Compatibility of Serratia ureylitica Su_YN1, Malaria Transmission-Blocking Bacterium, with the Anopheles aquasalis Vector
Abstract
1. Introduction
2. Materials and Methods
2.1. Anopheles aquasalis
2.2. Bacteria
2.3. Bacterial Culture and Introduction into Mosquitoes
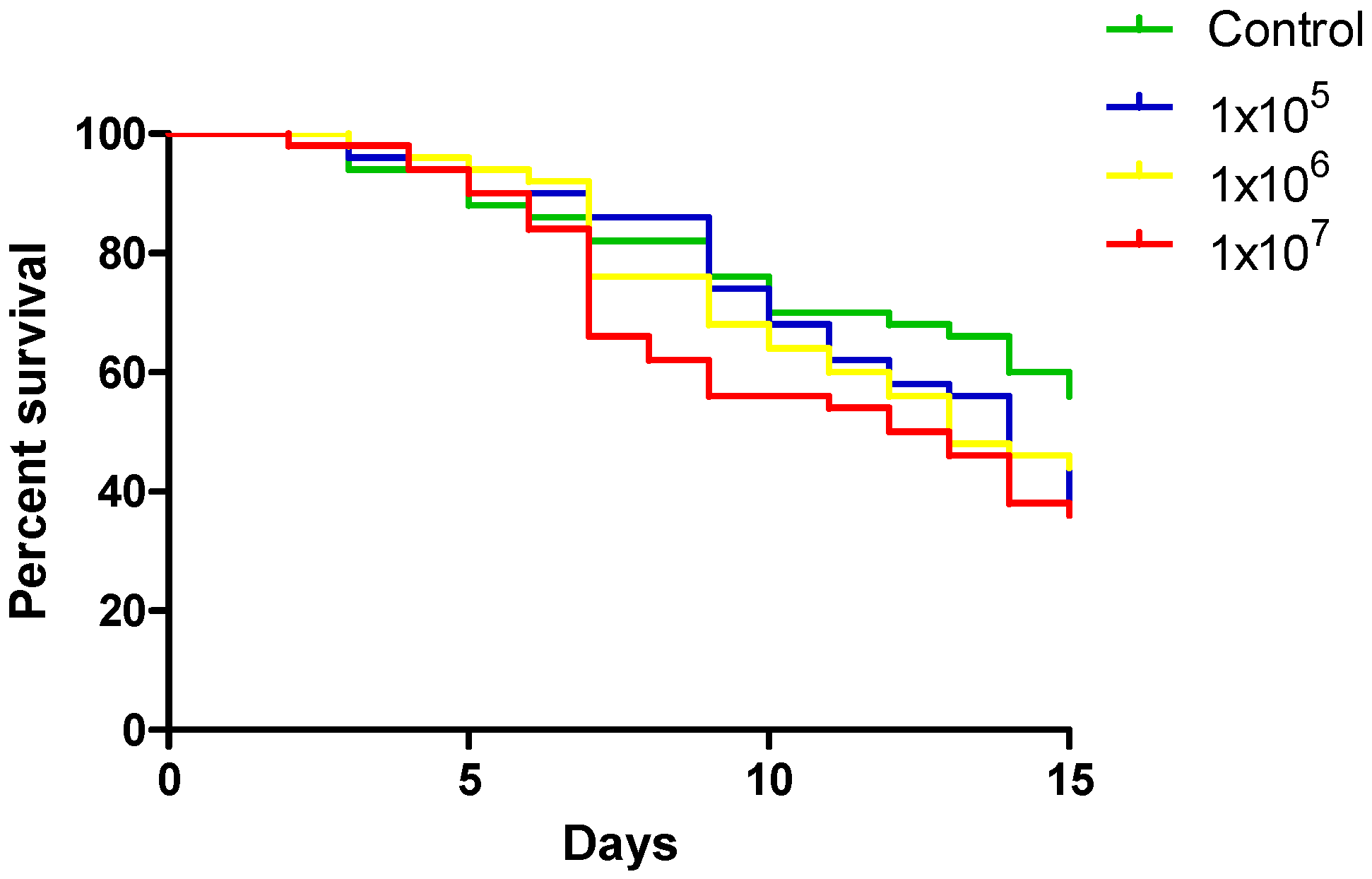
2.4. Effects of Different Concentrations of YN1 on the Survival of A. aquasalis
2.5. Effects of YN1 on the Survival of Female and Male A. aquasalis
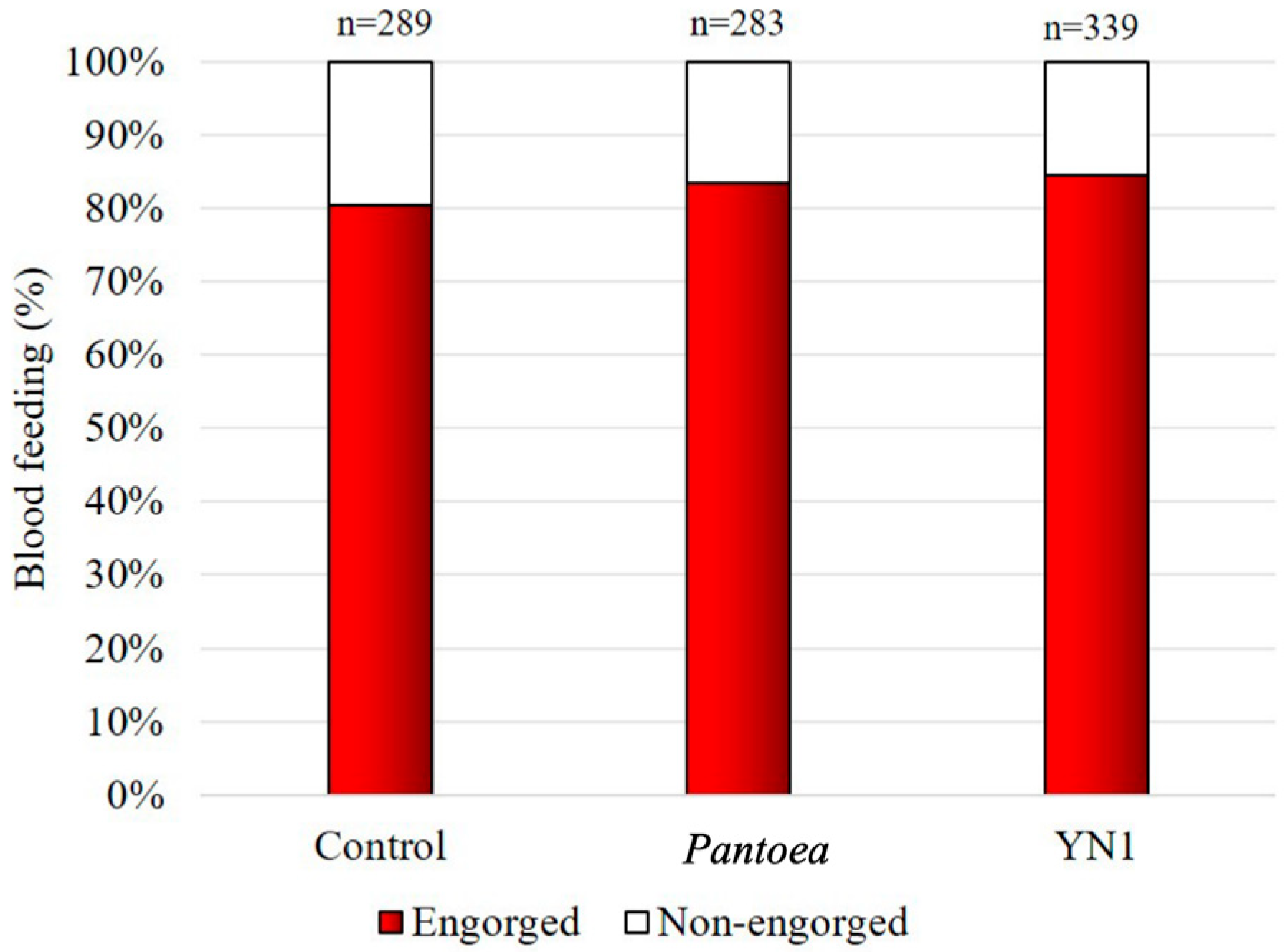
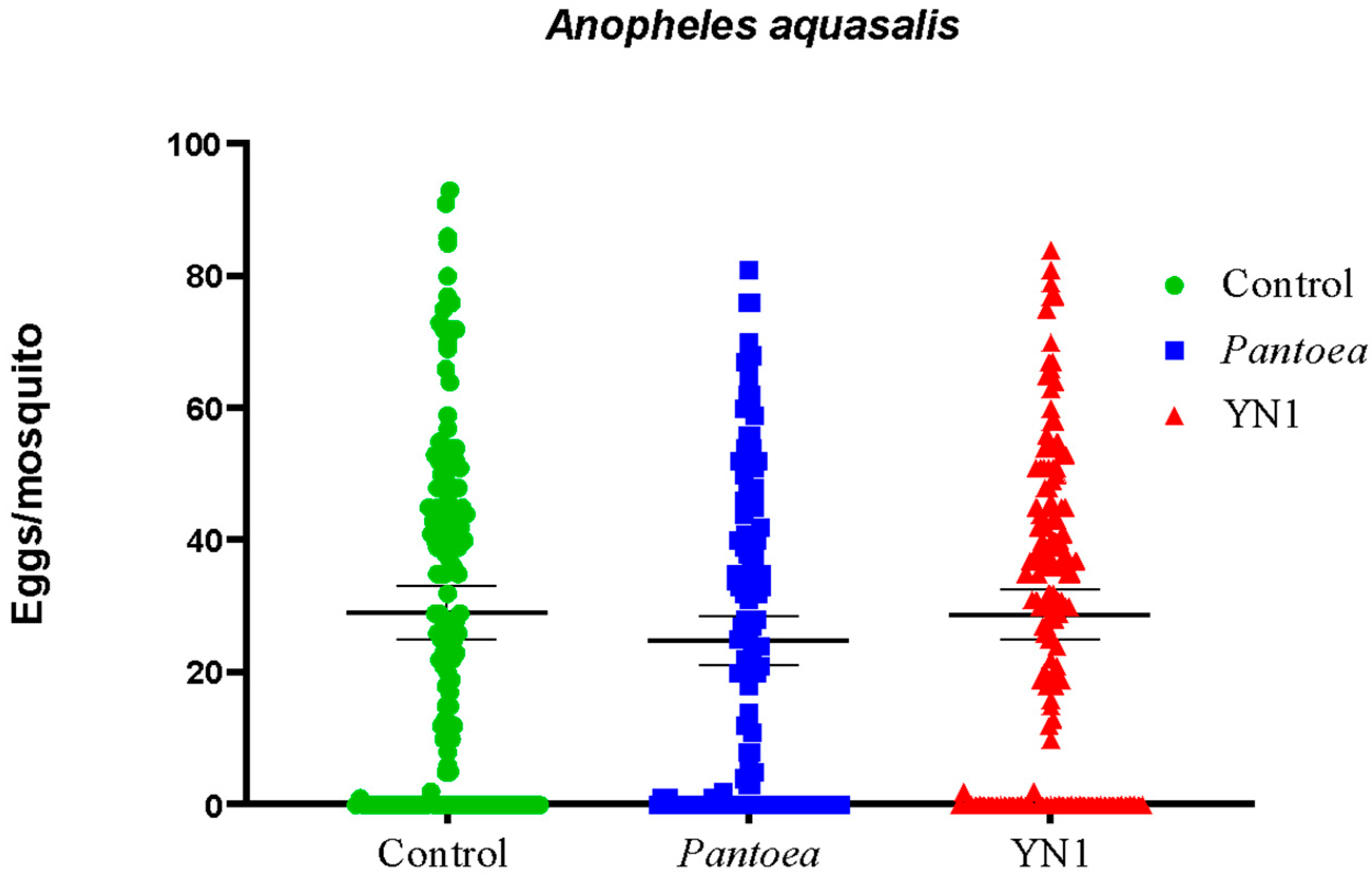
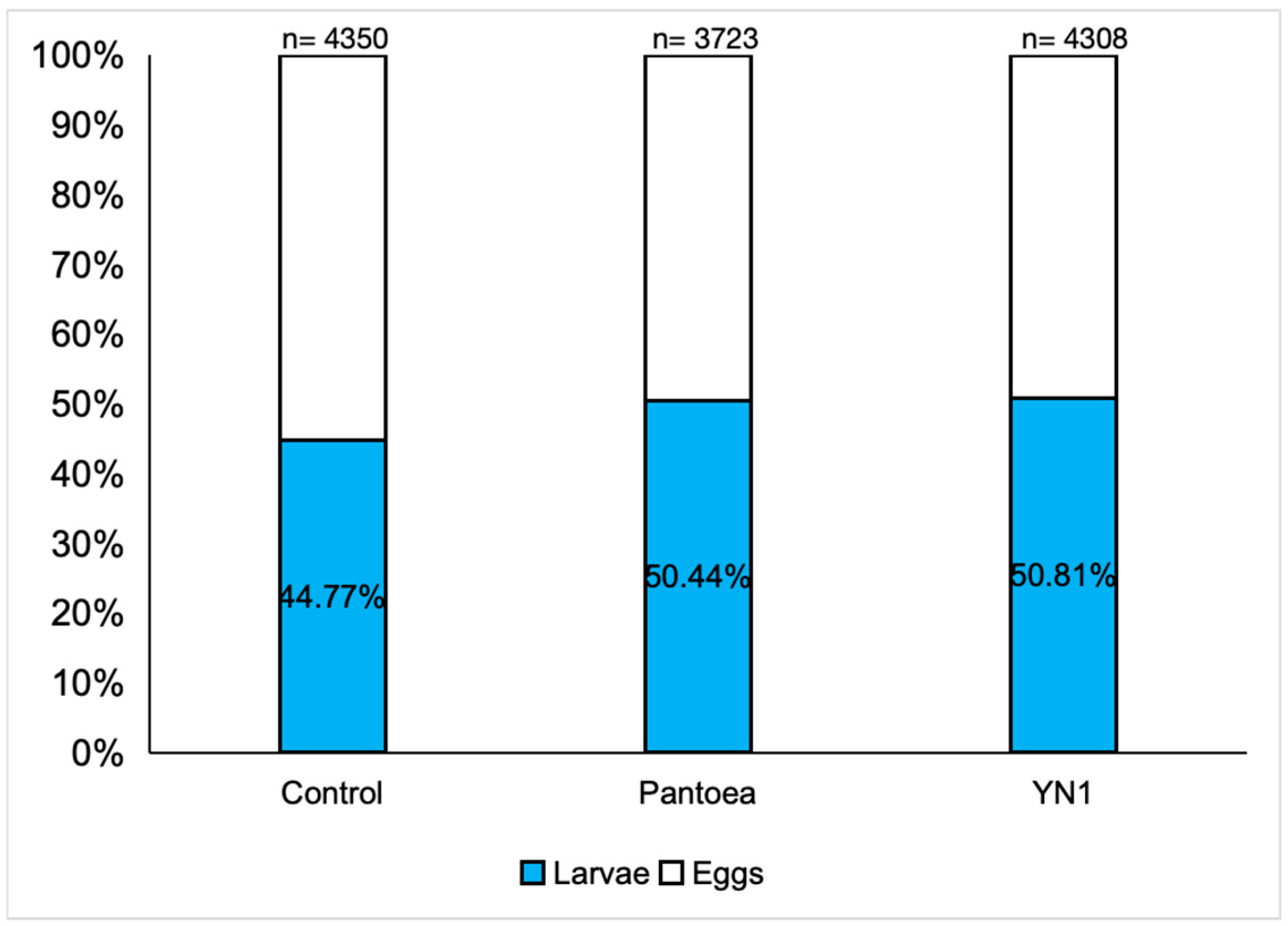
2.6. Effects of YN1 on Mosquito Blood Feeding, Fecundity and Fertility
2.7. Statistical Analysis
2.8. Ethical Statements
3. Results
3.1. Effect of Different YN1 Concentrations on the Survival of A. aquasalis
3.2. Effect of YN1 on Adult Male and Female A. aquasalis Survival
3.3. Effects of YN1 on Mosquito Blood-Feeding Behavior
3.4. Effects of YN1 on Mosquito Fecundity
3.5. Effects of YN1 on Mosquito Fertility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Malaria World Report. 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 30 April 2025).[Green Version]
- Ghosh, A.; Edwards, M.J.; Jacobs-Lorena, M. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol. Today 2000, 16, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Baton, L.A.; Ranford-Cartwright, L.C. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 2005, 21, 573–580. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Jacobs-Lorena, M. Plasmodium sporozoite invasion of the mosquito salivary gland. Curr. Opin. Microbiol. 2009, 12, 394–400. [Google Scholar] [CrossRef]
- Smith, R.C.; Vega-Rodriguez, J.; Jacobs-Lorena, M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz. 2014, 109, 644–661. [Google Scholar] [CrossRef]
- Bahia, A.C.; Dong, Y.; Blumberg, B.J.; Mlambo, G.; Tripathi, A.; BenMarzouk-Hidalgo, O.J.; Chandra, R.; Dimopoulos, G. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ. Microbiol. 2014, 16, 2980–2994. [Google Scholar] [CrossRef]
- Tchioffo, M.T.; Boissière, A.; Churcher, T.S.; Abate, L.; Gimonneau, G.; Nsango, S.E.; Awono-Ambéné, P.H.; Christen, R.; Berry, A.; Morlais, I. Modulation of Malaria Infection in Anopheles gambiae Mosquitoes Exposed to Natural Midgut Bacteria. PLoS ONE 2013, 8, e81663. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Habtewold, T.; Groom, Z.; Christophides, G.K. Immune resistance and tolerance strategies in malaria vector and non-vector mosquitoes. Parasit. Vectors 2017, 10, 186. [Google Scholar] [CrossRef]
- Romoli, O.; Gendrin, M. The tripartite interactions between the mosquito, its microbiota and Plasmodium. Parasit. Vectors 2018, 11, 200. [Google Scholar] [CrossRef]
- Kajla, M.; Choudhury, T.P.; Kakani, P.; Gupta, K.; Dhawan, R.; Gupta, L.; Kumar, S. Silencing of Anopheles stephensi Heme Peroxidase HPX15 Activates Diverse Immune Pathways to Regulate the Growth of Midgut Bacteria. Front. Microbiol. 2016, 7, 1351. [Google Scholar] [CrossRef]
- Huang, W.; Rodrigues, J.; Bilgo, E.; Tormo, J.R.; Challenger, J.D.; De Cozar-Gallardo, C.; Pérez-Victoria, I.; Reyes, F.; Castañeda-Casado, P.; Gnambani, E.J.; et al. Delftia tsuruhatensis TC1 symbiont suppresses malaria transmission by anopheline mosquitoes. Science 2023, 381, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.; Wang, D.; Huang, Z.; Li, X.; et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol. 2021, 6, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Carlos, B.C.; Rona, L.D.P.; Christophides, G.K.; Souza-Neto, J.A. A comprehensive analysis of malaria transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasit. Vectors 2012, 5, 69. [Google Scholar] [CrossRef]
- Rios-Velásquez, C.M.; Martins-Campos, K.M.; Simoes, R.C.; Izzo, T.; Santos, E.V.; Pessoa, F.A.C.; Lima, J.B.P.; Monteiro, W.M.; Secundino, N.F.C.; Lacerda, M.V.G.; et al. Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar. J. 2013, 12, 460. [Google Scholar] [CrossRef]
- Pimenta, P.F.P.; Orfano, A.S.; Bahia, A.C.; Duarte, A.P.M.; Ríos-Velásquez, C.M.; Melo, F.F.; Pessoa, F.A.C.; Oliveira, G.A.; Campos, K.M.M.; Villegas, L.M. An overview of malaria transmission from the perspective of Amazon Anopheles vectors. Mem. Inst. Oswaldo Cruz. 2015, 110, 23–47. [Google Scholar] [CrossRef]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739. [Google Scholar] [CrossRef]
- Gonzales, M.F.; Brooks, T.; Pukatzki, S.U.; Provenzano, D. Rapid Protocol for Preparation of Electrocompetent Escherichia coli and Vibrio cholerae. J. Vis. Exp. 2013, 80, 50684. [Google Scholar] [CrossRef]
- Olah, Z.; Lehel, C.; Jakab, G.; Anderson, W.B. A cloning and ϵ-epitope-tagging insert for the expression of polymerase chain reaction-generated cDNA fragments in Escherichia coli and mammalian cells. Anal. Biochem. 1994, 221, 94–102. [Google Scholar] [CrossRef]
- Wright, C.A.; Beattie, G.A. Bacterial species specificity in proU osmoinducibility and nptII and lacZ expression. J. Mol. Microbiol. Biotechnol. 2004, 8, 201–208. [Google Scholar]
- Alav, I.; Kobylka, J.; Kuth, M.S.; Pos, K.M.; Picard, M.; Blair, J.M.; Bavro, V.N. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in gram-negative bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef] [PubMed]
- Ezemuoka, L.C.; Akorli, E.A.; Aboagye-Antwi, F.; Akorli, J. Mosquito midgut Enterobacter cloacae and Serratia marcescens affect the fitness of adult female Anopheles gambiae s.l. PLoS ONE 2020, 15, e0238931. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Vatandoost, H.; Karimian, F.; Choubdar, N.; Abai, M.R.; Oshaghi, M.A. Effect of Serratia AS1 (Enterobacteriaceae: Enterobacteriales) on the Fitness of Culex pipiens (Diptera: Culicidae) for Paratransgenic and RNAi Approaches. J. Med. Entomol. 2018, 56, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dos-Santos, A.L.A.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.A.d.S.; Martins, E.S.d.R.; Katak, R.d.M.; Campos, K.M.M.; Rocha, E.M.; Roque, R.A.; Lalwani, P.J.; Silva, L.A.; Carmo, E.J.d.; de Andrade, P.P.; et al. Compatibility of Serratia ureylitica Su_YN1, Malaria Transmission-Blocking Bacterium, with the Anopheles aquasalis Vector. Trop. Med. Infect. Dis. 2025, 10, 249. https://doi.org/10.3390/tropicalmed10090249
Ferreira MAdS, Martins ESdR, Katak RdM, Campos KMM, Rocha EM, Roque RA, Lalwani PJ, Silva LA, Carmo EJd, de Andrade PP, et al. Compatibility of Serratia ureylitica Su_YN1, Malaria Transmission-Blocking Bacterium, with the Anopheles aquasalis Vector. Tropical Medicine and Infectious Disease. 2025; 10(9):249. https://doi.org/10.3390/tropicalmed10090249
Chicago/Turabian StyleFerreira, Marília Andreza da Silva, Elen Sabrina dos Reis Martins, Ricardo de Melo Katak, Keillen Monick Martins Campos, Elerson Matos Rocha, Rosemary Aparecida Roque, Pritesh Jaychand Lalwani, Luciete Almeida Silva, Edson Júnior do Carmo, Paulo Paes de Andrade, and et al. 2025. "Compatibility of Serratia ureylitica Su_YN1, Malaria Transmission-Blocking Bacterium, with the Anopheles aquasalis Vector" Tropical Medicine and Infectious Disease 10, no. 9: 249. https://doi.org/10.3390/tropicalmed10090249
APA StyleFerreira, M. A. d. S., Martins, E. S. d. R., Katak, R. d. M., Campos, K. M. M., Rocha, E. M., Roque, R. A., Lalwani, P. J., Silva, L. A., Carmo, E. J. d., de Andrade, P. P., Wang, S., Moreira, L. A., Jacobs-Lorena, M., & Ríos-Velásquez, C. M. (2025). Compatibility of Serratia ureylitica Su_YN1, Malaria Transmission-Blocking Bacterium, with the Anopheles aquasalis Vector. Tropical Medicine and Infectious Disease, 10(9), 249. https://doi.org/10.3390/tropicalmed10090249






