1. Introduction
Tick-borne diseases (TBDs) are a significant public health concern globally, but also in the United States (US) [
1]. Among these, Lyme disease, caused by
Borrelia burgdorferi and transmitted primarily by
Ixodes scapularis ticks in the northeastern and upper Midwest U.S., is the most prevalent [
2]. However, there are many TBDs in the United States that contribute to morbidity and mortality [
3,
4]. The true global burden of TBDs is likely underestimated. There is concern about underreporting, misdiagnosis, and limited diagnostic sensitivity. Studies suggest that Lyme disease cases may be underestimated by as much 12–15-fold [
5].
Climate change is also expected to have a significant impact on the prevalence and geographic distribution of TBDs [
6]. Increasing temperatures, milder winters, and changes in precipitation have all expanded regions of the United States that can be inhabited by tick vectors and their animal hosts. This expansion is currently seen in the Northeast and Upper Midwest, where reforestation and suburban changes to wooded areas have increased human exposure to tick habitats [
7,
8]. For example, the white-tailed deer is a host for the
Ixodes scapularis and has seen a drastic rise in population following centuries of deforestation, adding to the increased risk of tick population expansion [
9].
Historically, estimates of TBD burden have relied on reporting to public health entities. This method does not provide much understanding of the impact that these conditions have on the healthcare system and does not discriminate between mild and severe TBD cases. Hospitalization data offers the ability to quantify the burden of severe cases and delineate geographic and seasonal trends related to cases that are severe enough to require an inpatient stay [
10]. Moreover, outpatient reporting is dependent on socioeconomic differences in access to care; therefore, severe cases requiring admission likely bypass this constraint. This highlights the need for robust data sources, like inpatient records, to better understand the impact of TBDs on healthcare systems and public health initiatives.
Therefore, this study utilizes a nationally represented inpatient database to characterize trends in hospitalizations for TBDs in the United States. This database identifies diagnoses using ICD codes, which are standardized alphanumeric codes used to classify and record diagnoses during healthcare encounters. The insights from this study are crucial for informing public health surveillance initiatives, bringing awareness in the clinical environment, and providing strategies to mitigate the impact of these diseases.
2. Methods
This research utilized a retrospective observational study design analyzing data from the Healthcare Utilization Project (HCUP) nationwide inpatient sample (NIS) database, covering hospitalizations across various regions in the United States between 2002 and 2021. The NIS database is a publicly available de-identified database and contains a sample of 20% of all inpatient admissions in the United States. The data is systematically collected at the time of discharge to allow clustering and weighting in order to extrapolate results to represent 100% of the hospital admissions during the calendar year and the total of all hospital admissions in the United States can be reliably reported. The database contains data from approximately 7 million hospital admissions per year, representing approximately 35 million weighted admissions each year, or more than 700 million admissions during the study time period.
Data variables include the total number of admissions each year, demographics such as age, sex, race/ethnicity, payor, hospital type (academic vs. non-academic), and setting (rural vs. urban). Average household income within the zip code of the patient’s residence is used as a surrogate for the patients’ actual household income status. In-hospital mortality data is available; however, data on the rates of mortality after discharge is not available. Length of stay and total hospital charges are included. All billing and procedure codes for each hospitalization (up to 100 codes) are recorded. Codes are prioritized by the attending physicians as well as hospital coders, ranked based on their importance and relevance to the hospital admission.
Data was analyzed by the Western Michigan University Homer Stryker, MD School of Medicine biostatistics department, which has extensive experience working with this database. The data was managed securely in accordance with the WMed institutional policies, HIPAA regulations, and HCUP requirements. The data was rendered anonymous before analysis, with only the necessary information being retained for research purposes. All data was stored in encrypted databases, with access restricted to authorized research personnel. Data analysis included descriptive statistics to examine hospitalization trends, with regression models employed to assess socio-demographic and geographic factors associated with severe outcomes. Per NIS requirements, cells containing fewer than ten patients cannot be reported to the researchers or published due to the potential risk of re-identifying patients. These cells are noted with an *. Cells with zero cases can be reported.
Inclusion criteria (
Table 1) were any admissions that had a diagnostic code for at least one of the tick-borne diseases (Lyme disease, babesiosis, anaplasmosis, ehrlichiosis, rickettsial diseases and tularemia and other TBDs) in one of the first two diagnoses at the time of discharge. ICD9 and ICD10 diagnostic codes for tick-borne diseases were categorized and listed. Data from the year 2015 were excluded due to changes in coding from ICD9 to ICD10 in October of that year, as well as incomplete data. Anaplasmosis was previously known as granulocytic ehrlichiosis and coded as ehrlichiosis in ICD9 prior to 2015. The addition of anaplasmosis as a diagnostic code in ICD10 required combining anaplasmosis with ehrlichiosis into one category since both were likely coded as ehrlichiosis prior to 2015. Additionally, changes in codes in the less-common TBDs along with the lack of specificity of some codes in ICD9 prevented the detailed analysis of those diseases. As a result, a single category of “other TBDs” was created.
Descriptive analysis was used to assess the frequencies of total and individual tick-borne diseases. The frequencies and proportions (95% confidence intervals [CI]) are reported for the study period. Categorical patient characteristics were reported as frequencies and percentages, while continuous patient variables are reported as medians and interquartile ranges. Data on four geographic regions—as defined by the HCUP protocol (Northeast, Midwest, South and West)—of the country was provided. More-detailed individual state-level data is not available.
Trends over time were plotted. The lengths of each season of admissions (recorded in months) for tick-borne diseases were analyzed using trends in standard deviations for each year, with more than 1000 hospital admissions identified for TBDs. A non-Poisson linear regression line was used to identify the best fit trend line. Weighted frequencies were reported, and all analyses were completed using weighted estimates in accordance with the NIS sampling methodology. SAS Studio software version 9.4 was used for the analysis.
3. Results
There was a total of 261,630 weighted hospital admissions for TBDs during the study period (
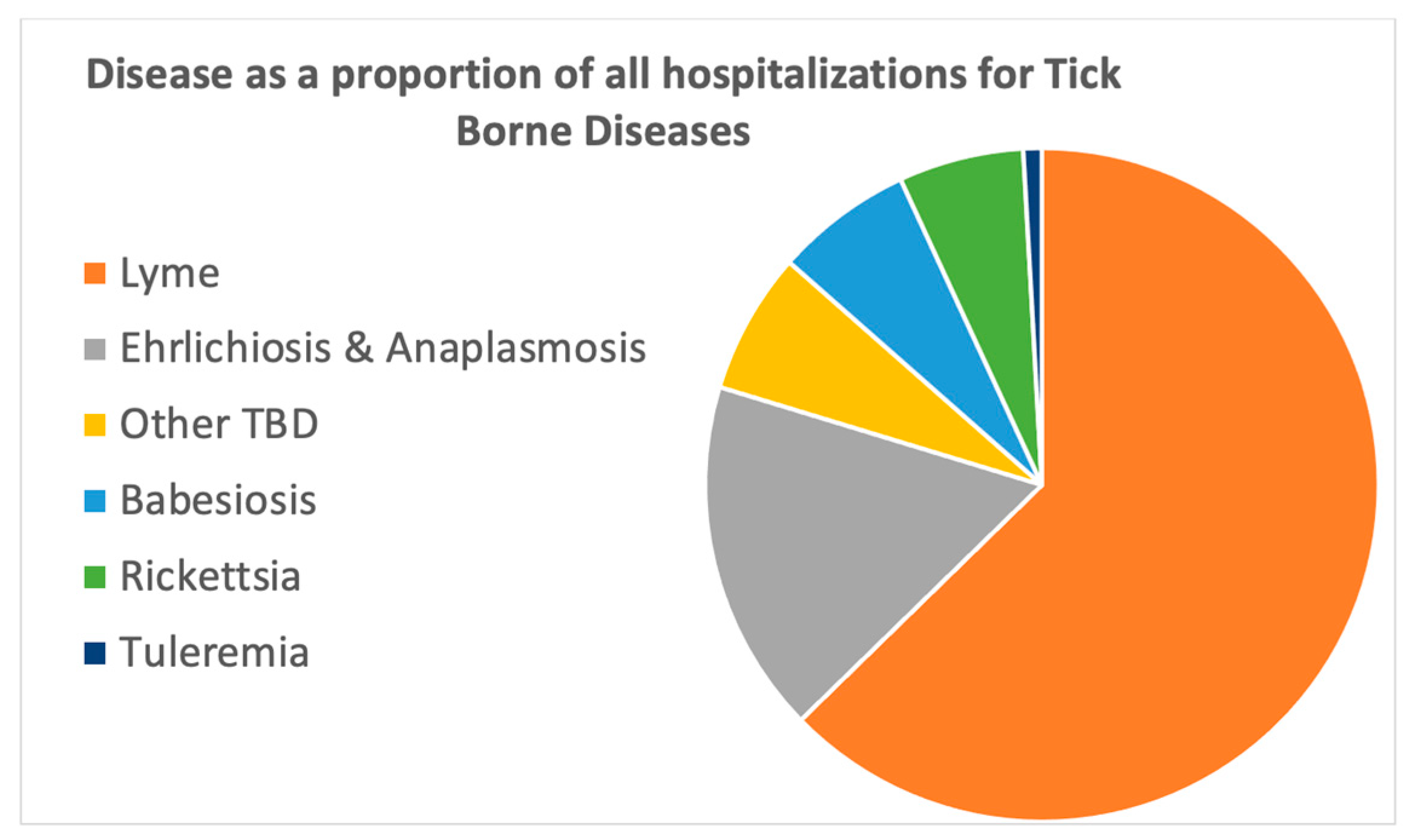
Table 2). Lyme disease made up almost 2/3 (65%) of this total, with 171,328 hospital admissions (
Table 3). Ehrlichiosis/Anaplasmosis was next most common, followed by babesiosis, rickettsia and tularemia (
Figure 1). The “other TBD” group had 19,435 admissions and was the third-largest group; however, the individual diseases in this group had fewer admissions, and there was evidence that many of the diseases in this group were miscoded as other diseases within this same group.
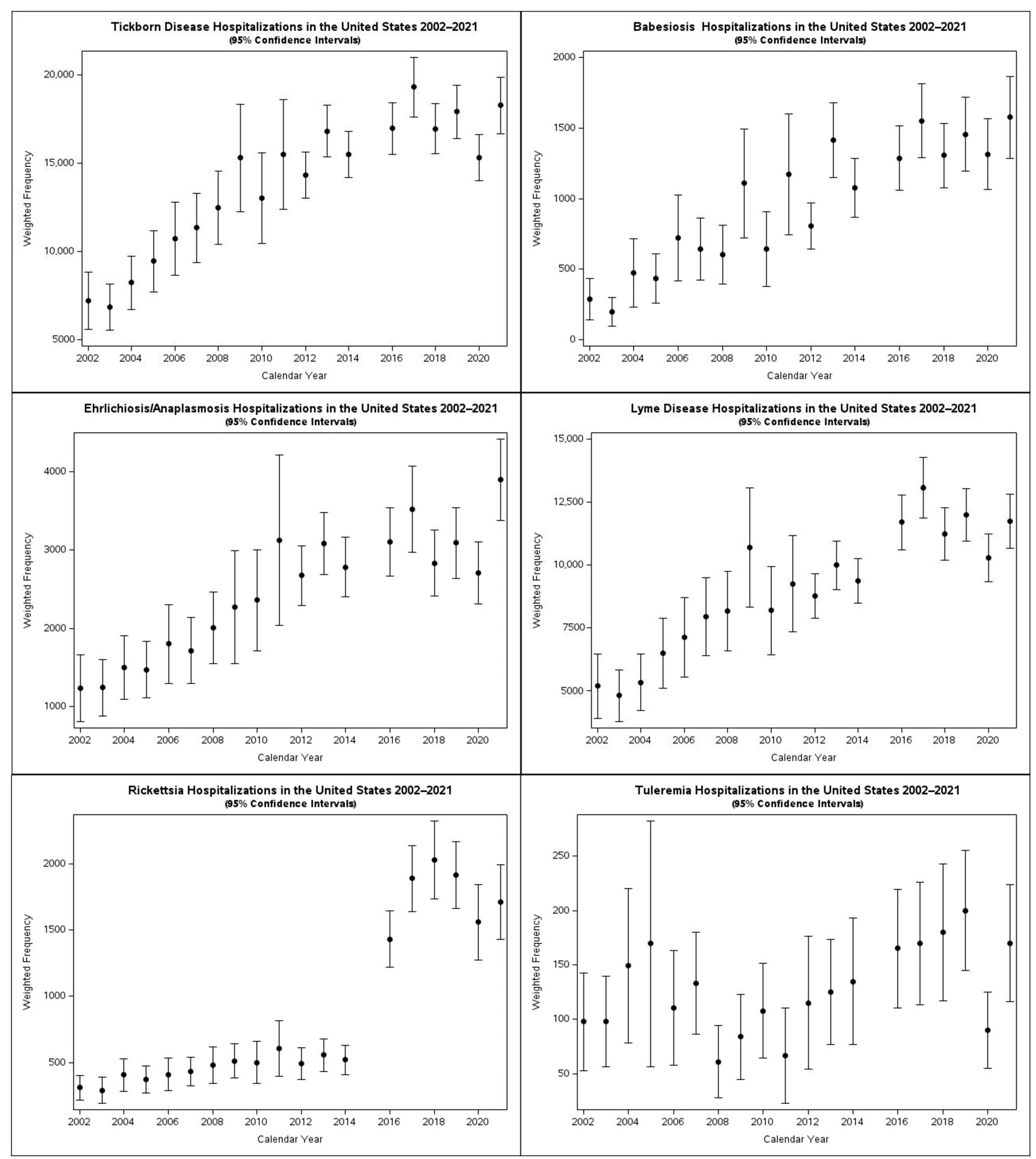
Frequency over time: During the study period, the total number of TBDs increased when pooled, as well as for each of the individual tick-borne diseases, although the confidence intervals for trends for tularemia are too wide to demonstrate a trend (
Figure 2). The total number of admissions for TBDs increased in frequency by a factor of 2.5 over the 20-year study period. The trends for Lyme, babesiosis, and ehrlichiosis/anaplasmosis have been relatively stable over the last five years of the study period.
Gender and TBD—Males were affected more often than females—53.9% vs. 46.1%. (
Table 2) These differences varied across all tick-borne diseases. Lyme disease had a slight female predominance of less than 1% difference, while hospital admissions for the others ranged from 59.9% to 66.3% male.
Race: Caucasians are over-represented in recorded numbers of hospitalizations overall (87%). It is important to note that the US Census number and NIS number for the Caucasian population is around 65%. Black and Hispanic patients showed a reciprocal under-representation for all TBDs, except for rickettsia. This disease showed an increase in cases in Hispanic patients (19.5%), which is consistent with the US census population data, but higher than the overall NIS racial distribution (11.5%).
Income: There were significant differences in frequencies of TBD hospitalizations among income levels when examined by home zip codes. Overall, patients from wealthier zip codes were more likely to be hospitalized with TBDs, with the highest-quartile income level having more than twice the number of TBDs compared with the lowest-quartile income level. This was not a consistent finding for all TBDs (
Table 4). The impact of income among all TBDs pooled together was strongly influenced by an even greater difference with Lyme disease, the most frequent TBD admitted to the hospital, where the highest income quartile had nearly four times the frequency of Lyme disease hospitalization compared with the lowest income quartile. Babesiosis, while less common than Lyme disease, had an even greater difference, with the highest income quartile having ten times the frequency of the lowest income quartile; moreover, ehrlichiosis and anaplasmosis were twice as common in the highest vs. lowest income quartiles. Interestingly, for tularemia, rickettsial diseases, and other TBD hospitalizations, the opposite pattern was true, showing a negative association with income. Hospitalizations due to these diseases were two to three times more common in the lowest income quartile compared to the highest. For these diseases, the trends were linear across income groups.
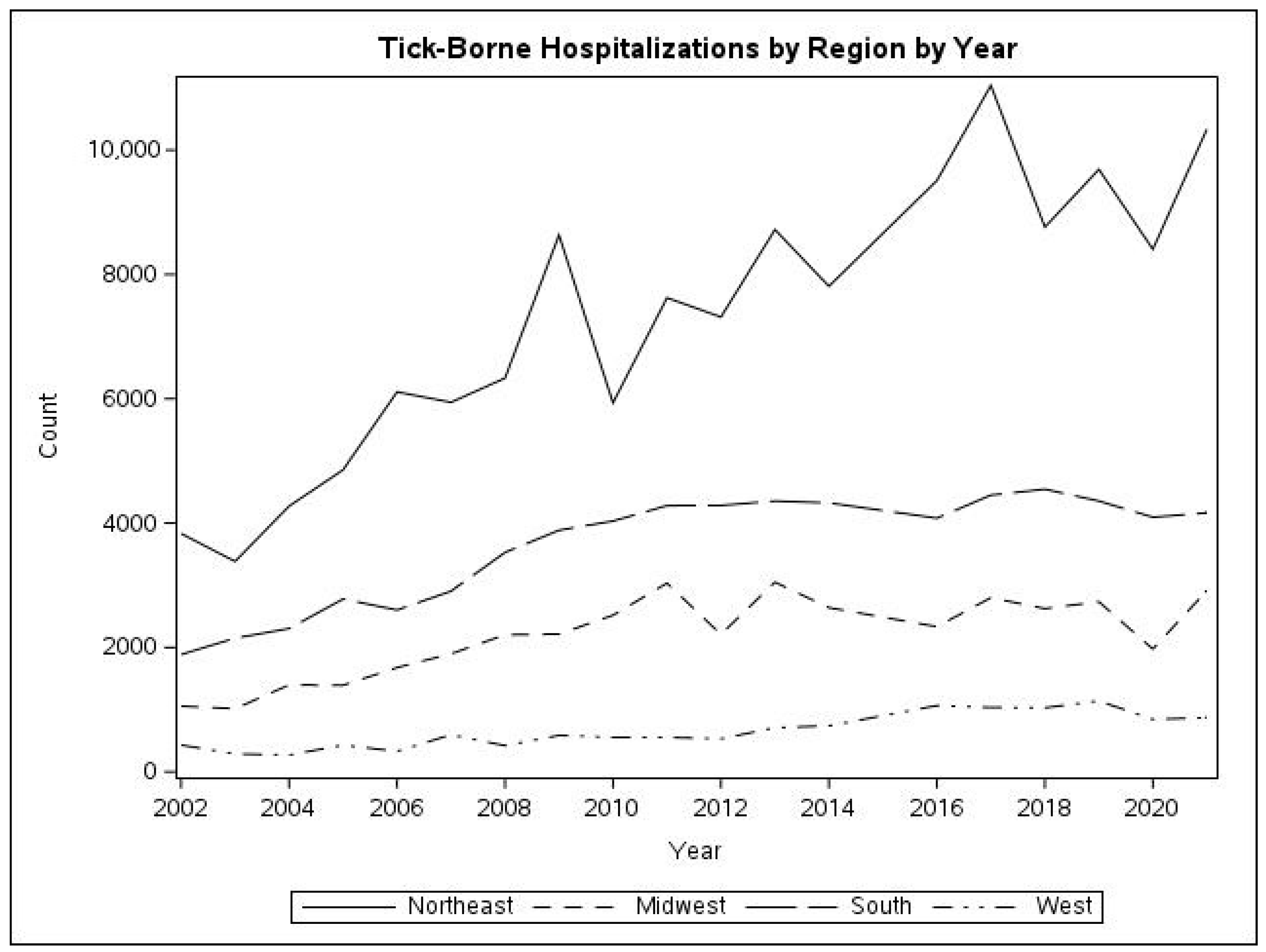
Geographic distribution: Analysis of the geographic distribution of TBD admissions shows that more than half of all hospitalizations for TBDs occur in the Northeast. This was also strongly influenced by Lyme disease and babesiosis, with 60.1% and 86.9%, respectively, of hospitalizations for these diseases occurring in the Northeast. Ehrlichiosis and anaplasmosis were also more common in this area, while tularemia, rickettsia, and other TBDs were most common in the South.
Figure 3 shows the temporal changes in frequency of all TBDs. There are similar increases in TBDs in all regions relative to their 2002 frequency.
Taken together, the income and geographic data suggests that in the Northeast, TBDs were positively correlated with income, while in the South, TBDs were negatively correlated with income. The data on income in each of the regions was not directly analyzed in this study.
Setting: Nearly 5 times as many hospitalizations for TBDs occurred in urban areas compared to rural areas. This pattern was true for all TBDs. The database does not include the term ‘suburban’. ‘Suburban’ is included in the urban category.
The financial burden of hospitalizations for TBDs: The financial burden of TBD hospitalization was analyzed. The median charges per hospitalization involving tick-borne diseases rose from USD 9433 to USD 35,161 (
Table 5). The total charges for all admissions involving TBDs rose from USD 114 million in 2002 to USD 1.27 billion in 2021.
Co-infections: Analysis of the data on co-infections showed that concurrent infections with two or more TBD organisms are relatively common during hospitalizations with any TBD.
Table 6 and
Table 7. Rates varied depending on the disease considered. Overall, 5.5% of patients with Lyme disease had a co-infection with another TBD. Babesiosis had the highest co-infection rate—35.8% of patients admitted with babesiosis had at least one other TBD. Anaplasmosis/ehrlichiosis had a co-infection rate of 15.6%. Although co-infections with both anaplasmosis and ehrlichiosis are likely, due to coding issues in ICD9, we were unable to identify the co-infections between these two organisms. The frequency of co-infections increased throughout the study period.
Mortality: The overall mortality for all hospitalizations with a TBD was low, at 1.1%. This was also relatively consistent across all types of TBD, with babesiosis having the highest rate at 2.06%. With babesiosis having the highest frequency of co-infection and the highest mortality, the possibility that co-infections had a higher rate of mortality was investigated. The odds ratio for mortality in patients with two or more concurrent TBD infections relative to those with a single infection was not significant [1.18 (95% CI 0.76, 1.83)]. Males had a slightly higher mortality rate compared with females [1.20 (95% CI 1.01, 1.42)]. There were no significant differences in mortality between household income quartiles.
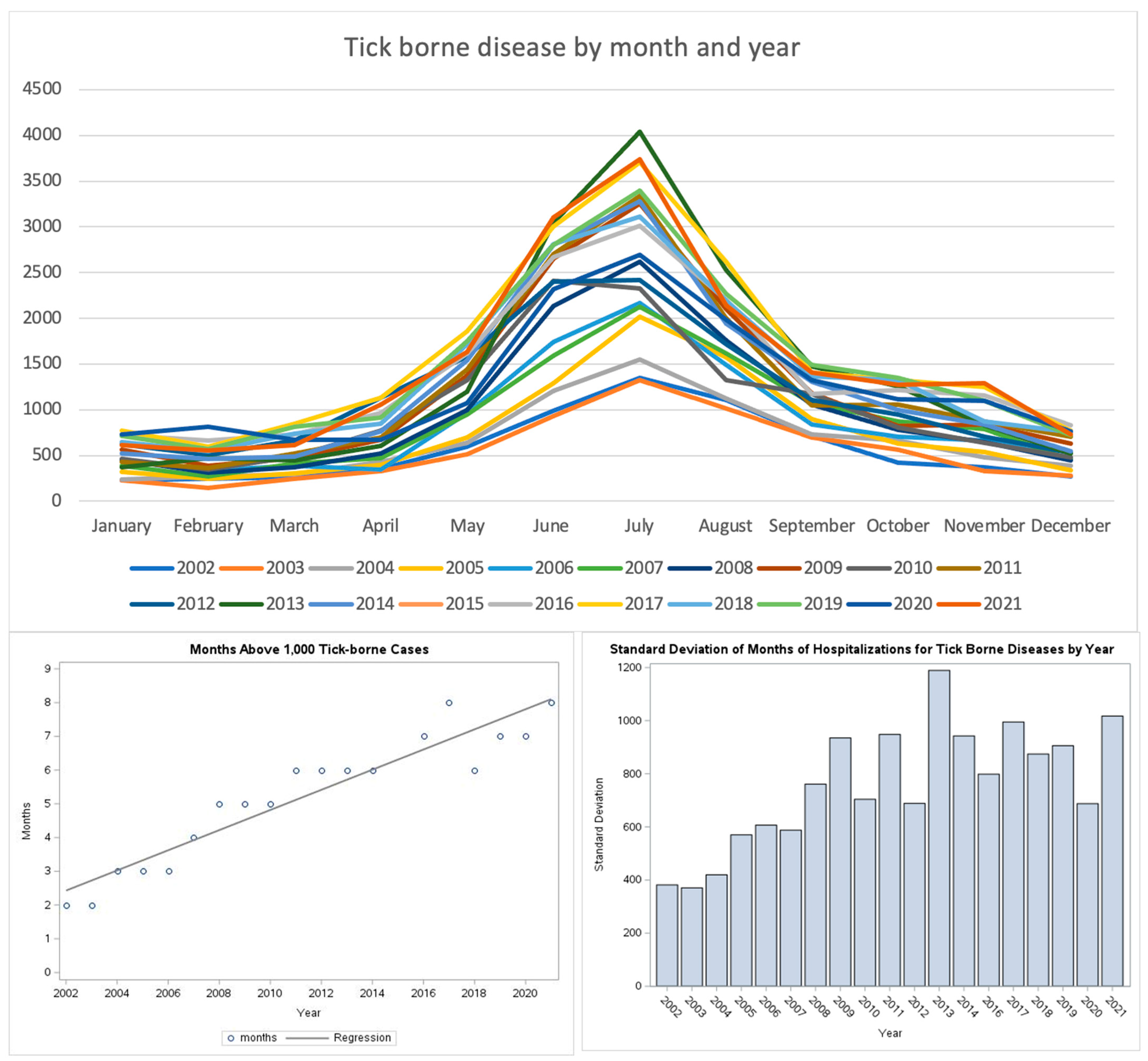
Seasonality: Analysis of seasonality and length showed that hospitalizations for TBDs occurred year-round since the beginning of the study period; there were consistent peaks for all TBDs in the summer, with July having the highest frequency for almost every year (
Figure 4). The lengthening of the season was addressed in two ways. The standard deviation of the peak frequency in number of months for each year was plotted, showing a general upward trend. In a second analysis, the number of months in which there were at least 1000 hospital admissions were plotted. A line of best fit was plotted, showing a significant rise in the number of months in which 1000 hospitalizations for TBDs occurred. In 2000, approximately 2.5 months of the year with at least 1000 hospitalizations to almost 8 months in the year in 2021.
4. Discussion
The findings of our study highlight the overall increase in hospitalizations related to TBDs in the United States over the study period from 2002 to 2021. Lyme disease accounted for most hospitalizations. However, interesting findings were noted for the other TBDs, including ehrlichiosis, anaplasmosis, babesiosis, and Rocky Mountain Spotted Fever (RMSF), with each demonstrating regional clustering, changes during the seasons, and various spikes overtime. Similar patterns of spread for TBDs have been found in other studies [
11,
12].
Geographic analysis showed that the burden of the disease increases was shown in the Northeast and Upper Midwest regions. The
Ixodes scapularis tick is endemic to these regions, and with the changes in land use and climate over the study period, there is reasonable suspicion to believe that those factors have contributed to the amplification of tick activity and populations. The Pacific region did not show drastic changes, and any increase could be due to variations in endemic species of vectors and other host ecology. It is important to note that the resurgence of the white-tailed deer population, specifically in the Northeast and Upper Midwest, may have played a role in contributing to the expanding tick populations and, therefore, the increased hospitalization burden of TBDs in these regions [
9,
13].
The highest numbers of TBD hospitalizations occur in June and July. TBD hospitalizations are increasing year-round, with the fastest rate of increase in the summer months. Our study cannot attribute a cause to this, but multiple factors may play a role. These include demographic changes such as population increase. Other factors may include expanding endemic range and the increased population of host species. The present study shows a strong association between the increasing length of the season of hospitalizations for tick-borne diseases and time over the past 20 years. The changes in climate factors are known to not only favor expansion of geographic range, population, and season of the tick vectors, but also increase the factors impacting tick activity, including increased feeding and the length of time of attachment. Richard Ostfeld and Jesse Brunner also suggested that increased tick activity is due to climate change through their mechanistic and phenomenological models, suggesting the need for more studies on the influence of climate in tick-borne pathogen dynamics [
14].
Demographic analysis showed that most hospital cases were among older adults, with the majority of patients being white, male, and privately insured individuals. This could simply be a representation of overall population demographics but raises the question of the effect of healthcare access on hospitalization for tick-borne disease. It remains unclear whether these groups are more likely to receive inpatient care when infected.
There is an overwhelmingly Caucasian predominance among TBD hospitalizations at 88% of hospitalizations, followed distantly by Hispanic and Black patients at about 4% each. All TBDs have a strong Caucasian predominance—between 88 and 91%—for Rickettsial hospitalizations, which are 66% Caucasian. About 20% of rickettsial cases are seen in Hispanic populations in our data (
Table 3). NIS race determination is based on self-identification and uses only White, Black, Hispanic, Asian/Pacific Islander, Native American, and Other. This is different from the US census bureau, which is more complicated and detailed, where Hispanic falls into an ethnic category rather than a racial category. The census data includes people who identify as White alone, white Hispanic, and non-white Hispanic, making it difficult to compare. However, in the 2020 census, Hispanic or Latino populations represented 19.5% of the US population, identical to the rickettsial diseases group. Male patients, in general, are shown to present with TBDs more frequently than female patients. The higher incidence of TBDs in white Caucasians has been attributed to their higher indulgence of outdoor activities like hiking, camping, gardening, etc., which could also explain their higher incidence in males [
15]. Studies of rickettsial diseases like Rocky Mountain Spotted Fever have revealed a higher incidence among White males; however, a high incidence of these diseases has not been seen among Hispanic populations as in our data. Rather, many studies have shown that the rate of rickettsial infections is increasing in Native Americans [
16,
17]. Despite TBDs being more commonly seen in Caucasians, there are worse outcomes in Black patients and other minorities due to difficulty in seeing rashes like erythema migrans or failure to seek early medical care [
18].
Our study also highlights the growing burden of hospitalization for TBDs in the United States. The median cost per patient has increased from USD 9333 in 2002 to USD 35,161 in 2021, amounting to a weighted total cost of more than USD 1 billion. This highlights the growing need for prevention as well as early diagnosis and treatment of TBDs.
Routine testing for coinfection in patients with Lyme disease is not currently recommended by US guidelines [
19]. However, coinfection testing is currently recommended when a patient with Lyme disease has uncharacteristic symptoms indicating risk of coinfection or persistent fever after starting the appropriate antibiotics. This study revealed a high proportion of coinfection between Lyme, babesiosis, and ehrlichiosis/anaplasmosis among patients hospitalized with TBDs. Notably, babesiosis requires a different treatment regimen than Lyme or ehrlichiosis/anaplasmosis. These results suggest that further study into the utility of routine coinfection testing for hospitalized patients with one of these TBDs should be carried out.
Importantly, the study addresses a gap in the literature. Passive surveillance systems such as the CDC’s National Notifiable Diseases Surveillance System are known to underestimate case counts [
5]. Our use of the inpatient data offers an additional perspective that helps further characterize the burden of TBDs, especially for severe cases where hospitalization is required.
The limitations of this study are related to the nature of the HCUP database. The database only includes inpatient data, and many TBDs are treated in an outpatient setting. Disease identification relies on ICD codes. These codes can be entered inaccurately by treating physicians and hospital coders. The study time period includes the transition between ICD9 and ICD10, and it is challenging to match diagnoses across these coding systems. For example, there is not a code for anaplasmosis in ICD9, while there is one in ICD10. The change to ICD10 occurred in 2015, which caused the data from that year to be incomplete and unusable in our analysis. Each hospitalization is counted as a separate entry in the HCUP database; thus, the same patient could be coded twice if admitted more than once for the same condition.
Ultimately, the findings of this study show increasing morbidity associated with TBDs, necessitating ongoing research into prevention, accurate diagnosis, and therapeutics. Furthermore, public health messaging should evolve, given regional and seasonal changes in TBD hospitalizations. Clinician awareness of these findings is key to maintaining vigilance for these increasingly common conditions.
5. Conclusions and Future Directions
This study highlights the rising burden of TBD hospitalizations across the United States. As ecosystems around the country adapt to climate changes every year, the public health sector needs to be aware of these changes. Lyme disease is still a predominant TBD that requires hospitalization, but it is not the only TBD. Hospitalizations from other TBDs like babesiosis, ehrlichiosis, and anaplasmosis warrant attention. Coinfection is also common among TBD hospitalizations. Seasonal and regional distribution of all TBDs in the United States is expanding.
This lays the foundation for future research into TBD prevention, diagnosis, and treatment. Changing geographic and seasonal patterns necessitate adjustments to surveillance and prevention strategies. This study helps characterize the inpatient burden of TBDs; however, similar reviews of the burden in an outpatient setting are warranted. Racial disparities in TBD hospitalization require further characterization with an emphasis on understanding whether there are social determinants of health preventing minority patients from seeking medical care for TBDs. The high incidence of coinfection suggests investigation into routine coinfection testing or cascading testing algorithms.
Author Contributions
Conceptualization, T.A.M.; methodology, S.N., K.H., M.S., E.E. and T.A.M.; software, K.H.; validation, K.H.; formal analysis, K.H.; investigation, S.N., K.H., M.S., E.E. and T.A.M.; resources, S.N., K.H., M.S., E.E. and T.A.M.; data curation, S.N., K.H., M.S., E.E. and T.A.M.; writing-original draft preparation, S.N.; writing-review and editing, S.N., K.H., M.S., E.E. and T.A.M.; visualization, K.H. and T.A.M.; supervision, T.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Research involving data pulled from the HCUP database do not need to be submitted to the WMed IRB because the research doesn’t meet the federal definition of human subjects’ research. Per the HCUP Database Website: “HCUP Databases Are Limited Data Sets. HCUP databases conform to the definition of a limited data set. A limited data set is healthcare data in which 16 direct identifiers, specified in the Privacy Rule, have been removed. Under HIPAA, review by an institutional review board (IRB) is not required for use of limited data sets”.
Informed Consent Statement
The NIS database is de- identified and available to the public. Thus, no informed consents were required or obtained.
Data Availability Statement
The data presented in this study are openly available in HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- CDC-Lyme Disease. Available online: https://www.cdc.gov/lyme/data-research/facts-stats/index.html (accessed on 7 May 2025).
- CDC-Ehrlichiosis. Available online: https://www.cdc.gov/ehrlichiosis/data-research/facts-stats/index.html (accessed on 7 May 2025).
- CDC-Rocky Mountain Spotted Fever. Available online: https://www.cdc.gov/rocky-mountain-spotted-fever/data-research/facts-stats/index.html (accessed on 7 May 2025).
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogden, N.H.; Lindsay, L.R. Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rochlin, I.; Kenney, J.; Little, E.; Molaei, G. Public health significance of the white-tailed deer (Odocoileus virginianus) and its role in the eco-epidemiology of tick- and mosquito-borne diseases in North America. Parasit. Vectors 2025, 18, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Gayle, A.; Ringdahl, E. Tick-borne diseases. Am. Fam. Physician 2001, 64, 461–466. [Google Scholar] [PubMed]
- Spach, D.H.; Liles, W.C.; Campbell, G.L.; Quick, R.E.; Anderson, D.E., Jr.; Fritsche, T.R. Tick-borne diseases in the United States. N. Engl. J. Med. 1993, 329, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Dobson, A.D.M.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.M.; Fish, D.; et al. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostfeld, R.S.; Brunner, J.L. Climate change and Ixodes tick-borne diseases of humans. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fix, A.D.; Peña, C.A.; Strickland, G.T. Racial differences in reported Lyme disease incidence. Am. J. Epidemiol. 2000, 152, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Borski, J.; Wang, H.H.; Donaldson, T.G.; Michalk, A.; Montgomery, A.; Heldman, S.; Mogg, M.; Derouen, Z.; Grant, W.E.; et al. Increasing Incidence of Spotted Fever Group Rickettsioses in the United States, 2010–2018. Vector Borne Zoonotic Dis. 2022, 22, 491–497, Erratum in: Vector Borne Zoonotic Dis. 2024, 24, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, J.J.; Swerdlow, D.L.; Krebs, J.W.; Holman, R.C.; Mandel, E.; Harvey, A.; Haberling, D.; Massung, R.F.; McQuiston, J.H. Rocky mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am. J. Trop. Med. Hyg. 2010, 83, 174–182, Erratum in: Am. J. Trop. Med. Hyg. 2010, 83, 729–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gould, L.H.; Fathalla, A.; Moïsi, J.C.; Stark, J.H. Racial and ethnic disparities in Lyme disease in the United States. Zoonoses Public Health 2024, 71, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. 2020, 72, e1–e48. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).