Development of a Microfluidic Point-of-Care Platform for HPV Detection Based on Helicase-Dependent Amplification
Abstract
1. Introduction
- Vaccinate 90% of girls under 15 years of age.
- Ensure that 70% of women receive high-throughput screening in two rounds: the first before age 35 and the second before age 45.
- Provide appropriate treatment for 90% of women diagnosed with HPV infection, precancerous lesions, or invasive cervical cancer.
2. Materials and Methods
2.1. Ethical Considerations
2.2. Sample Collection and Processing
2.3. Genotyping of DNA Samples
2.4. Standardization of the HDA Reaction Conditions
2.5. Design and Fabrication of the Microfluidic Device
2.6. Performance and Analysis of HDA Assays on the Microfluidic Platform
2.7. POC Platform
2.8. Statistical Analysis
3. Results
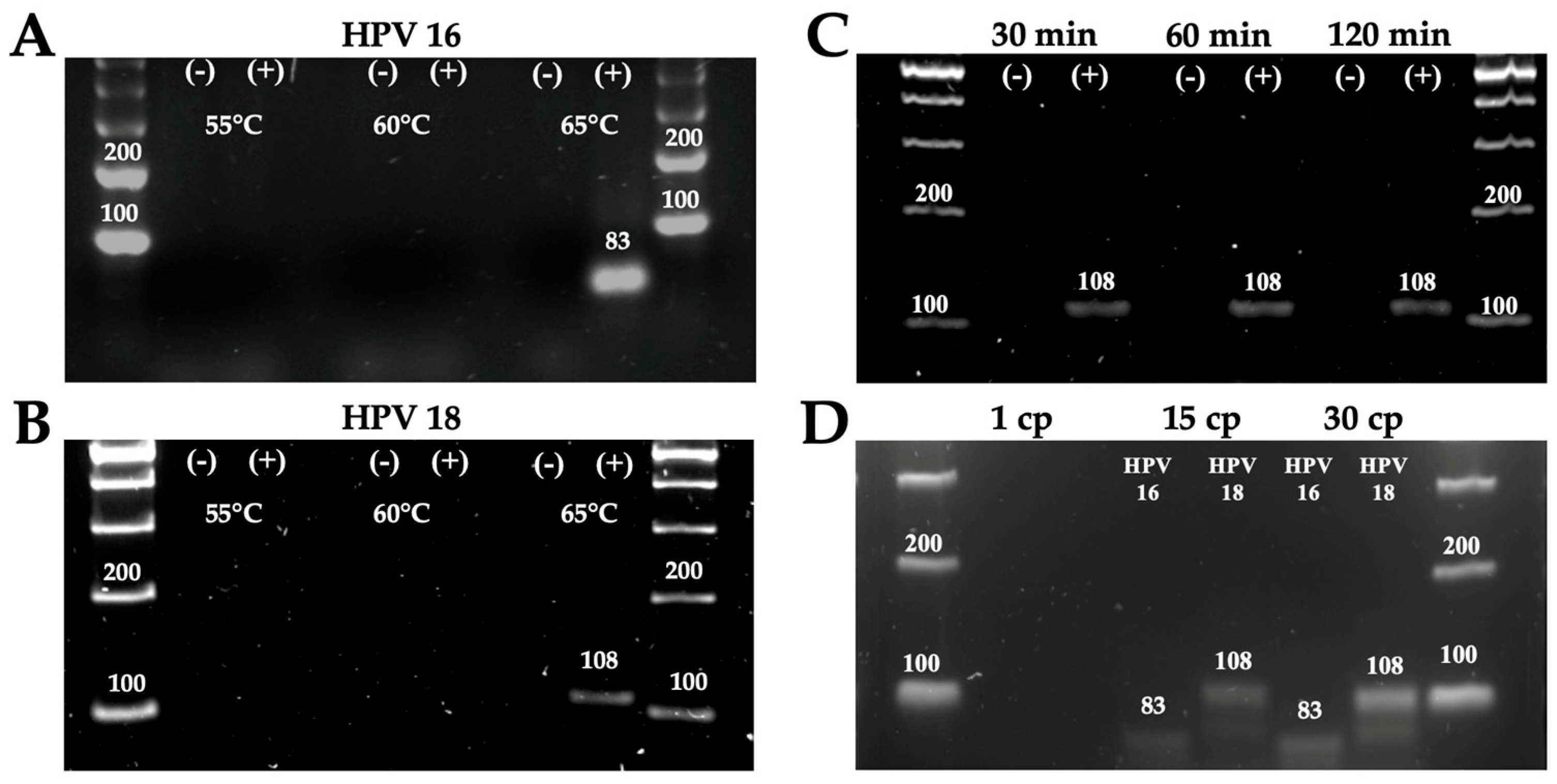
3.1. Standardization of Reaction Conditions
3.2. Detection of HPV Genotypes 16 and 18 Using the POC Platform
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bp | Base Pairs |
| cp | Copies |
| CI | Confidence interval |
| HDA | Helicase-Dependent Amplification |
| HPV | Human Papillomavirus |
| LOD | Limit of detection |
| PAHO | Pan American Health Organization |
| POC | Point-of-care |
| PMMA | Poly(methyl methacrylate) PMMA |
| RPA | Recombinase polymerase amplification |
| SSB | Single-Stranded DNA Binding Proteins |
| WHO | World Health Organization |
References
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; Desalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989. [Google Scholar] [CrossRef]
- Quinlan, J.D. Human Papillomavirus: Screening, Testing, and Prevention Human Papillomavirus. Am. Fam. Physician 2021, 104, 152–159. [Google Scholar]
- de Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet. Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 22 July 2025).
- Human Papillomavirus (HPV) Vaccine—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/human-papillomavirus-hpv-vaccine (accessed on 6 November 2023).
- Williams, J.; Kostiuk, M.; Biron, V.L. Molecular Detection Methods in HPV-Related Cancers. Front. Oncol. 2022, 12, 864820. [Google Scholar] [CrossRef]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Naderi Ghale-noie, Z.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed]
- Kundrod, K.A.; Smith, C.A.; Hunt, B.; Schwarz, R.A.; Schmeler, K.; Richards-Kortum, R. Advances in technologies for cervical cancer detection in low-resource settings. Expert Rev. Mol. Diagn. 2019, 19, 695–714. [Google Scholar] [CrossRef]
- Bartosik, M.; Moranova, L.; Izadi, N.; Strmiskova, J.; Sebuyoya, R.; Holcakova, J.; Hrstka, R. Advanced technologies towards improved HPV diagnostics. J. Med. Virol. 2024, 96, e29409. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; Carrasco-González, J.A.; Linhares, D.C.L.; Corzo, C.A.; Campos-Villalobos, J.I.; Henao-Díaz, A.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; González-González, R.B.; Parra-Saldívar, R.; et al. Emergent Molecular Techniques Applied to the Detection of Porcine Viruses. Vet. Sci. 2023, 10, 609. [Google Scholar] [CrossRef]
- Yadav, S.K.; Gupta, R. Point-of-Care Testing. Clinical Laboratory Management; Springer: Cham, Switzerland, 2023; pp. 109–111. [Google Scholar] [CrossRef]
- Mota, D.S.; Guimarães, J.M.; Gandarilla, A.M.D.; Filho, J.C.B.S.; Brito, W.R.; Mariúba, L.A.M. Recombinase polymerase amplification in the molecular diagnosis of microbiological targets and its applications. Can. J. Microbiol. 2022, 68, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Otoo, J.A.; Schlappi, T.S. REASSURED Multiplex Diagnostics: A Critical Review and Forecast. Biosensors 2022, 12, 124. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Venturoli, S.; Rösl, F.; Rincon-Orozco, B. Detection of high-risk human papillomavirus type 16 and 18 using isothermal helicase-dependent amplification. Diagn. Microbiol. Infect. Dis. 2014, 79, 178–182. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Dong, M.; Kshirsagar, A.; Politza, A.J.; Guan, W. High Fidelity Machine Learning-Assisted False Positive Discrimination in Loop-Mediated Isothermal Amplification Using Nanopore-Based Sizing and Counting. ACS Nano 2024, 18, 7170. [Google Scholar] [CrossRef]
- De Falco, M.; Colella, S.; Abdelkarim, J.A.Y.; Antonacci, A.; Scognamiglio, V.; De Felice, M. Helicase-dependent isothermal amplification turns biosensing into modern diagnostic technologies. Microchem. J. 2025, 216, 114772. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, E.; Trujillo-Rodríguez, G.d.J.; Rodríguez-Sánchez, I.P.; Ancer-Rodríguez, J.; Pérez-Maya, A.A.; Alvarez-Cuevas, S.; Martinez-Fierro, M.L.; Marino-Martínez, I.A.; Garza-Veloz, I. Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives. Pathogens 2024, 13, 653. [Google Scholar] [CrossRef]
- Weigl, B.; Domingo, G.; LaBarre, P.; Gerlach, J. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip 2008, 8, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lyu, W.; Yu, M.; Wang, Q.; Qu, H.; Ismagilov, R.F.; Han, X.; Lai, D.; Shen, F. Self-partitioning SlipChip for slip-induced droplet formation and human papillomavirus viral load quantification with digital LAMP. Biosens. Bioelectron. 2020, 155, 112107. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Yang, W.; Peng, J.; Huang, J.; Mi, S. An integrated microfluidic detection system for the automated and rapid diagnosis of high-risk human papillomavirus. Analyst 2021, 146, 5102–5114. [Google Scholar] [CrossRef]
- Yin, K.; Pandian, V.; Kadimisetty, K.; Ruiz, C.; Cooper, K.; You, J.; Liu, C. Synergistically enhanced colorimetric molecular detection using smart cup: A case for instrument-free HPV-associated cancer screening. Theranostics 2019, 9, 2637–2645. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Y.; Gao, L.; Wang, T.; Zhang, X.; Hu, J.; Ding, L.; Zhang, Y.; Wang, Q.; Wang, L.; et al. A portable all-in-one microfluidic device with real-time colorimetric LAMP for HPV16 and HPV18 DNA point-of-care testing. Biosens. Bioelectron. 2024, 248, 115968. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Jin, Y.; Li, C.; Xin, M.; Jiang, X.; Wan, J. Lambda exonuclease assisted helicase-dependent amplification CRISPR/Cas12a detection of Listeria monocytogenes. Biochimie 2025, 235, 106–112. [Google Scholar] [CrossRef]
- Yu, L.; Tang, Y.; Sun, Y.; Wang, H.; Yi, H.; Zhong, Y.; Shao, Z.; Zhou, S.; He, S.; Cao, K.; et al. DMSO enhanced one-pot HDA-CRISPR/Cas12a biosensor for ultrasensitive detection of Monkeypox virus. Talanta 2025, 287, 127660. [Google Scholar] [CrossRef]
- Jenison, R.; Jaeckel, H.; Klonoski, J.; Latorra, D.; Wiens, J. Rapid amplification/detection of nucleic acid targets utilizing a HDA/thin film biosensor. Analyst 2014, 139, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Mahalanabis, M.; Do, J.; Almuayad, H.; Zhang, J.Y.; Klapperich, C.M. An integrated disposable device for DNA extraction and helicase dependent amplification. Biomed. Microdevices 2010, 12, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, W.; Li, H.; Cui, J.; Xie, Y.; Wu, B.; Chen, R. Advances in isothermal nucleic acid amplification methods for hepatitis B virus detection. Analyst 2023, 148, 3708–3718. [Google Scholar] [CrossRef]
- Ramalingam, N.; San, T.C.; Kai, T.J.; Mak, M.Y.M.; Gong, H.Q. Microfluidic devices harboring unsealed reactors for real-time isothermal helicase-dependent amplification. Microfluid. Nanofluidics 2009, 7, 325–336. [Google Scholar] [CrossRef]
- Attia, M.A.; Chang, W.T.; Tandon, R. Heterogeneity Aware Two-Stage Group Testing. IEEE Trans. Signal Process. 2021, 69, 3977. [Google Scholar] [CrossRef]
- Söderlund-Strand, A.; Carlson, J.; Dillner, J. Modified General Primer PCR System for Sensitive Detection of Multiple Types of Oncogenic Human Papillomavirus. J. Clin. Microbiol. 2009, 47, 541. [Google Scholar] [CrossRef] [PubMed]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Helicase-dependent isothermal amplification: A novel tool in the development of molecular-based analytical systems for rapid pathogen detection. Anal. Bioanal. Chem. 2017, 410, 679. [Google Scholar] [CrossRef]
- Lamsisi, M.; Benlghazi, A.; Kouach, J.; Laraqui, A.; Ennaji, M.M.; Chauleur, C.; Bourlet, T.; Li, G. Isothermal Nucleic Acid Amplification for Point-of-Care Primary Cervical Cancer Screening. Viruses 2024, 16, 1852. [Google Scholar] [CrossRef]
- Kumvongpin, R.; Jearanaikool, P.; Wilailuckana, C.; Sae-ung, N.; Prasongdee, P.; Daduang, S.; Wongsena, M.; Boonsiri, P.; Kiatpathomchai, W.; Swangvaree, S.S.; et al. High sensitivity, loop-mediated isothermal amplification combined with colorimetric gold-nanoparticle probes for visual detection of high risk human papillomavirus genotypes 16 and 18. J. Virol. Methods 2016, 234, 90–95. [Google Scholar] [CrossRef]
- Gulinaizhaer, A.; Yang, C.; Zou, M.; Ma, S.; Fan, X.; Wu, G. Detection of monkeypox virus using helicase dependent amplification and recombinase polymerase amplification combined with lateral flow test. Virol. J. 2023, 20, 274. [Google Scholar] [CrossRef]
- Yamket, W.; Sathianpitayakul, P.; Santanirand, P.; Ratthawongjirakul, P. Implementation of helicase-dependent amplification with SYBR Green I for prompt naked-eye detection of bacterial contaminants in platelet products. Sci. Rep. 2023, 13, 3238. [Google Scholar] [CrossRef]
- Ma, B.; Fang, J.; Lin, W.; Yu, X.; Sun, C.; Zhang, M. A simple and efficient method for potential point-of-care diagnosis of human papillomavirus genotypes: Combination of isothermal recombinase polymerase amplification with lateral flow dipstick and reverse dot blot. Anal. Bioanal. Chem. 2019, 411, 7451–7460. [Google Scholar] [CrossRef]
- 6 Ways to Minimize Contamination During PCR|labclinics.com. Available online: https://www.labclinics.com/2019/07/22/six-ways-to-minimize-contamination-during-pcr/?lang=en (accessed on 29 May 2025).
- HPV Tests For Cervical Cancer Screening—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/cervical-cancer/hpv-tests-cervical-cancer-screening (accessed on 22 July 2025).
- González-González, E.; Garcia-Ramirez, R.; Díaz-Armas, G.G.; Esparza, M.; Aguilar-Avelar, C.; Flores-Contreras, E.A.; Rodríguez-Sánchez, I.P.; Delgado-Balderas, J.R.; Soto-García, B.; Aráiz-Hernández, D.; et al. Automated elisa on-chip for the detection of anti-SARS-CoV-2 antibodies. Sensors 2021, 21, 6785. [Google Scholar] [CrossRef] [PubMed]
- González-González, E.; Lara-Mayorga, I.M.; Rodríguez-Sánchez, I.P.; Zhang, Y.S.; Martínez-Chapa, S.O.; Santiago, G.T.D.; Alvarez, M.M. Colorimetric loop-mediated isothermal amplification (LAMP) for cost-effective and quantitative detection of SARS-CoV-2: The change in color in LAMP-based assays quantitatively correlates with viral copy number. Anal. Methods 2021, 13, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kundrod, K.A.; Barra, M.; Wilkinson, A.; Smith, C.A.; Natoli, M.E.; Chang, M.M.; Coole, J.B.; Santhanaraj, A.; Lorenzoni, C.; Mavume, C.; et al. An integrated isothermal nucleic acid amplification test to detect HPV16 and HPV18 DNA in resource-limited settings. Sci. Transl. Med. 2023, 15, eabn4768. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Robson, J.M.; Fan, A.; Bono, M.S.; Furst, A.L.; Klapperich, C.M. Electrochemical Strategy for Low-Cost Viral Detection. ACS Cent. Sci. 2021, 7, 963–972. [Google Scholar] [CrossRef]
- Hsiang, E.; Little, K.M.; Haguma, P.; Hanrahan, C.F.; Katamba, A.; Cattamanchi, A.; Davis, J.L.; Vassall, A.; Dowdy, D. Higher cost of implementing Xpert® MTB/RIF in Ugandan peripheral settings: Implications for cost-effectiveness. Int. J. Tuberc. Lung Dis. 2016, 20, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Putting HIV and HCV to the Test, 2nd ed.; Médecins Sans Frontières Access Campaign: Geneva, Switzerland, 2015; Available online: https://msfaccess.org/putting-hiv-and-hcv-test-2nd-ed-2015 (accessed on 22 July 2025).


| Samples | Results/Genotype | ||
|---|---|---|---|
| 16 | 18 | 45 | |
| 1 | − | − | − |
| 2 | + | − | − |
| 3 | − | − | − |
| 4 | − | − | − |
| 5 | + | − | − |
| 6 | − | − | − |
| 7 | − | − | − |
| 8 | − | − | − |
| 9 | − | + | − |
| 10 | − | + | − |
| 11 | − | + | − |
| 12 | − | + | − |
| 13 | + | − | − |
| 14 | + | − | − |
| 15 | − | + | − |
| 16 | − | + | − |
| 17 | + | − | − |
| 18 | − | + | − |
| 19 | − | − | − |
| 20 | + | − | − |
| 21 | − | + | − |
| 22 | + | − | − |
| 23 | − | − | − |
| 24 | + | − | − |
| 25 | − | + | − |
| 26 | + | − | − |
| 27 | − | + | − |
| 28 | − | + | − |
| 29 | + | − | − |
| 30 | − | − | − |
| 31 | − | − | − |
| 32 | − | − | − |
| 33 | − | − | − |
| Name | Sequence | Size Amplicon | Reference |
|---|---|---|---|
| Forward HPV-16 | AAGCAGAACCGGACAGAGCCCA | 83 bp | [15] |
| Reverse HPV-16 | GCTTTGTACGCACAACCGAAGCG | ||
| Forward HPV-18 | ACCAGCCCGACGAGCCGAACC | 108 bp | [15] |
| Reverse HPV-18 | GCTCGAAGGTCGTCTGCTGAGCTTT |
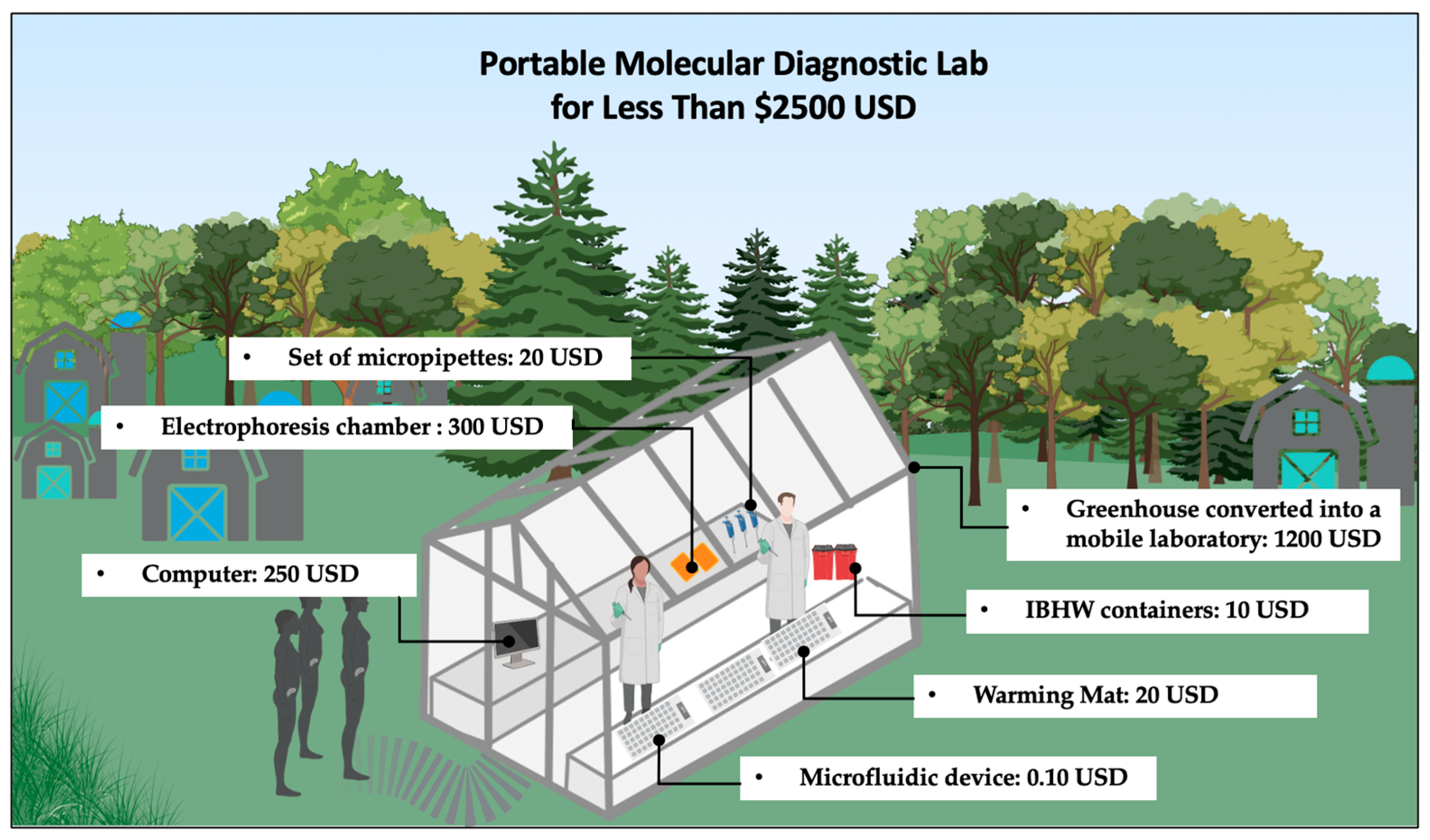
| Component | Cost (USD) |
|---|---|
| Microfluidic device (PMMA) | 0.10 |
| Portable warming mat | 20 |
| Electrophoresis chamber | 300 |
| Total | 320.10 |
| HDA Assay | Real-Time PCR | Total | Sensitivity | Specificity | Kappa (95% CI, p-Value) | |
|---|---|---|---|---|---|---|
| + | − | |||||
| + | 20 | 0 | 20 | 95.52% | 100% | 0.936 (84.24–99.92, p < 0.05) |
| − | 1 | 12 | 13 | |||
| Total | 21 | 12 | 33 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, E.; Flores-Contreras, E.A.; Trujillo-Rodríguez, G.d.J.; Jiménez-Martínez, M.L.; Rodríguez-Sánchez, I.P.; Ancer-Arellano, A.; Alvarez-Cuevas, S.; Martinez-Fierro, M.L.; Marino-Martínez, I.A.; Garza-Veloz, I. Development of a Microfluidic Point-of-Care Platform for HPV Detection Based on Helicase-Dependent Amplification. Trop. Med. Infect. Dis. 2025, 10, 272. https://doi.org/10.3390/tropicalmed10090272
González-González E, Flores-Contreras EA, Trujillo-Rodríguez GdJ, Jiménez-Martínez ML, Rodríguez-Sánchez IP, Ancer-Arellano A, Alvarez-Cuevas S, Martinez-Fierro ML, Marino-Martínez IA, Garza-Veloz I. Development of a Microfluidic Point-of-Care Platform for HPV Detection Based on Helicase-Dependent Amplification. Tropical Medicine and Infectious Disease. 2025; 10(9):272. https://doi.org/10.3390/tropicalmed10090272
Chicago/Turabian StyleGonzález-González, Everardo, Elda A. Flores-Contreras, Gerardo de Jesús Trujillo-Rodríguez, Mariana Lizbeth Jiménez-Martínez, Iram P. Rodríguez-Sánchez, Adriana Ancer-Arellano, Salomon Alvarez-Cuevas, Margarita L. Martinez-Fierro, Iván A. Marino-Martínez, and Idalia Garza-Veloz. 2025. "Development of a Microfluidic Point-of-Care Platform for HPV Detection Based on Helicase-Dependent Amplification" Tropical Medicine and Infectious Disease 10, no. 9: 272. https://doi.org/10.3390/tropicalmed10090272
APA StyleGonzález-González, E., Flores-Contreras, E. A., Trujillo-Rodríguez, G. d. J., Jiménez-Martínez, M. L., Rodríguez-Sánchez, I. P., Ancer-Arellano, A., Alvarez-Cuevas, S., Martinez-Fierro, M. L., Marino-Martínez, I. A., & Garza-Veloz, I. (2025). Development of a Microfluidic Point-of-Care Platform for HPV Detection Based on Helicase-Dependent Amplification. Tropical Medicine and Infectious Disease, 10(9), 272. https://doi.org/10.3390/tropicalmed10090272








