Praziquantel Pretreatment Reduces Schistosoma japonicum Infection in Mice by Targeting Immature Worm Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Animals, Parasites, and Drugs
2.3. PZQ Pretreatment, and Challenge Infection
2.4. Worm Burdens and Liver Egg Counting Assessment
2.5. Data Analysis
3. Results
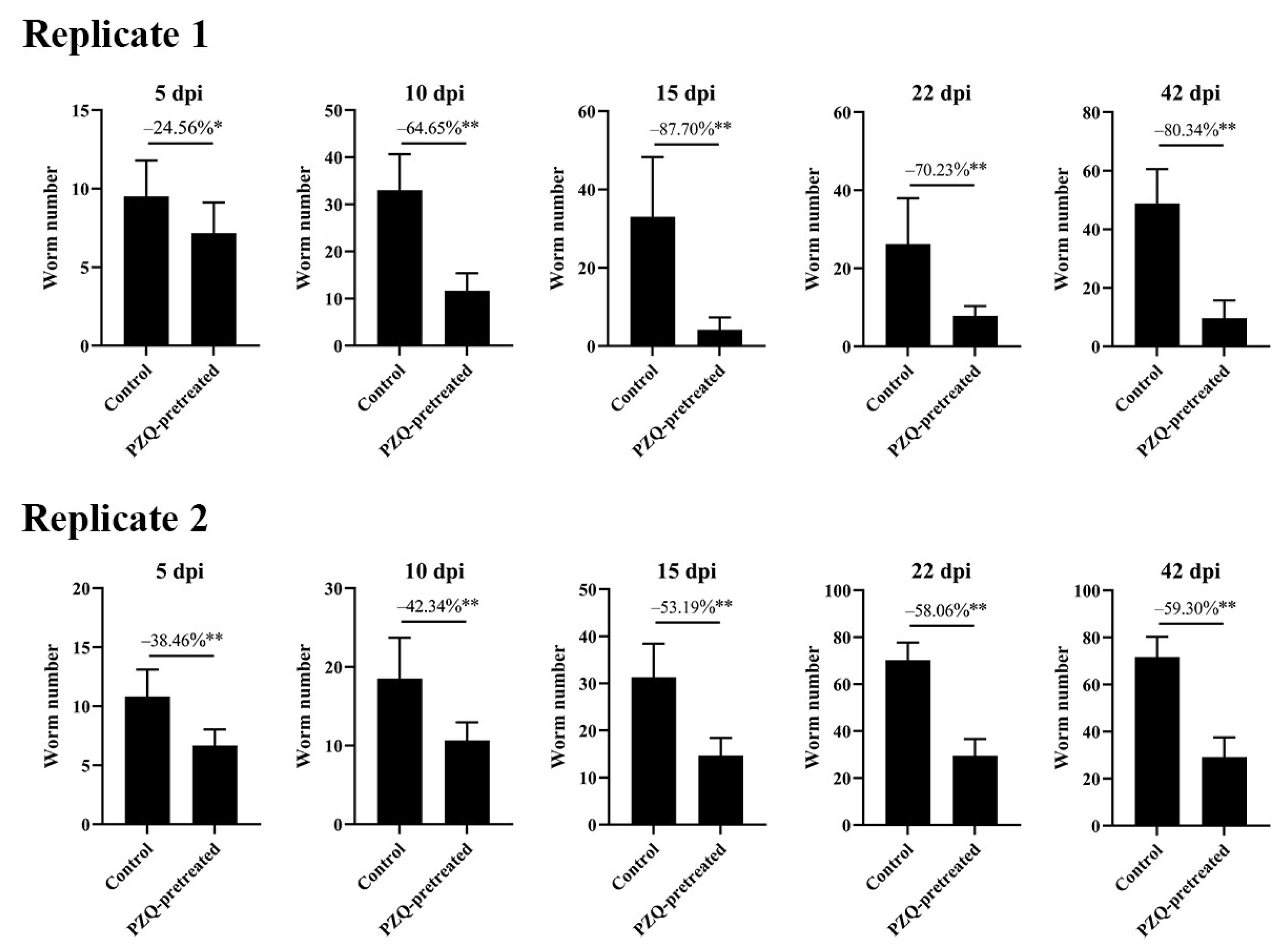
3.1. PZQ Pretreatment Significantly Reduces Worm Burdens and Liver Egg Counts at Various Infection Intensities
3.2. Target Stages of Schistosome Mortality in PZQ-Pretreated Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buonfrate, D.; Ferrari, T.C.A.; Akim Adegnika, A.; Russell Stothard, J.; Gobbi, F.G. Human schistosomiasis. Lancet 2025, 405, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Williams, G.M.; Gray, D.J.; Clements, A.C.A.; Zhou, X.N.; Li, Y.; Utzinger, J.; Kurscheid, J.; Forsyth, S.; Addis Alene, K.; et al. Schistosomiasis in the People’s Republic of China—Down but not out. Parasitology 2022, 149, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Nation, C.S.; Da’dara, A.A.; Marchant, J.K.; Skelly, P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020, 14, e0007951. [Google Scholar] [CrossRef] [PubMed]
- Grover, E.; Paull, S.; Kechris, K.; Buchwald, A.; James, K.; Liu, Y.; Carlton, E.J. Predictors of bovine Schistosoma japonicum infection in rural Sichuan, China. Int. J. Parasitol. 2022, 52, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A. Schistosomiasis then and now: What has changed in the last 100 years? Parasitology 2020, 147, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Jiz, M.; Mingala, C.; Fu, Z.Q.; Adriatico, M.; Lu, K.; Jarilla, B.; Sagliba, M.; Moreno, A.; Park, S.; Lin, J.J.; et al. High prevalence of Schistosoma japonicum by perfusion in naturally exposed water buffalo in a region of the Philippines endemic for human schistosomiasis. PLoS Negl. Trop. Dis. 2021, 15, e0009796. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, N.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, N.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nature reviews. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Nogi, T.; Zhang, D.; Chan, J.D.; Marchant, J.S. A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel beta subunits: Subversion of flatworm regenerative polarity. PLoS Negl. Trop. Dis. 2009, 3, e464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chan, J.D.; Nogi, T.; Marchant, J.S. Opposing roles of voltage-gated Ca2+ channels in neuronal control of regenerative patterning. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15983–15995. [Google Scholar] [CrossRef] [PubMed]
- Driciru, E.; Koopman, J.P.R.; Cose, S.; Siddiqui, A.A.; Yazdanbakhsh, M.; Elliott, A.M.; Roestenberg, M. Immunological Considerations for Schistosoma Vaccine Development: Transitioning to Endemic Settings. Front. Immunol. 2021, 12, 635985. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.J.; McManus, D.P.; Li, Y.; Williams, G.M.; Bergquist, R.; Ross, A.G. Schistosomiasis elimination: Lessons from the past guide the future. The Lancet. Infect. Dis. 2010, 10, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Gui, X.; Lu, Z.; Lv, R.; Li, H.; Lu, K.; Hong, Y.; Fu, Z.; Jin, Y.; Lin, J.; et al. Praziquantel promotes protection against Schistosoma japonicum infection in mice. Acta Trop. 2023, 241, 106874. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Shao, B.; Zhong, H.; Lv, R.; Li, H.; Lu, K.; Hong, Y.; Fu, Z.; Lu, Z.; Xu, M.; et al. Effect of Praziquantel on Preventing Delayed Infection of Schistosoma japonicum in Buffaloes and Goats. Microorganisms 2024, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Bergquist, R.; Li, S.-Z.; Zhou, X.-N. “Farewell to the God of Plague”: The Importance of Political Commitment Towards the Elimination of Schistosomiasis. Trop. Med. Infect. Dis. 2018, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, L.; Yang, F.; Dang, H.; Li, Y.; Guo, S.; Li, S.; Cao, C.; Xu, J.; Li, S. Progress of schistosomiasis control in People’s Republic of China in 2023. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2024, 36, 221–227. (In Chinese) [Google Scholar]
- Lv, S.; Xu, J.; Li, Y.-L.; Bao, Z.-P.; Zhang, L.-J.; Yang, K.; Lin, D.-D.; Liu, J.-B.; Wang, T.-P.; Ren, G.-H.; et al. Snail control as a crucial approach to schistosomiasis elimination: Evidence from the People’s Republic of China. Infect. Dis. Poverty 2025, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Mwinzi, P.N.; Chimbari, M.; Sylla, K.; Odiere, M.R.; Midzi, N.; Ruberanziza, E.; Mupoyi, S.; Mazigo, H.D.; Coulibaly, J.T.; Ekpo, U.F.; et al. Priority knowledge gaps for schistosomiasis research and development in the World Health Organization Africa Region. Infect. Dis. Poverty 2025, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar] [CrossRef] [PubMed]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, X.; Lv, R.; Zhong, H.; Li, H.; Lu, K.; Fu, Z.; Jin, Y.; Liu, J. Praziquantel Pretreatment Reduces Schistosoma japonicum Infection in Mice by Targeting Immature Worm Stages. Trop. Med. Infect. Dis. 2025, 10, 262. https://doi.org/10.3390/tropicalmed10090262
Gui X, Lv R, Zhong H, Li H, Lu K, Fu Z, Jin Y, Liu J. Praziquantel Pretreatment Reduces Schistosoma japonicum Infection in Mice by Targeting Immature Worm Stages. Tropical Medicine and Infectious Disease. 2025; 10(9):262. https://doi.org/10.3390/tropicalmed10090262
Chicago/Turabian StyleGui, Xiang, Rongxue Lv, Haoran Zhong, Hao Li, Ke Lu, Zhiqiang Fu, Yamei Jin, and Jinming Liu. 2025. "Praziquantel Pretreatment Reduces Schistosoma japonicum Infection in Mice by Targeting Immature Worm Stages" Tropical Medicine and Infectious Disease 10, no. 9: 262. https://doi.org/10.3390/tropicalmed10090262
APA StyleGui, X., Lv, R., Zhong, H., Li, H., Lu, K., Fu, Z., Jin, Y., & Liu, J. (2025). Praziquantel Pretreatment Reduces Schistosoma japonicum Infection in Mice by Targeting Immature Worm Stages. Tropical Medicine and Infectious Disease, 10(9), 262. https://doi.org/10.3390/tropicalmed10090262







