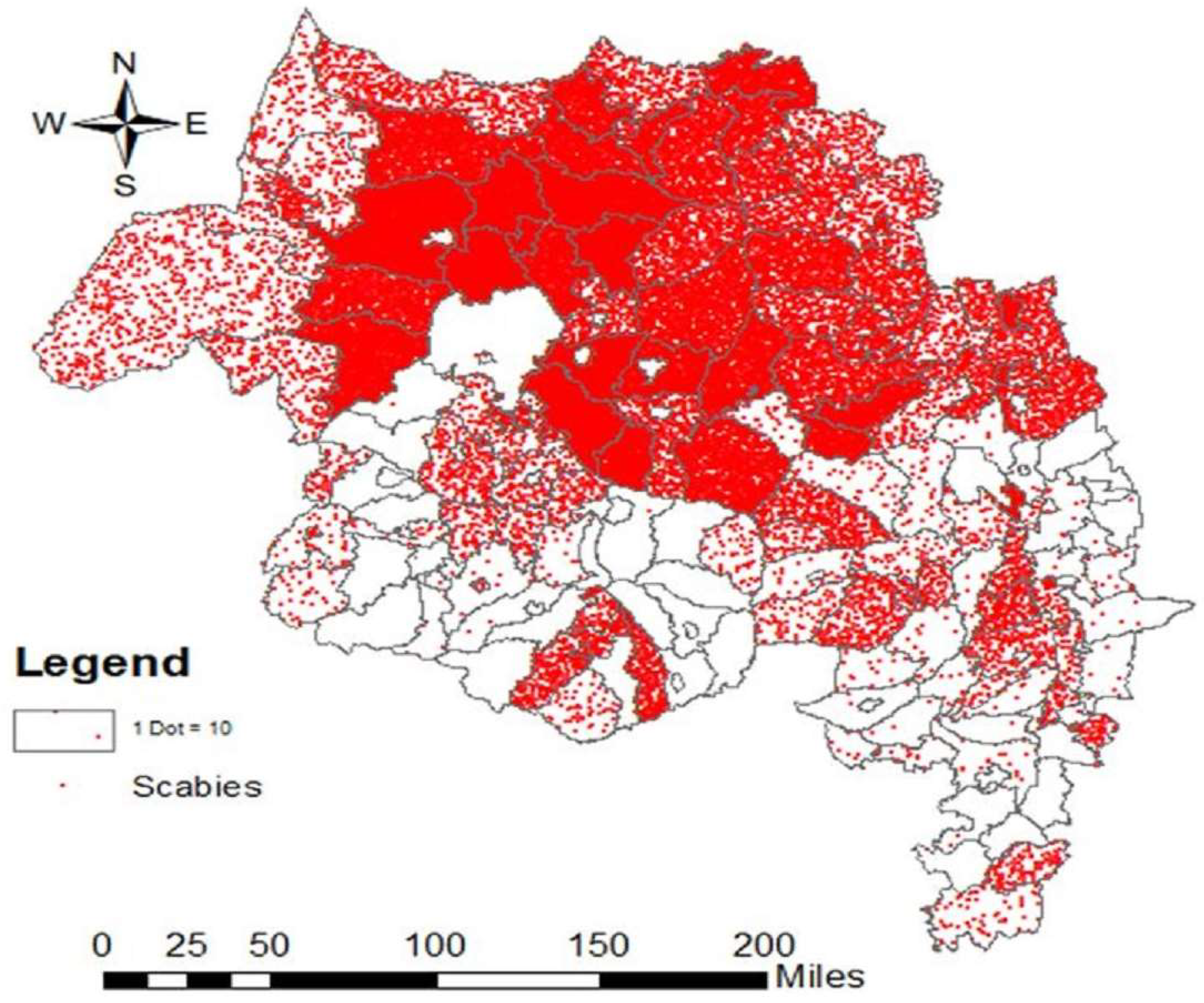
3.1. Phase 1
3.2. Phase 2
4. Discussion
- The study used an aggregated report of the census with a limited data set (scabies case, contact, age, and sex) which could be a limitation of the study, especially for risk factor analysis.
- In the house-to-house census of scabies cases, the examining HEWs had no other relevant experience apart from the one-day training described, which may have led to missed cases. The validation study focused only on specificity of the diagnosis.
- Both in the census and in the validation study, the diagnosis of scabies and impetigo was made on the basis of clinical history and skin examination alone. Skin scrapings for direct microscopy or other confirmatory tests were not used.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hicks, M.I.; Elston, D.M. Scabies. Dermatol. Ther. 2009, 22, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Feldmeier, H. Scabies. Lancet 2006, 367, 1767–1774. [Google Scholar] [CrossRef]
- Ugbomoiko, U.; Oyedeji, S.; Babamale, O.; Heukelbach, J. Scabies in resource-poor communities in Nasarawa State, Nigeria: Epidemiology, clinical features and factors associated with infestation. Trop. Med. Infect. Dis. 2018, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Epidemiology and Management of Common Skin Diseases in Children in Developing Countries; WHO: Geneva, Switzerland, 2005; Available online: whqlibdoc.who.int/hq/2005/WHO_FCH_CAH_05.12_eng.pdf (accessed on 23 July 2018).
- WHO. El Nino and Health; WHO Report 2016; WHO: Geneva, Switzerland, 2016; Available online: who_el_nino_and_health_global_report_21jan2016.pdf (accessed on 4 June 2017).
- EM-DAT: The OFDA/CRED International Disaster Database; Universite Catholique de Louvain: Brussels, Belgium. Available online: http://www.emda.be/result-disaster-profiles?disgroup=natural&period=1900%242012&dis_type=Drought&Submit=Display+Disaster+Profile (accessed on 4 June 2017).
- UNISDR. Global Assessment Report on Disaster Risk Reduction: Revealing Risk, Redefining Development; United Nations International Strategy for Disaster Reduction: Geneva, Switzerland, 2011. [Google Scholar]
- Scabies Outbreak Preparedness and Response Plan; FMOH: Addis Ababa, Ethiopia, 2015; Available online: www.humanitarianresponse.info (accessed on 23 July 2018).
- Interim-Guideline for Multi Sectorial Scabies Outbreak Emergency Response; FMOH: Addis Ababa, Ethiopia, 2015.
- Andersen, L.K.; Davis, M.D. The effects of the El Nino Southern Oscillation on skin and skin-related diseases: A message from the International Society of Dermatology Climate Change Task Force. Int. J. Dermatol. 2015, 54, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Steer, A.C.; Whitfeld, M.J.; Kaldor, J.M. Prevalence of scabies and impetigo worldwide: A systematic review. Lancet Infect. Dis. 2015, 15, 960–967. [Google Scholar] [CrossRef]
- Engelman, D.; Andrew, I.D.; Steer, C. Control strategies for scabies. Trop. Med. Infect. Dis. 2018, 3, 98. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, S.; Barnabas, G.A.; Farese, P.; Padovese, V.; Terranova, M.; Racalbuto, V.; Morrone, A. Skin disorders and disease profile of poverty: Analysis of medical records in Tigray, northern Ethiopia, 2005-2007. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, D.; Keita, S.M.; Pönnighaus, J.M.; Tounkara, C. Epidemiologic aspects of scabies in Mali, Malawi, and Cambodia. Int. J. Dermatol. 1998, 37, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Connors, C.; Yarmirr, D.; Krause, V.; Currie, B.J. Success of a scabies control program in an Australian aboriginal community. Pediatr. Infect. Dis. J. 1997, 16, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.K.; Joseph, A.; Kandamuthan, M. Epidemic scabies. Indian J. Med. Res. 1977, 65, 513–518. [Google Scholar] [PubMed]
- Dos Santos, M.M.; Amaral, S.; Harmen, S.P.; Joseph, H.M.; Fernandes, J.L.; Counahan, M.L. The prevalence of common skin infections in four districts in Timor-Leste: A cross-sectional survey. BMC Infect. Dis. 2010, 10, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, C.; Colombara, D.V.; Drucker, A.M.; Norton, S.A.; Hay, R.; Engelman, D.; Steer, A.; Whitfeld, M.; Naghavi, M.; Dellavalle, R.P. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1247–1254. [Google Scholar] [CrossRef]
- Hay, R.J.; Steer, A.C.; Engelman, D.; Walton, S. Scabies in the developing world—Its prevalence, complications, and management. Clin. Microbiol. Infect. 2012, 18, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.G.; Burkhart, C.N.; Burkhart, K.M. An epidemiologic and therapeutic reassessment of scabies. Cutis 2000, 65, 233–240. [Google Scholar] [PubMed]
- Kouotou, E.A.; Nansseu, J.R.N.; Sieleunou, I.; Defo, D.; Bissek, A.C.Z.K.; Ndam, E.C.N. Features of human scabies in resource-limited settings: The Cameroon case. BMC Dermatol. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.S.; Marks, M.; Sokana, O.; Solomon, A.W.; Mabey, D.C.; Romani, L.; Engelman, D. The prevalence of scabies and impetigo in the Solomon Islands: A population-based survey. PLoS Negl. Trop. Dis. 2016, 10, e0004803. [Google Scholar] [CrossRef] [PubMed]
- Swe, P.M.; Zakrzewski, M.; Kelly, A.; Krause, L.; Fischer, K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl. Trop. Dis. 2014, 8, e2897. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. The AIM study: A field trial of co-administration of azithromycin and ivermectin mass drug administration (MDA), CRF skin examination clinical record form, version 1, 2015. Available online: https://kirby.unsw.edu.au/project/aim-study (accessed on 23 July 2018).
- Mahé, A.; Faye, O.; N’Diaye, H.T.; Ly, F.; Konare, H.; Keita, S.; Hay, R. Definition of an algorithm for the management of common skin diseases at primary health care level in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C.; Tikoduadua, L.V.; Manalac, E.M.; Colquhoun, S.; Carapetis, J.R.; Maclennan, C. Validation of an integrated management of childhood illness algorithm for managing common skin conditions in Fiji. Bull WHO 2009, 87, 173–179. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 9th NTD-STAG Global Working Group Meeting on Monitoring and Evaluation of Neglected Tropical Diseases. 2018. Available online: http://www.who.int/neglected_diseases/events/STAG_Working_Group_on_Monitoring_Evaluation/en/ (accessed on 12 August 2018).
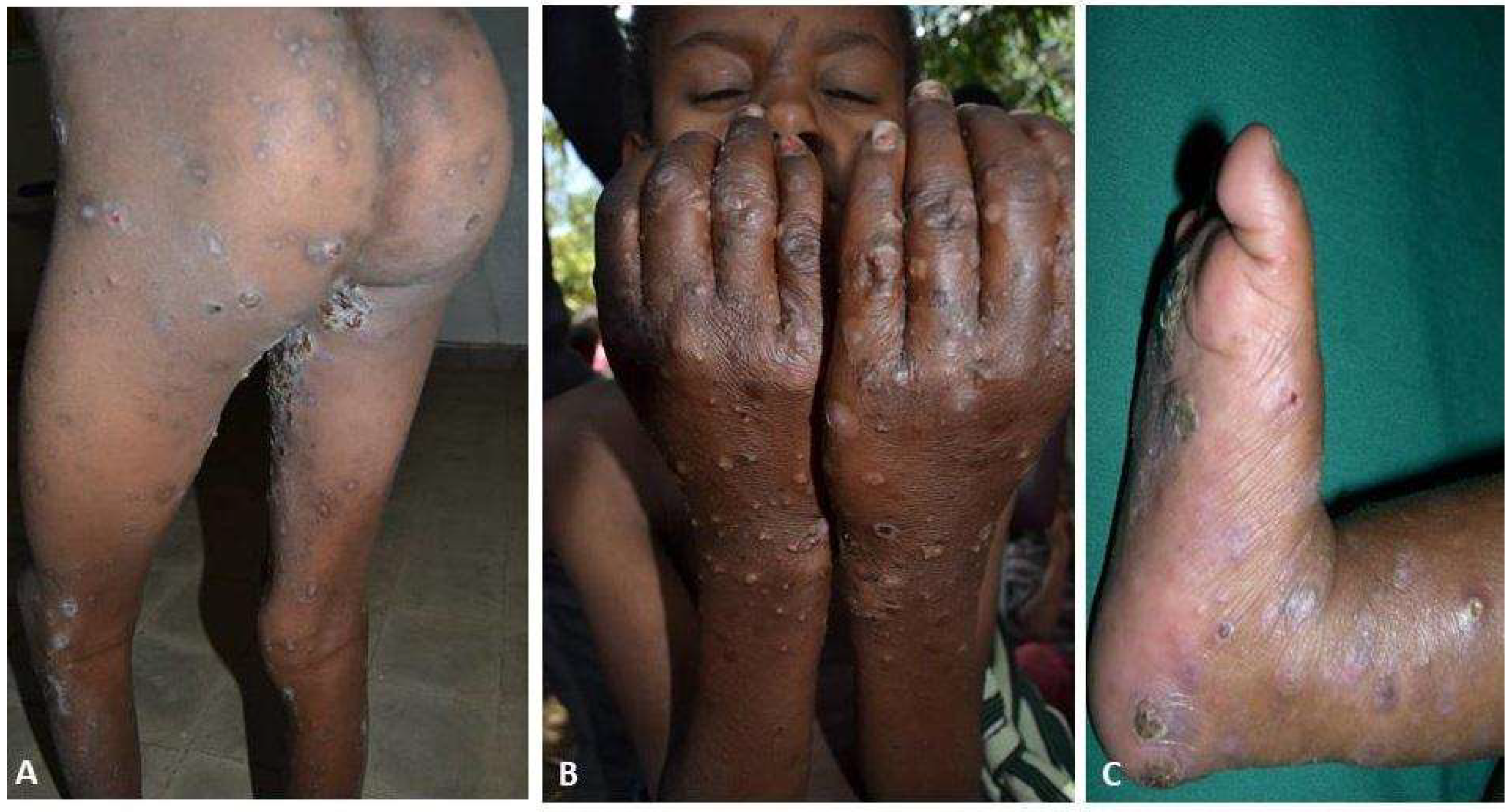
| Scabies | Presence of itching with typical lesions on hands, inter-digital, and/or genitalia and/or itching and close contact with an individual who has itching or typical lesions in a typical distribution. |
| Contact | A contact is a person who does not fulfill the clinical criteria for infestation with scabies (above) or a person without signs and symptoms consistent with scabies, who has had direct contact (particularly prolonged, direct, skin-to-skin contact) with a suspected or confirmed case in the two months preceding the onset of scabies signs and symptoms in the index case |
| Variable | Total (N) | Affected (n) | Prevalence | OR * | p Value |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 585,400 | 195,665 | 33.4 | Ref | Ref |
| Male | 540,370 | 183,335 | 33.9 | 1.05 | 0.8 |
| Total | 1,125,770 | 379,000 | 33.7 | ||
| Age group (years) ** | |||||
| <2 | 60,792 | 27,909 | 45.9 | 2.5 | 0.01 |
| 2 to 18 | 518,980 | 249,535 | 48.1 | 2.4 | 0.01 |
| >18 | 545,998 | 101,556 | 18.6 |
| Variables | N (%) |
|---|---|
| Sign and symptom | |
| Itching | 466 (100%) |
| Classic scabies lesion | 466 (100%) |
| Bacterial infection | 116 (25%) |
| Crusted scabies | 14 (3%) |
| Severity of the skin lesion | |
| Mild | 13 (28%) |
| Moderate | 191 (41%) |
| Severe | 144 (31%) |
| Variable | Scabies Cases (N) | Infection (N) | Infection % (95% CI) | Odds Ratio | p Value |
|---|---|---|---|---|---|
| <2 years | 46 | 7 | 15.2 (11.8–18.2) | 1.8 | 0.012 |
| 2–18 years | 288 | 101 | 35.1 (29.3–40.9) | 4.2 | <0.001 |
| >18 years | 132 | 11 | 8.3 (6.9–9.7) | Ref. | |
| Total | 466 | 119 | 25.5 (21.3–29.7) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enbiale, W.; Ayalew, A. Investigation of a Scabies Outbreak in Drought-Affected Areas in Ethiopia. Trop. Med. Infect. Dis. 2018, 3, 114. https://doi.org/10.3390/tropicalmed3040114
Enbiale W, Ayalew A. Investigation of a Scabies Outbreak in Drought-Affected Areas in Ethiopia. Tropical Medicine and Infectious Disease. 2018; 3(4):114. https://doi.org/10.3390/tropicalmed3040114
Chicago/Turabian StyleEnbiale, Wendemagegn, and Ashenafi Ayalew. 2018. "Investigation of a Scabies Outbreak in Drought-Affected Areas in Ethiopia" Tropical Medicine and Infectious Disease 3, no. 4: 114. https://doi.org/10.3390/tropicalmed3040114
APA StyleEnbiale, W., & Ayalew, A. (2018). Investigation of a Scabies Outbreak in Drought-Affected Areas in Ethiopia. Tropical Medicine and Infectious Disease, 3(4), 114. https://doi.org/10.3390/tropicalmed3040114





