Melioidosis in Singapore: Clinical, Veterinary, and Environmental Perspectives
Abstract
1. Introduction
2. Clinical Aspects
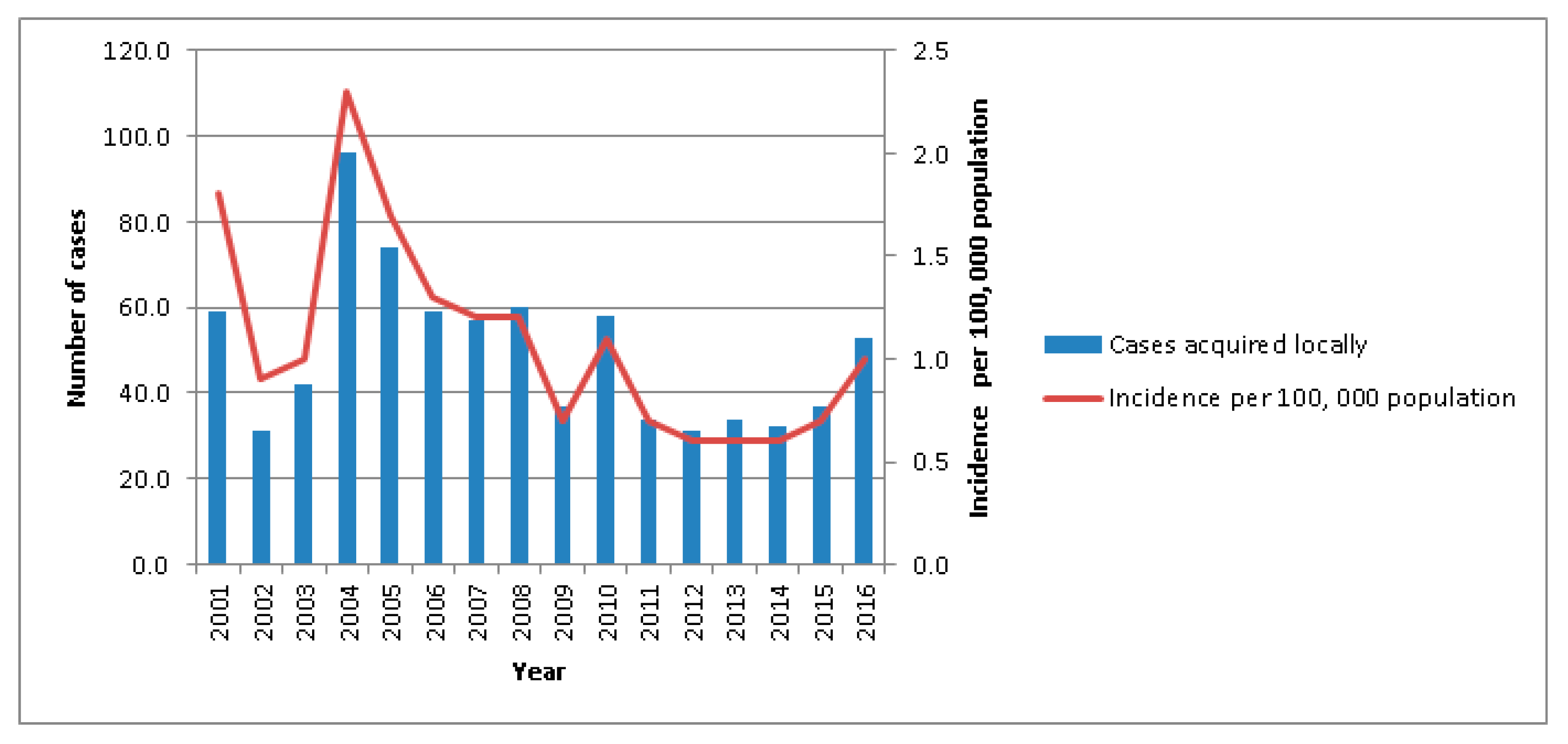
2.1. Disease Epidemiology
2.2. Clinical Features of Melioidosis Patients
2.3. Diagnosis
2.4. Treatment
3. Veterinary Aspects
3.1. Prevalence and Surveillance of Animal Melioidosis
3.2. Laboratory Diagnosis for Animal Melioidosis
3.3. Treatment and Prevention of Melioidosis in Zoo Animals
4. Environmental Melioidosis
4.1. Prevalence and Environmental Surveillance of B. pseudomallei
4.2. Detection of B. pseudomallei in Environmental Samples
4.3. Correlation between Environmental Isolates and Animal/Clinical Isolates
4.4. Characterization of Environmental B. pseudomallei Isolates from Singapore
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dance, D.A. Melioidosis: The tip of the iceberg? Clin. Microbiol. Rev. 1991, 4, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Spinks, G.A.; D’Arcy, T.L.; Norton, J.H.; Trueman, K.F. Evaluation of four serological tests for the diagnosis of caprine melioidosis. Aust. Vet. J. 1988, 65, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef]
- Chan, H.P.; Yip, H.S. Mediastinal lymphadenopathy: Melioidosis mimicking tuberculosis. Trop. Med. Health 2015, 43, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, S.; Prasath, T.; Corea, E.M.; Elwitigala, J.P. Melioidosis presenting as lymphadenitis: A case report. BMC Res. Notes 2014, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.L.; Mayo, M.; Janmaat, A.; Currie, B.J. Animal melioidosis in Australia. Acta. Trop. 2000, 74, 153–158. [Google Scholar] [CrossRef]
- Dance, D.A. Melioidosis as an emerging global problem. Acta. Trop. 2000, 74, 115–119. [Google Scholar] [CrossRef]
- Elschner, M.C.; Hnizdo, J.; Stamm, I.; El-Adawy, H.; Mertens, K.; Melzer, F. Isolation of the highly pathogenic and zoonotic agent Burkholderia pseudomallei from a pet green iguana in Prague, Czech Republic. BMC Vet. Res. 2014, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Tuanyok, A.; Woods, D.E. Survival of Burkholderia pseudomallei in water. BMC Res. Notes 2008, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Pumpuang, A.; Chantratita, N.; Wikraiphat, C.; Saiprom, N.; Day, N.P.; Peacock, S.J.; Wuthiekanun, V. Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.T.; Fletcher, W. Melioidosis. Studies from the institute of medical research, Federated Malay States. Bull 1932, 21, 8–9. [Google Scholar]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Ang, B.S.; Ong, Y.Y. Melioidosis: Epidemiology and antibiogram of cases in Singapore. Singap. Med. J. 1990, 31, 335–337. [Google Scholar]
- Lim, M.K.; Tan, E.H.; Soh, C.S.; Chang, T.L. Burkholderia pseudomallei infection in the Singapore Armed Forces from 1987 to 1994 - an epidemiological review. Ann. Acad. Med. Singap. 1997, 26, 13–17. [Google Scholar] [PubMed]
- Heng, B.H.; Goh, K.T.; Yap, E.H.; Loh, H.; Yeo, M. Epidemiological surveillance of melioidosis in Singapore. Ann. Acad Med. Singap. 1998, 27, 478–484. [Google Scholar] [PubMed]
- Lo, T.J.; Ang, L.W.; James, L.; Goh, K.T. Melioidosis in a tropical city state, Singapore. Emerg. Infect. Dis. 2009, 15, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Harris, P.N.A.; Seiler, R.L.; Ooi, P.L.; Cutter, J.; Goh, K.T.; Cook, A.R.; Fisher, D.; Chai, L.Y.A. Melioidosis, Singapore, 2003–2014. Emerg. Infect. Dis. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Loh, J.P.; Aw, L.T.; Yap, E.P.; Lee, M.A.; Ooi, E.E. Rapid molecular typing of Burkholderia pseudomallei, isolated in an outbreak of melioidosis in Singapore in 2004, based on variable-number tandem repeats. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.K.; Ang, B.S.; Tan, J. Clinics in diagnostic imaging (57). Singap. Med. J. 2001, 42, 41–43. [Google Scholar]
- Tan, A.P.; Pui, M.H.; Tan, L.K. Imaging patterns in melioidosis. Australas. Radiol. 1995, 39, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.M.; Kwan, M.K.; Merican, A.M. Melioidotic osteomyelitis treated with antibiotic-calcium hydroxyapatite composite: Case report with four-year follow-up. Singap. Med. J. 2006, 47, 71–74. [Google Scholar]
- Amadasi, S.; Dal Zoppo, S.; Bonomini, A.; Bussi, A.; Pedroni, P.; Balestrieri, G.; Signorini, L.; Castelli, F. A case of melioidosis probably acquired by inhalation of dusts during a helicopter flight in a healthy traveler returning from Singapore. J. Travel. Med. 2015, 22, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Chau, C.H.; Wong, P.C. Melioidosis mycotic aneurysm: An uncommon complication of an uncommon disease. Respir. Med. Case Rep. 2015, 14, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Kaushal, A.S.; Hoong, C.K. Abdominal aortic pseudoaneurysm secondary to melioidosis. Asian J. Surg. 2009, 32, 64–69. [Google Scholar] [CrossRef]
- Chlebicki, M.P.; Kurup, A.; Sin, Y.K. Burkholderia pseudomallei meningitis following inadequate treatment of melioidotic mycotic aneurysm. Singap. Med. J. 2008, 49, e219–221. [Google Scholar]
- Lath, R.; Rajshekhar, V.; George, V. Brain abscess as the presenting feature of melioidosis. Br. J. Neurosurg. 1998, 12, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.R.; Ang, B.; Sitoh, Y.Y.; Lee, C.C. Cerebral melioidosis in Singapore: A review of five cases. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 72–76. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Peacock, S.J. Melioidosis: A clinical overview. Br. Med. Bull. 2011, 99, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.S. Dermatology in the military field: What physicians should know? World J. Clin. Cases 2013, 1, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.W.; Tan, N.W.; Chong, C.Y.; Thoon, K.C.; Tee, N.W.; Koh, M.J. Melioidosis in children: A retrospective study. Int. J. Dermatol. 2015, 54, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Novak, R.T.; Gal, D.; Kaestli, M.E.; Mayo, M.; Hanson, J.P.; Spencer, E.; Glass, M.B.; Gee, J.E.; Wilkins, P.P.; et al. Clinical evaluation of a type III secretion system real-time PCR assay for diagnosing melioidosis. J. Clin. Microbiol. 2006, 44, 3028–3030. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Wuthiekanun, V.; Limmathurotsakul, D.; Thanwisai, A.; Chantratita, W.; Day, N.P.; Peacock, S.J. Prospective clinical evaluation of the accuracy of 16s rRNA real-time PCR assay for the diagnosis of melioidosis. Am. J. Trop. Med. Hyg. 2007, 77, 814–817. [Google Scholar] [PubMed]
- Wuthiekanun, V.; Chierakul, W.; Langa, S.; Chaowagul, W.; Panpitpat, C.; Saipan, P.; Thoujaikong, T.; Day, N.P.; Peacock, S.J. Development of antibodies to Burkholderia pseudomallei during childhood in melioidosis-endemic northeast Thailand. Am. J. Trop. Med. Hyg. 2006, 74, 1074–1075. [Google Scholar] [PubMed]
- Yap, E.H.; Chan, Y.C.; Ti, T.Y.; Thong, T.W.; Tan, A.L.; Yeo, M.; Ho, L.C.; Singh, M. Serodiagnosis of melioidosis in Singapore by the indirect haemagglutination test. Singap. Med. J. 1991, 32, 211–213. [Google Scholar]
- Liu, Y.; Sim, S.H.; Wang, D. IHA results on serum samples collected from culture-confirmed melioidosis patients, suspected melioidosis cases presenting to local hospitals and healthy volunteers from 2004 to 2016. Defence Medical and Environmental Research Institute, DSO National Laboratories: Singapore, Unpublished work. 2018. [Google Scholar]
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Chierakul, W.; Anunnatsiri, S.; Short, J.M.; Maharjan, B.; Mootsikapun, P.; Simpson, A.J.; Limmathurotsakul, D.; Cheng, A.C.; Stepniewska, K.; Newton, P.N.; et al. Two randomized controlled trials of ceftazidime alone versus ceftazidime in combination with trimethoprim-sulfamethoxazole for the treatment of severe melioidosis. Clin. Infect. Dis. 2005, 41, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.T.; Lee, C.H.; Li, C.J.; Lu, H.I.; Ko, S.F.; Liu, J.W. Development of ceftazidime resistance in Burkhoderia pseudomallei in a patient experiencing melioidosis with mediastinal lymphadenitis. Ann. Acad. Med. Singap. 2010, 39, 945-3. [Google Scholar] [PubMed]
- Fisher, D. Division of infectious diseases, Department of Medicine, National University Health System, Singapore. Unpublished work. 2008. [Google Scholar]
- Suputtamongkol, Y.; Chaowagul, W.; Chetchotisakd, P.; Lertpatanasuwun, N.; Intaranongpai, S.; Ruchutrakool, T.; Budhsarawong, D.; Mootsikapun, P.; Wuthiekanun, V.; Teerawatasook, N.; et al. Risk factors for melioidosis and bacteremic melioidosis. Clin. Infect. Dis. 1999, 29, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Foo, G.; Lim, W.P.; Ravikumar, S.; Sim, S.H.; Win, M.S.; Goh, J.G.; Lim, J.H.; Ng, Y.H.; Fisher, D.; et al. Sulphonylurea usage in melioidosis is associated with severe disease and suppressed immune response. PLoS Negl. Trop. Dis. 2014, 8, e2795. [Google Scholar] [CrossRef] [PubMed]
- Annual Report 2016/17: Handled with Care; Agri-Food & Veterinary Authority of Singapore: Singapore, 2017.
- Pet Care in Singapore, Industry Overview; Euromonitor International: Singapore, 2016; pp. 1–55.
- Sprague, L.D.; Neubauer, H. Melioidosis in animals: A review on epizootiology, diagnosis and clinical presentation. J. Vet. Med. B. Infect. Dis. Vet. Public Health 2004, 51, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Tonpitak, W.; Sornklien, C.; Chawanit, M.; Pavasutthipaisit, S.; Wuthiekanun, V.; Hantrakun, V.; Amornchai, P.; Thaipadungpanit, J.; Day, N.P.; Yingst, S.; et al. Fatal melioidosis in goats in Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2014, 91, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.H.; Yu, Y.; Lin, C.H.; Karuturi, R.K.; Wuthiekanun, V.; Tuanyok, A.; Chua, H.H.; Ong, C.; Paramalingam, S.S.; Tan, G.; et al. The core and accessory genomes of Burkholderia pseudomallei: Implications for human melioidosis. PLoS Pathog. 2008, 4, e1000178. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Fisher, D.A.; Howard, D.M.; Burrow, J.N.; Selvanayagam, S.; Snelling, P.L.; Anstey, N.M.; Mayo, M.J. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000, 74, 121–127. [Google Scholar] [CrossRef]
- Ketterer, P.J.; Webster, W.R.; Shield, J.; Arthur, R.J.; Blackall, P.J.; Thomas, A.D. Melioidosis in intensive piggeries in south eastern Queensland. Aust. Vet. J. 1986, 63, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Xie, S. Conservation, Research and Veterinary Services, Wildlife Reserves Singapore, Singapore. Unpublished work. 2018. [Google Scholar]
- Walsh, A.L.; Wuthiekanun, V.; Smith, M.D.; Suputtamongkol, Y.; White, N.J. Selective broths for the isolation of Pseudomonas pseudomallei from clinical samples. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 124. [Google Scholar] [CrossRef]
- Supaprom, C.; Wang, D.; Leelayuwat, C.; Thaewpia, W.; Susaengrat, W.; Koh, V.; Ooi, E.E.; Lertmemongkolchai, G.; Liu, Y. Development of real-time PCR assays and evaluation of their potential use for rapid detection of Burkholderia pseudomallei in clinical blood specimens. J. Clin. Microbiol. 2007, 45, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Thin, R.N.; Groves, M.; Rapmund, G.; Mariappan, M. Pseudomonas pseudomallei in the surface water of Singapore. Singap. Med. J. 1971, 12, 181–182. [Google Scholar]
- Yap, E.H.; Thong, T.W.; Tan, A.L.; Yeo, M.; Tan, H.C.; Loh, H.; Teo, T.P.; Thong, K.T.; Singh, M.; Chan, Y.C. Comparison of Pseudomonas pseudomallei from humans, animals, soil and water by restriction endonuclease analysis. Singap. Med. J. 1995, 36, 60–62. [Google Scholar]
- Strauss, J.M.; Groves, M.G.; Mariappan, M.; Ellison, D.W. Melioidosis in Malaysia. Ii. Distribution of Pseudomonas pseudomallei in soil and surface water. Am. J. Trop. Med. Hyg. 1969, 18, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.E.L.; Koh, V.W.H.; Tan, Y.K.; Wong, M.S.Y.; Chew, J.S.W. Environmental surveillance of B. pseudomallei in Singapore between 2000 and 2013. Defence Medical and Environmental Research Institute, DSO National Laboratories: Singapore, Unpublished work. 2018. [Google Scholar]
- Chantratita, N.; Wuthiekanun, V.; Boonbumrung, K.; Tiyawisutsri, R.; Vesaratchavest, M.; Limmathurotsakul, D.; Chierakul, W.; Wongratanacheewin, S.; Pukritiyakamee, S.; White, N.J.; et al. Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J. Bacteriol. 2007, 189, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Wuthiekanun, V.; Amornchai, P.; Wongsuwan, G.; Day, N.P.; Peacock, S.J. Effectiveness of a simplified method for isolation of Burkholderia pseudomallei from soil. Appl. Environ. Microbiol. 2012, 78, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Price, E.P.; Mayo, M.; Kaestli, M.; Theobald, V.; Harrington, I.; Harrington, G.; Sarovich, D.S. Use of whole-genome sequencing to link Burkholderia pseudomallei from air sampling to mediastinal melioidosis, Australia. Emerg. Infect. Dis. 2015, 21, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Nandi, T.; Holden, M.T.; Didelot, X.; Mehershahi, K.; Boddey, J.A.; Beacham, I.; Peak, I.; Harting, J.; Baybayan, P.; Guo, Y.; et al. Burkholderia pseudomallei sequencing identifies genomic clades with distinct recombination, accessory, and epigenetic profiles. Genome Res. 2015, 25, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.E.L. Correlation of clinical and environmental B. pseudomallei isolates from an island off Singapore. Defence Medical and Environmental Research Institute, DSO National Laboratories: Singapore, Unpublished work. 2018. [Google Scholar]
- Sivalingam, S.P.; Sim, S.H.; Aw, L.T.; Ooi, E.E. Antibiotic susceptibility of 50 clinical isolates of Burkholderia pseudomallei from Singapore. J. Antimicrob. Chemother. 2006, 58, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Tan, M.L. Melioidosis: Antibiogram of cases in Singapore 1987–2007. Trans. R. Soc. Trop. Med. Hyg. 2008, 102 (Suppl. 1), S101–S102. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chen, Y.; Ouyang, X.; Gan, Y.H. Identification of tomato plant as a novel host model for Burkholderia pseudomallei. BMC Microbiol. 2010, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Wuthiekanun, V.; Limmathurotsakul, D.; Vesaratchavest, M.; Thanwisai, A.; Amornchai, P.; Tumapa, S.; Feil, E.J.; Day, N.P.; Peacock, S.J. Genetic diversity and microevolution of Burkholderia pseudomallei in the environment. PLoS Negl. Trop. Dis. 2008, 2, e182. [Google Scholar] [CrossRef] [PubMed]
| Study Groups | Subject Information/Year of Blood Collection | Number (%) of Subjects with Different Levels of IHA Titer | |||
|---|---|---|---|---|---|
| Very High Positive ≥1:512 | High Positive 1:128 to 1:256 | Low Positive 1:16 to 1:64 | Negative ≤1:8 | ||
| Culture-confirmed melioidosis (n = 31) | Acute, relapsed and recovered cases with a mean age of 52.5 years old/2004–2013 | 17 (54.8%) | 12 (38.7%) | 2 (6.5%) | 0 (0%) |
| Clinically unconfirmed infection (n = 992) | Patients with an active infection/2006 | 31 (3.1%) | 21 (2.1%) | 54 (5.4%) | 886 (89.4%) |
| Clinically unconfirmed infection (n = 1027) | Patients with an active infection/2016 | 18 (1.8%) | 20 (1.9%) | 101 (9.8%) | 888 (86.5%) |
| Healthy volunteers (n = 109) | Age range: 18–60 mean age: 33.1 years old/2004–2013 | 0 (0%) | 1 (0.9%) | 23 (21.1%) | 85 (78.0%) |
| Species | Number | Year of Diagnosis | Organ from Which B. pseudomallei Was Isolated | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Lung | Liver | Kidney | Spleen | Gonad | Skin | ||||
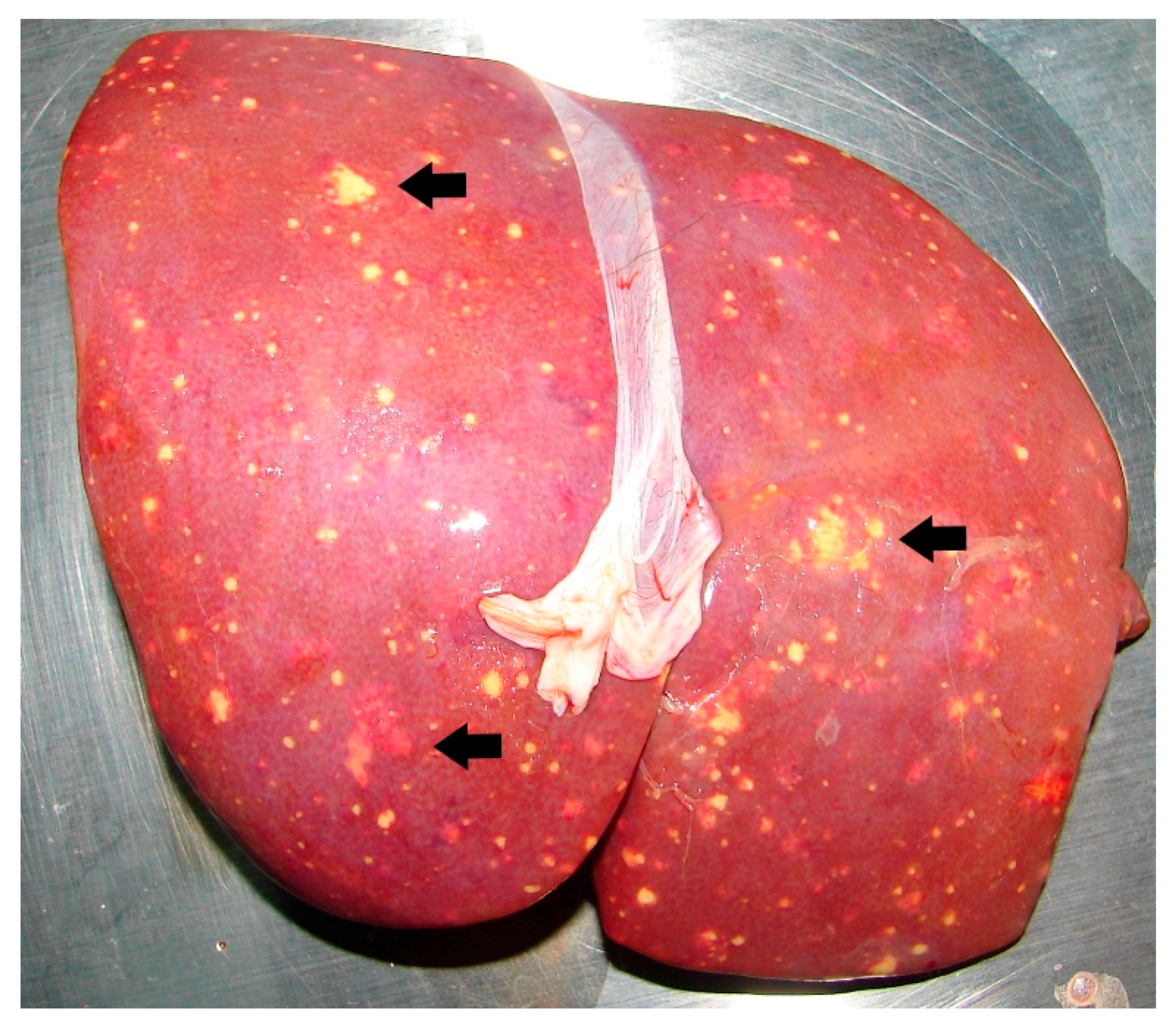
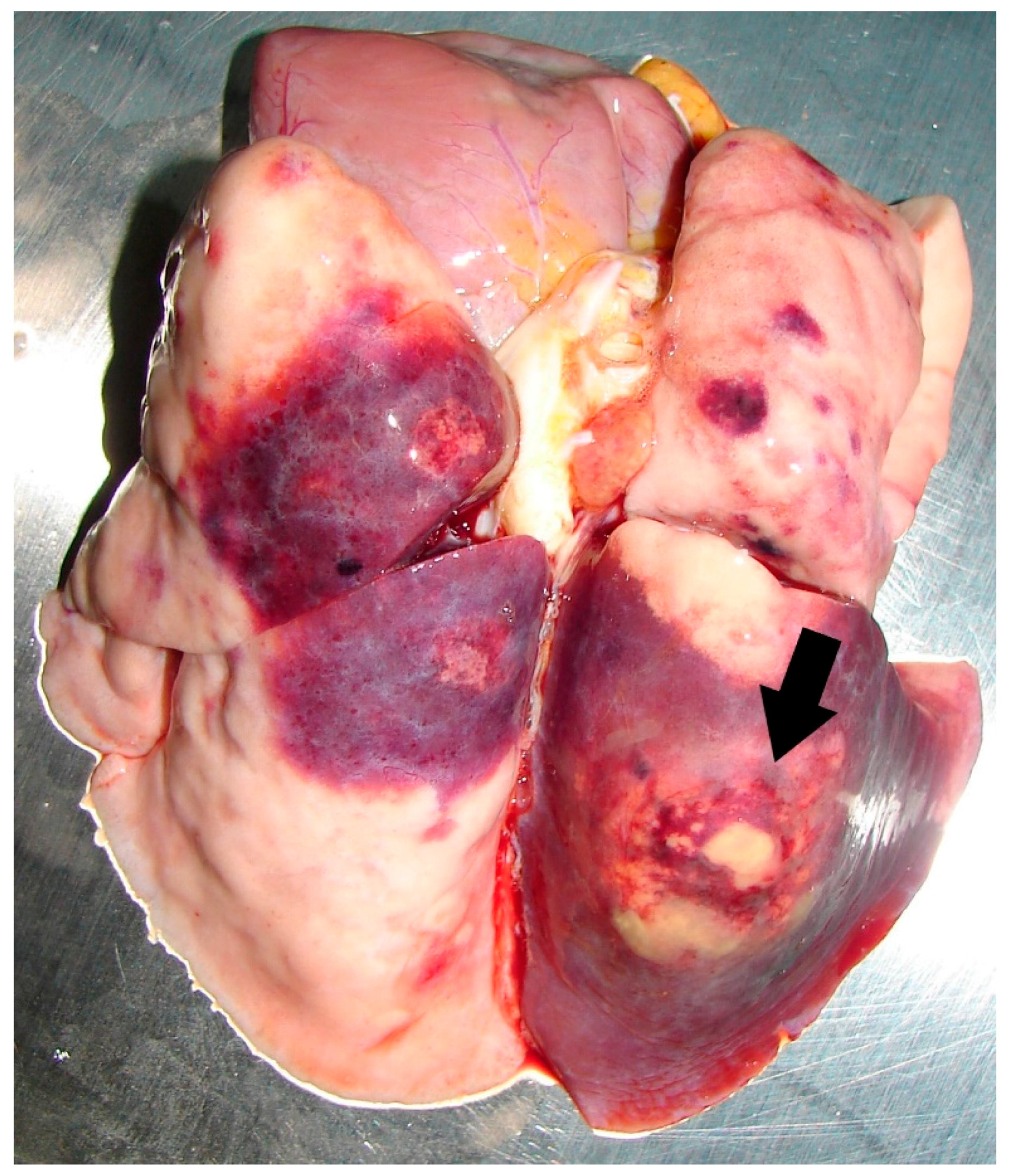
| Primates | Gorilla (Gorilla gorilla) | 5 | 1983/1992 | x | x | x | x | |||
| Southern pig-tailed macaque (Macaca nemestrina) | 1 | 1992 | x | |||||||
| Chimpanzee (Pan troglodytes) | 2 | 1985/1990 | x | x | ||||||
| Müller’s Bornean gibbon (Hylobates muelleri) | 2 | 1989/1992 | x | x | ||||||
| Mandrill (Mandrillus sphinx) | 1 | 1990 | x | |||||||
| Golden lion tamarin (Leontopithecus rosalia) | 2 | 1995/1996 | x | x | ||||||
| Siamang (Symphalangus syndactylus) | 1 | 2005 | x | |||||||
| Lesser spot-nosed guenon (Cercopithecus petaurista) | 1 | 1996 | x | |||||||
| Debrazza’s monkey (Cercopithecus neglectus) | 1 | 1998 | x | |||||||
| Douc langur (Simia nemaeus) | 1 | 1992 | x | |||||||
| Herbivores | Eastern grey kangaroo (Macropus giganteus) | 2 | 1986/1989 | x | x | |||||
| Indochinese hog deer (Hyelaphus annamiticus) | 1 | 2013 | x | |||||||
| Camel (Camelus dromedaries) | 1 | 1994 | x | |||||||
| Llama (Lama glama) | 1 | 1994 | x | |||||||
| Nile hippopotamus (Hippopotamus amphibious) | 1 | 1996 | x | |||||||
| Red lechwe (Kobus leche) | 2 | 1998/2007 | x | x | ||||||
| Indian sambar (Rusa unicolor) | 1 | 2003 | x | |||||||
| Chinese goral (Naemorhedus griseus) | 1 | 2008 | x | |||||||
| Carnivores | Cape hunting dog (Lycaon pictus pictus) | 1 | 1990 | x | ||||||
| Birds | Southern cassowary (Casuarius casuarius) | 1 | 1985 | x | ||||||
| Southern crowned pigeon (Goura scheepmakeri) | 1 | 1987 | x | |||||||
| Moustached parakeet (Psittacula alexandri) | 1 | 1998 | x | |||||||
| Palm cockatoo (Proboscigera terrimus) | 2 | 1991/1996 | x | |||||||
| Salmon-crested cockatoo (Cacatua moluccensis) | 2 | 1998/2014 | x | |||||||
| Humboldt penguin (Spheniscus humboldti) | 1 | 1987 | x | |||||||
| Bird of paradise (exact species unknown) | 1 | 1986 | x | |||||||
| Type of Environmental Sample | Terrain/Location | Isolation Protocol | No. Collected | No. Positive | Percentage (%) | Interesting Correlations * | Reference |
|---|---|---|---|---|---|---|---|
| Surface water | Forest around buildings, roadside drains, sports fields | Hamster inoculation method [55]; colony identification on MacConkey agar. | 21 44 43 28 | 1 0 2 5 | 4.8 0.0 4.6 18.0 | - - Rainfall Rainfall; low lying field | [53] |
| Different localities where melioidosis patients had sustained injuries or had direct contact prior to their onset of illness | TSB with crystal violet (5 mg/L) and colistin (20 mg/L); colony identification on Ashdown agar. | 46 | 0 | 0.0 | - | [16] | |
| Water | Moat within animal enclosure in Singapore Zoological Gardens (WRS) | Hamster inoculation method [55]; colony identification on MacConkey’s agar. | Unknown | 3 | N.D. | 4 gorillas succumbed to melioidosis; both animal and water isolates belonged to same RE II type. | [54] |
| Soil | Island off Singapore Residential compound | Hamster inoculation method [55]; colony identification on MacConkey agar. | Unknown 4 | 2 1 | N.D. 25.0 | 2 patients staying on the island died of melioidosis (see Table 5). Pet German shepherd succumbed to melioidosis; both animal and soil isolates belonged to same RE II type. | [54] |
| Different localities where melioidosis patients had sustained injuries or had direct contact prior to their onset of illness | TSB with crystal violet (5 mg/L) and colistin (20 mg/L) enrichment; colony identification on Ashdown agar. | 395 | 7 | 1.8 | Genotype (by REA-PFGE) of Bp isolated from 4 samples in 1 locality similar to that isolated from elbow abscess of adult who had sustained injury at same locality. | [16] |
| Sample Source | Terrain/Location | Year | No. Collected | No. Positive | Percentage Positive (%) | Type of Study | Interesting Correlations * |
|---|---|---|---|---|---|---|---|
| Soil | Fields, island | 2000/01 | 98 | 0 | 0 | Repeated Sampling at sites reported by Yap et al. [54] | Refer to Table 5 |
| Plantations, island | 2000/01 | 62 | 3 | 3.1 | Repeated Sampling at sites reported by Yap et al. [54] | ||
| Reclaimed land, island | 2000/01 | 28 | 0 | 0 | Repeated Sampling at sites reported by Yap et al. [54] | ||
| Animal enclosure 1 | 2001 | 18 | 0 | 0 | Collaboration with SZG. | ||
| Animal enclosure 2 | 2001 | 13 | 0 | 0 | Collaboration with SZG. | ||
| Animal enclosure 3 | 2001 | 8 | 0 | 0 | Collaboration with Dr. Paul A. Tambyah, NUH | ||
| Animal enclosure 4 | 2001 | 4 | 0 | 0 | Collaboration with Dr. Paul A. Tambyah, NUH | ||
| Park A | 2005 | 15 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Park B | 2005 | 5 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Park C | 2005 | 90 | 3 | 3.3 | Environmental Surveillance at 9 locations | ||
| Park D | 2005 | 100 | 1 | 1.0 | Environmental Surveillance at 9 locations | ||
| Nature Reserve A | 2005 | 79 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Nature Reserve B | 2005 | 30 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Nature Reserve C | 2005 | 90 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Disturbed soil area A | 2005 | 259 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Disturbed soil area B | 2005 | 63 | 0 | 0 | Environmental Surveillance at 9 locations | ||
| Forested hill, southern island | 2013 | 55 | 1 | 1.8 | Environmental Surveillance project in 2013 | ||
| Water | Water from streams/puddles, island | 2000/01 | 16 | 2 | 12.5 | Repeated sampling at sites reported by Yap et al. [54] | Refer to Table 5 |
| Moat within animal enclosure 2 | 2001 | 3 | 0 | 0 | Collaboration with SZG. | ||
| Water behind animal enclosure 3 | 2001 | 1 | 0 | 0 | Collaboration with Dr Paul A. Tambyah, NUH | ||
| Run-off from forested hill, southern island | 2013 | 1 | 0 | 0 | Environmental surveillance project in 2013 | ||
| Rainwater | Forested hill, southern island | 2013 | 9 | 0 | 0 | Environmental Surveillance project in 2013 |
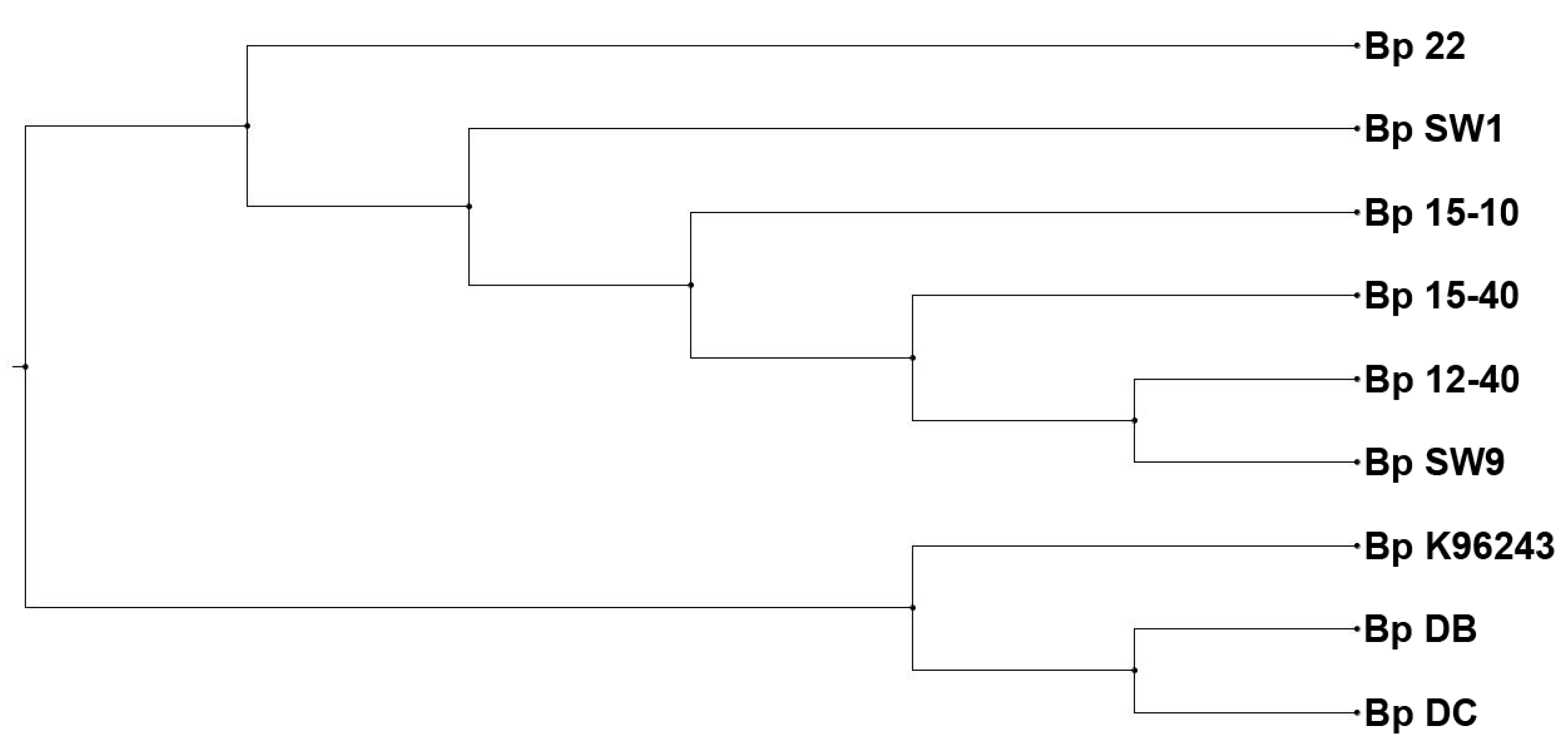
| Isolate | Source | Isolated by | Accessory Genome Clade [47] | Sequence Type (MLST) [60] | Genomic Clade (WGS) [60] |
|---|---|---|---|---|---|
| 22 | Human, clinical | Yap et al. (1995) [54] | Environmental clade | ST423 | N.D. |
| DB | Soil, environmental | Yap et al. (1995) [54] | Animal clade | ST51 | A |
| DC | Soil, environmental | Yap et al. (1995) [54] | Animal clade | ST51 | A |
| 15-10 | Soil, environmental | DSO 2000/01 | Environmental clade | ST423 | B |
| 15-40 | Soil, environmental | DSO 2000/01 | Environmental clade | ST423 | B |
| 12-40 | Soil, environmental | DSO 2000/01 | Environmental clade | ST423 | B |
| SW1 | Water, environmental | DSO 2000/01 | Environmental clade | ST423 | B |
| SW9 | Water, environmental | DSO 2000/01 | Environmental clade | ST423 | B |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, S.H.; Ong, C.E.L.; Gan, Y.H.; Wang, D.; Koh, V.W.H.; Tan, Y.K.; Wong, M.S.Y.; Chew, J.S.W.; Ling, S.F.; Tan, B.Z.Y.; et al. Melioidosis in Singapore: Clinical, Veterinary, and Environmental Perspectives. Trop. Med. Infect. Dis. 2018, 3, 31. https://doi.org/10.3390/tropicalmed3010031
Sim SH, Ong CEL, Gan YH, Wang D, Koh VWH, Tan YK, Wong MSY, Chew JSW, Ling SF, Tan BZY, et al. Melioidosis in Singapore: Clinical, Veterinary, and Environmental Perspectives. Tropical Medicine and Infectious Disease. 2018; 3(1):31. https://doi.org/10.3390/tropicalmed3010031
Chicago/Turabian StyleSim, Siew Hoon, Catherine Ee Ling Ong, Yunn Hwen Gan, Dongling Wang, Victor Wee Hong Koh, Yian Kim Tan, Michelle Su Yen Wong, Janet Seok Wei Chew, Sian Foong Ling, Brian Zi Yan Tan, and et al. 2018. "Melioidosis in Singapore: Clinical, Veterinary, and Environmental Perspectives" Tropical Medicine and Infectious Disease 3, no. 1: 31. https://doi.org/10.3390/tropicalmed3010031
APA StyleSim, S. H., Ong, C. E. L., Gan, Y. H., Wang, D., Koh, V. W. H., Tan, Y. K., Wong, M. S. Y., Chew, J. S. W., Ling, S. F., Tan, B. Z. Y., Ye, A. Z., Bay, P. C. K., Wong, W. K., Fernandez, C. J., Xie, S., Jayarajah, P., Tahar, T., Oh, P. Y., Luz, S., ... Tan, G. G. Y. (2018). Melioidosis in Singapore: Clinical, Veterinary, and Environmental Perspectives. Tropical Medicine and Infectious Disease, 3(1), 31. https://doi.org/10.3390/tropicalmed3010031






