Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti
Abstract
:1. Introduction
2. Methods
2.1. Data Set and Cohort Selection
2.2. Evaluation of Single Variables
2.3. Evaluation of Mortality during Quarantine
2.4. Risk Matrix
3. Results
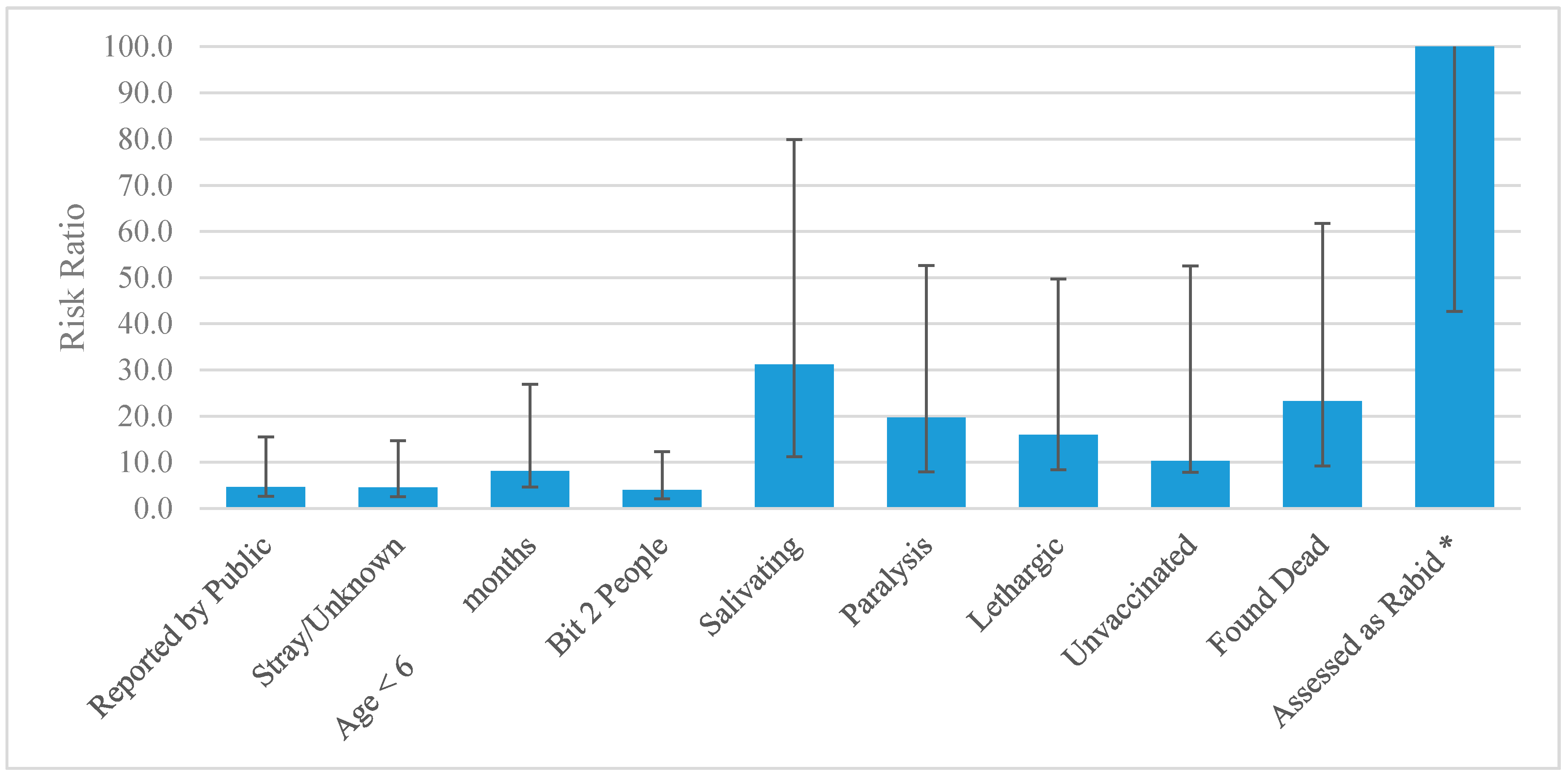
3.1. Single Variable Results
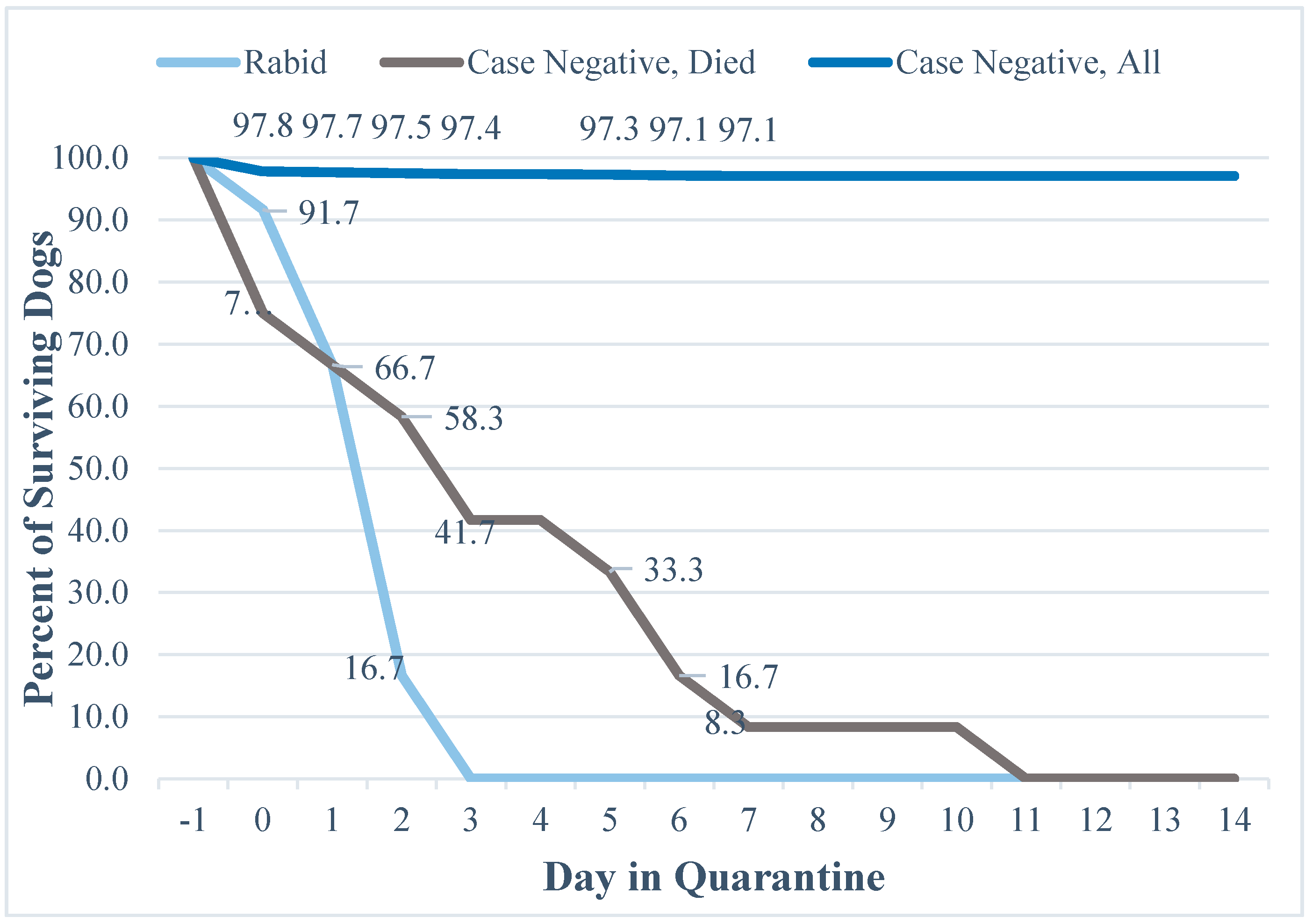
3.2. Mortality and Quarantine Results
4. Discussion
4.1. Objective of the Study
4.2. Characteristics of Rabid Dogs
4.3. Decision Making Based on the Variables
4.4. Evaluation of Mortality and Quarantine Data
4.5. Risk Matrix
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003786. [Google Scholar] [CrossRef] [PubMed]
- Tierkel, E.S. Chapter 8: Canine rabies. In The Natural History of Rabies; Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Wallace, R.M.; Reses, H.; Franka, R.; Dilius, P.; Fenelon, N.; Orciari, L.; Etheart, M.; Destine, A.; Crowdis, K.; Blanton, J.D.; et al. Establishment of a canine rabies burden in Haiti through the implementation of a novel surveillance program. PLoS Negl. Trop. Dis. 2015, 10, e0004245. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Briggs, D.; Brown, C.M.; Franka, R.; Katz, S.L.; Kerr, H.D.; Lett, S.M.; Levis, R.; Meltzer, M.I; William Schaffner, W.; et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2010, 59, 1–9. [Google Scholar] [PubMed]
- World Health Organization. WHO Guide for Rabies Pre and Post Exposure Prophylaxis in Humans. Updates 2014. Available online: http://www.who.int/rabies/PEP_Prophylaxis_guideline_15_12_2014.pdf (accessed on 11 November 2016).
- World Health Organization. WHO Expert Consultation on Rabies; Second Report; WHO Technical Report Series; WHO: Geneva, Switzerland, 2014; Volume 982, pp. 33–44. [Google Scholar]
- Shantavasinkul, P.; Wilde, H. Post-exposure prophylaxis for rabies in resource-limited/poor countries. Adv. Virus Res. 2011, 17, 291–307. [Google Scholar]
- Hampson, K.; Cleaveland, S.; Briggs, D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Undurraga, E.A.; Wallace, R.M.; Blanton, J.D.; Cleaton, J.; Franka, R. Elimination of dog-mediated human rabies deaths by 2030: Needs assessment and alternatives for progress based on dog vaccination. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Etheart, M. 2017–currently in review with Lancet Global Health. (Unpublished).
- Coetzer, A.; Kidane, A.H.; Bekele, M.; Hundera, D.D.; Pieracci, E.G.; Shiferaw, M.L.; Wallace, R.M.; Nel, L.H. The SARE tool for rabies control: Current experience in Ethiopia. Antivir. Res. 2016, 135, 74–80. [Google Scholar] [CrossRef] [PubMed]
- The Global Alliance for Rabies Control Website. Available online: rabiesalliance.org (accessed on 10 February 2017).
- Babes, V. Traité de la Rage (Treatise on Rabies); Baillière et Fils: Paris, France, 1912. [Google Scholar]
- Deeks, J.J.; Altman, D.G. Statistics Notes: Diagnostic tests 4: Likelihood ratios. BMJ 2004, 329, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Knust, B.; MacNeil, A.; Rollin, P.E. Hantavirus pulmonary syndrome clinical findings: Evaluating a surveillance case definition. Vector Borne Zoonotic Dis. 2011, 12, 393–399. [Google Scholar] [CrossRef] [PubMed]
- McGee, S. Simplifying Likelihood Ratios. J. Gen. Intern. Med. 2002, 17, 647–650. [Google Scholar] [CrossRef]
- Tepsumethanon, V.; Wilde, H.; Meslin, F.X. Six criteria for rabies diagnosis in living dogs. J. Med. Assoc. Thail. 2005, 88, 419–422. [Google Scholar]
- Indrayan, A. Basic Methods of Medical Research, 3rd ed.; Sensitivity-Specificity, Bayes’ Rule, and Predictives; AITBS Publishers: Krishan Nagar, Delhi, India, 2013. [Google Scholar]
- Abela-Ridder, B.; Knopf, L.; Martin, S.; Taylor, L.; Torres, G.; De Balough, K. 2016: The beginning of the end of rabies? Lancet Glob. Health 2016, 4, 780–781. [Google Scholar] [CrossRef]
- Benotti, P.; Wood, G.C.; Winegar, D.A.; Petrick, A.T.; Still, C.D.; Argyropoulos, G.; Gerhard, G.S. Risk factors associated with mortality after Roux-en-Y gastric bypass surgery. Ann. Surg. 2014, 259, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sriaroon, C.; Sriaroon, S.; Svastijaya, D.; Pakamatz, K.; Wilde, H. Retrospective: Animal attacks and rabies exposures in Thai children. Travel Med. Infect. Dis. 2005, 4, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Slavinski, S.; Ettestad, P.; Sidwa, T.J.; Sorhage, F.E. Compendium of animal rabies prevention and control: National Association of State Public Health Veterinarians, Inc. J. Am. Vet. Med. Assoc. 2011, 248, 1–13. [Google Scholar]
- Tepsumethanon, V.; Wilde, H.; Sitprija, V. Ten-day observation of live rabies suspected dogs. Dev. Biol. (Basel) 2008, 131, 543–546. [Google Scholar] [PubMed]
- Consales, C.A.; Bolzan, V.L. Rabies review: Immunopathology, clinical aspects and treatment. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 5–38. [Google Scholar] [CrossRef]
- Tipold, A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: A retrospective study. J. Vet. Intern. Med. 1995, 9, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Knopf, L. Partners for rabies prevention. surveillance of human rabies by national authorities-a global survey. Zoonoses Public Health 2015, 62, 543–552. [Google Scholar] [CrossRef] [PubMed]

| Variable | Strata | Specifics |
|---|---|---|
| Entity Reporting the Dog Bite Incident | All Health Sectors | Ministry of Health, Local Health Department, Hospitals |
| Veterinary Sectors | Veterinarians and Veterinary Agents | |
| Public | Notifications originating directly from a community member | |
| Ownership Status | Owned | |
| Stray/owner not identified | ||
| Number of People Bitten | 1 person bitten | |
| 2 people bitten | ||
| 3 or more people bitten | ||
| Age | Puppy | <6 months |
| Junior | 6 months–1 year | |
| Adult | >1 year | |
| Unknown | No age reported | |
| Sex | Female | |
| Male | ||
| Unknown | ||
| Aggression | Present or Absent | Aggression is determined by the rabies assessor |
| Hypersalivation | Present or Absent | Hypersalivation is determined by the rabies assessor |
| Paralysis | Present or Absent | Paralysis is determined by the rabies assessor |
| Lethargy | Present or Absent | Lethargy is determined by the rabies assessor |
| Vaccination Status | Vaccinated | Owner-reported that dog was vaccinated at least once |
| Not Vaccinated | Includes unvaccinated and unknown vaccination status | |
| Status of Dog at the Time of Investigation | Alive | |
| Dead | Hit by car, killed, died of natural causes | |
| Rabies Assessor’s Decision | Probably not Rabies | |
| Probably Rabies | ||
| Dead/Not Assessed |
| Variable | Test Group | Rabies Positive n = 48 | Case Negative n = 1361 | Risk Ratio and 95% CI | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Entity Reporting the Dog Bite Incident | All Health Sectors 1 | 19 | 39.6% | 1033 | 75.9% | REF |
| Veterinary Sectors 2 | 22 | 45.8% | 252 | 18.5% | 4.45 (2.44–8.09) | |
| Public | 7 | 14.6% | 76 | 5.6% | 4.67 (2.02–10.79) | |
| Ownership Status | Owned | 42 | 87.50% | 1324 | 97.28% | REF |
| Stray or Unknown | 6 | 12.50% | 37 | 2.72% | 4.54 (2.04–10.10) | |
| Number of People Bitten | Bit 1 | 36 | 75.00% | 1260 | 92.58% | REF |
| Bit 2 | 8 | 16.67% | 64 | 4.70% | 4.00 (1.93–8.29) | |
| Bit ≥ 3 | 4 | 8.33% | 37 | 2.72% | 3.51 (1.31–9.41) | |
| Sex | Female | 14 | 29.17% | 456 | 33.50% | REF |
| Male | 17 | 35.42% | 759 | 55.77% | 0.74 (0.37–1.48) | |
| Unknown | 17 | 35.42% | 146 | 10.73% | 3.50 (1.77–6.94) | |
| Age | Adult | 16 | 33.33% | 803 | 59.00% | REF |
| Puppy | 7 | 14.58% | 37 | 2.72% | 8.14 (3.53–18.76) | |
| Junior | 17 | 35.42% | 475 | 34.90% | 1.77 (0.90–3.47) | |
| Unknown | 8 | 16.67% | 46 | 3.38% | 7.58 (3.40–22.38) | |
| Aggression | Non-aggressive | 9 | 18.75% | 87 | 6.39% | REF |
| Aggressive | 39 | 81.25% | 1274 | 93.61% | 0.32 (0.16–0.63) | |
| Salivation | Normal Salivation | 29 | 60.42% | 1351 | 99.27% | REF |
| Hypersalivation | 19 | 39.58% | 10 | 0.73% | 31.18 (19.95–48.73) | |
| Paralysis | Non-paralytic | 37 | 77.08% | 1351 | 99.65% | REF |
| Paralytic | 11 | 22.92% | 10 | 0.35% | 19.65 (11.72–32.95) | |
| Lethargy | Non-lethargic | 44 | 91.67% | 1357 | 99.71% | REF |
| Lethargic | 4 | 8.33% | 4 | 0.29% | 15.92 (7.51–33.75) | |
| Vaccination Status | History of Rabies Vaccination | 2 | 4.17% | 434 | 31.89% | REF |
| Not vaccinated or unknown history | 46 | 95.83% | 927 | 68.11% | 10.31 (2.51–42.26) | |
| Status of Dog at Time of Investigation | Found Alive | 23 | 47.92% | 1323 | 97.21% | REF |
| Found Dead | 25 | 52.08% | 38 | 2.79% | 23.22 (13.99–38.55) | |
| Assessor's Decision | Dog not showing signs of Rabies | 1 | 2.08% | 1251 | 91.92% | REF |
| Dog likely to be rabid | 38 | 79.17% | 77 | 5.66% | 413.4 (57.33–2985) | |
| Dead/Not Assessed | 9 | 18.75% | 33 | 2.42% | 268.1 (34.79–2069) | |
| Variable | Test Group | SENS | SPEC | PPV | NPV | NLR−1 | PLR |
|---|---|---|---|---|---|---|---|
| Entity Reporting the Dog Bite Incident | All Health Sectors | 39.58% | 24.10% | 1.81% | 91.88% | 0.399 | 0.522 |
| Veterinary Sectors | 45.83% | 81.48% | 8.03% | 97.71% | 1.504 | 2.475 | |
| Public | 14.58% | 94.42% | 8.43% | 96.91% | 1.105 | 2.612 | |
| Ownership Status | Owned | 87.50% | 2.72% | 3.07% | 86.05% | 0.217 | 0.899 |
| Stray or Unknown | 12.50% | 97.28% | 13.95% | 96.93% | 1.112 | 4.598 | |
| Number of People Bitten | Bit 1 | 75.00% | 7.42% | 2.86% | 89.38% | 0.297 | 0.810 |
| Bit 2 | 16.67% | 95.30% | 12.50% | 97.01% | 1.144 | 3.544 | |
| Bit ≥ 3 | 8.33% | 97.28% | 10.81% | 96.78% | 1.061 | 3.065 | |
| Sex | Female | 29.17% | 66.50% | 2.98% | 96.38% | 0.939 | 0.871 |
| Male | 35.42% | 44.23% | 2.19% | 95.10% | 0.685 | 0.635 | |
| Unknown | 35.42% | 89.27% | 10.43% | 97.51% | 1.382 | 3.302 | |
| Age | Adult | 33.33% | 41.00% | 1.95% | 94.58% | 0.615 | 0.565 |
| Puppy | 14.58% | 97.28% | 15.91% | 97.00% | 1.139 | 5.364 | |
| Junior | 35.42% | 65.10% | 3.46% | 96.62% | 1.008 | 1.015 | |
| Unknown | 16.67% | 96.62% | 14.81% | 97.05% | 1.159 | 4.931 | |
| Aggression | Non-aggressive | 18.75% | 93.61% | 9.38% | 97.03% | 1.152 | 2.933 |
| Aggressive | 81.25% | 6.39% | 97.03% | 90.63% | 0.341 | 0.868 | |
| Salivation | Normal Salivation | 60.42% | 0.73% | 2.10% | 34.48% | 0.019 | 0.609 |
| Hypersalivation | 39.58% | 99.27% | 65.52% | 97.90% | 1.643 | 53.873 | |
| Paralysis | Non-paralytic | 77.08% | 0.73% | 2.67% | 47.62% | 0.008 | 0.027 |
| Paralytic | 22.92% | 99.27% | 52.38% | 97.33% | 1.288 | 31.190 | |
| Lethargy | Non-lethargic | 91.67% | 0.29% | 3.14% | 50.00% | 0.035 | 0.919 |
| Lethargic | 8.33% | 99.71% | 50.00% | 96.86% | 1.088 | 28.354 | |
| Vaccination Status | History of Vaccination | 4.17% | 79.96% | 0.46% | 95.74% | 0.834 | 0.208 |
| Not vaccinated or unknown history | 95.83% | 57.20% | 4.73% | 97.41% | 13.718 | 2.239 | |
| Status of Dog at Time of Investigation | Found Alive | 47.92% | 2.36% | 1.71% | 58.18% | 0.045 | 0.491 |
| Found Dead | 52.08% | 97.21% | 39.68% | 98.29% | 2.029 | 18.654 | |
| Assessor’s Decision | Dog not showing signs of Rabies | 2.08% | 8.08% | 0.08% | 99.86% | 1.020 | 14.496 |
| Dog likely to be rabid | 79.17% | 91.92% | 33.04% | 99.23% | 4.764 | 102.446 | |
| Dead/Not Assessed | 18.75% | 97.58% | 21.43% | 97.15% | 1.196 | 6.572 |
| Objective Risk Matrix | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Consideration | Probability of Rabies Based on Clinical Categorization of Bite (Babes) | Limited Surveillance Program Capacity | Advanced Surveillance Program Capacity | |||||||
| Dog Symptomatic | Dog Dead At Follow-up | Dog Bite Was Not Provoked | Stray Dog | Dog Bit Multiple People | Dog Not Vaccinated | Dog Healthy and Available for Quarantine | Dog Healthy 10 Days Post-Bite | Tested Negative | ||
| Bite to head/neck | 45.00% | 27.99% | 17.87% | 6.75% | 6.26% | 4.77% | 2.12% | 0.04% | 0.00% | 0.00% |
| Multiple severe bite wounds | 27.50% | 17.11% | 10.92% | 4.13% | 3.82% | 2.92% | 1.29% | 0.02% | 0.00% | 0.00% |
| Bites to young children | 27.50% | 17.11% | 10.92% | 4.13% | 3.82% | 2.92% | 1.29% | 0.02% | 0.00% | 0.00% |
| Bites to extremities | 5.00% | 3.11% | 1.99% | 0.75% | 0.70% | 0.53% | 0.24% | 0.00% | 0.00% | 0.00% |
| Minor bites (no break in skin) | 1.00% | 0.62% | 0.40% | 0.15% | 0.14% | 0.11% | 0.05% | 0.00% | 0.00% | 0.00% |
| Category II | 1.00% | 0.62% | 0.40% | 0.15% | 0.14% | 0.11% | 0.05% | 0.00% | 0.00% | 0.00% |
| Medley et al. probability of rabies 2 | 62.20% | 39.70% | 15.00% | 13.90% | 10.60% | 4.70% | 0.08% | 0.00% | 0.00% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medley, A.M.; Millien, M.F.; Blanton, J.D.; Ma, X.; Augustin, P.; Crowdis, K.; Wallace, R.M. Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti. Trop. Med. Infect. Dis. 2017, 2, 14. https://doi.org/10.3390/tropicalmed2020014
Medley AM, Millien MF, Blanton JD, Ma X, Augustin P, Crowdis K, Wallace RM. Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti. Tropical Medicine and Infectious Disease. 2017; 2(2):14. https://doi.org/10.3390/tropicalmed2020014
Chicago/Turabian StyleMedley, Alexandra M., Max Francois Millien, Jesse D. Blanton, Xiaoyue Ma, Pierre Augustin, Kelly Crowdis, and Ryan M. Wallace. 2017. "Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti" Tropical Medicine and Infectious Disease 2, no. 2: 14. https://doi.org/10.3390/tropicalmed2020014
APA StyleMedley, A. M., Millien, M. F., Blanton, J. D., Ma, X., Augustin, P., Crowdis, K., & Wallace, R. M. (2017). Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti. Tropical Medicine and Infectious Disease, 2(2), 14. https://doi.org/10.3390/tropicalmed2020014





