Long-Term Durability and Public Health Impact of City-Wide wMel Wolbachia Mosquito Releases in Niterói, Brazil, During a Dengue Epidemic Surge
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mosquito Production
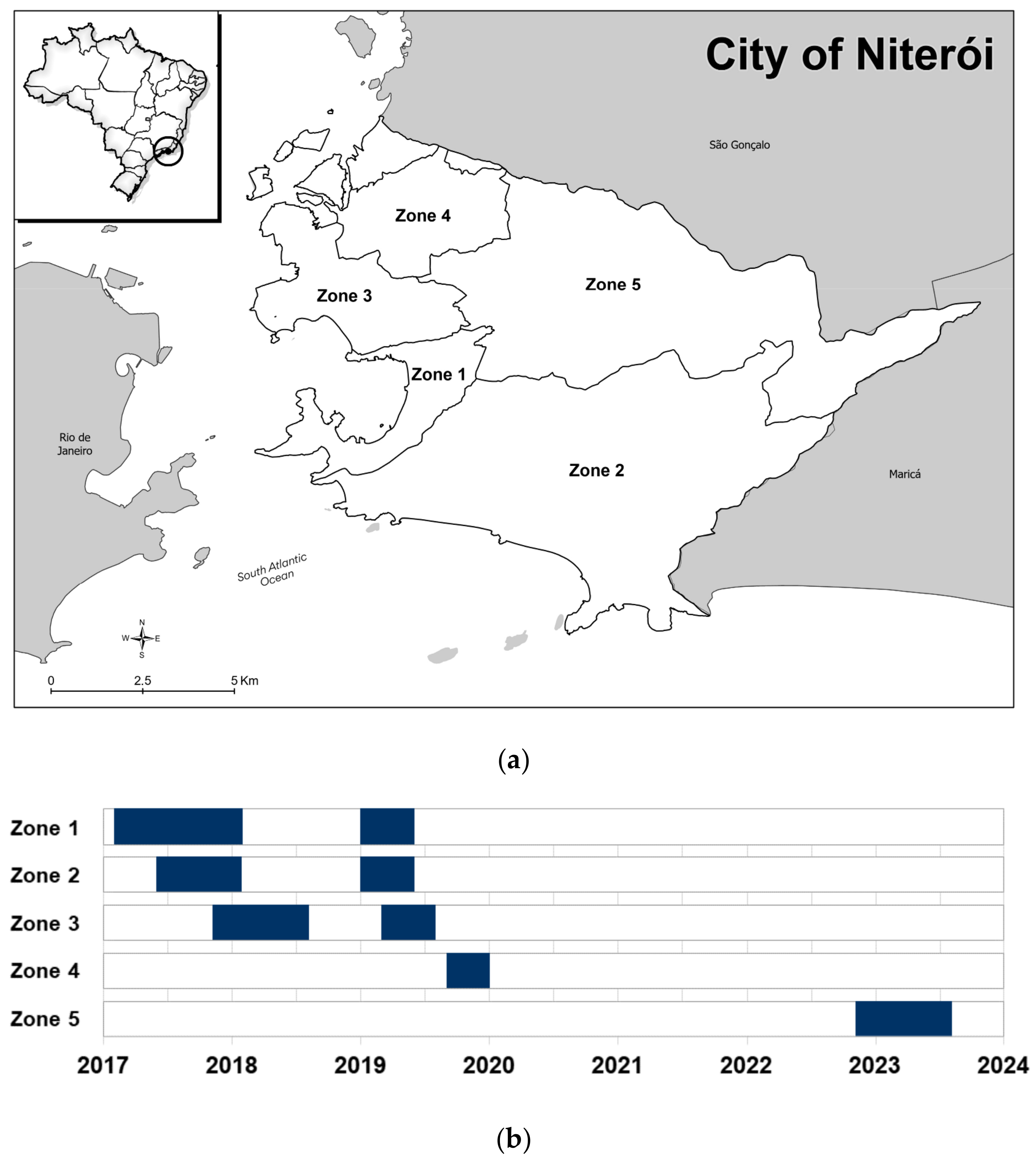
2.3. wMel Deployment in Niterói
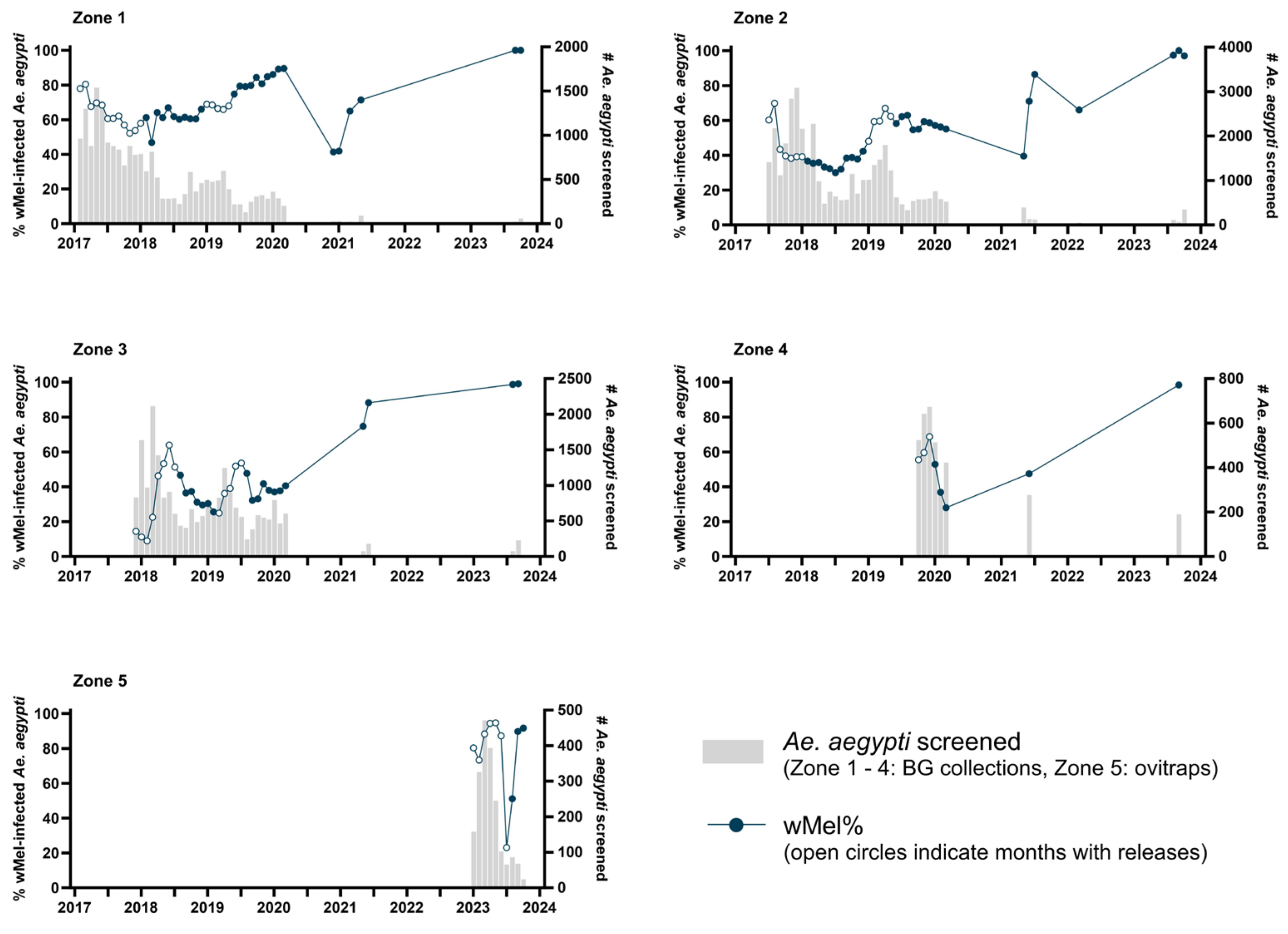
2.4. wMel Monitoring
2.5. Dengue Case Notifications and Population Data
2.6. Statistical Methods
3. Results
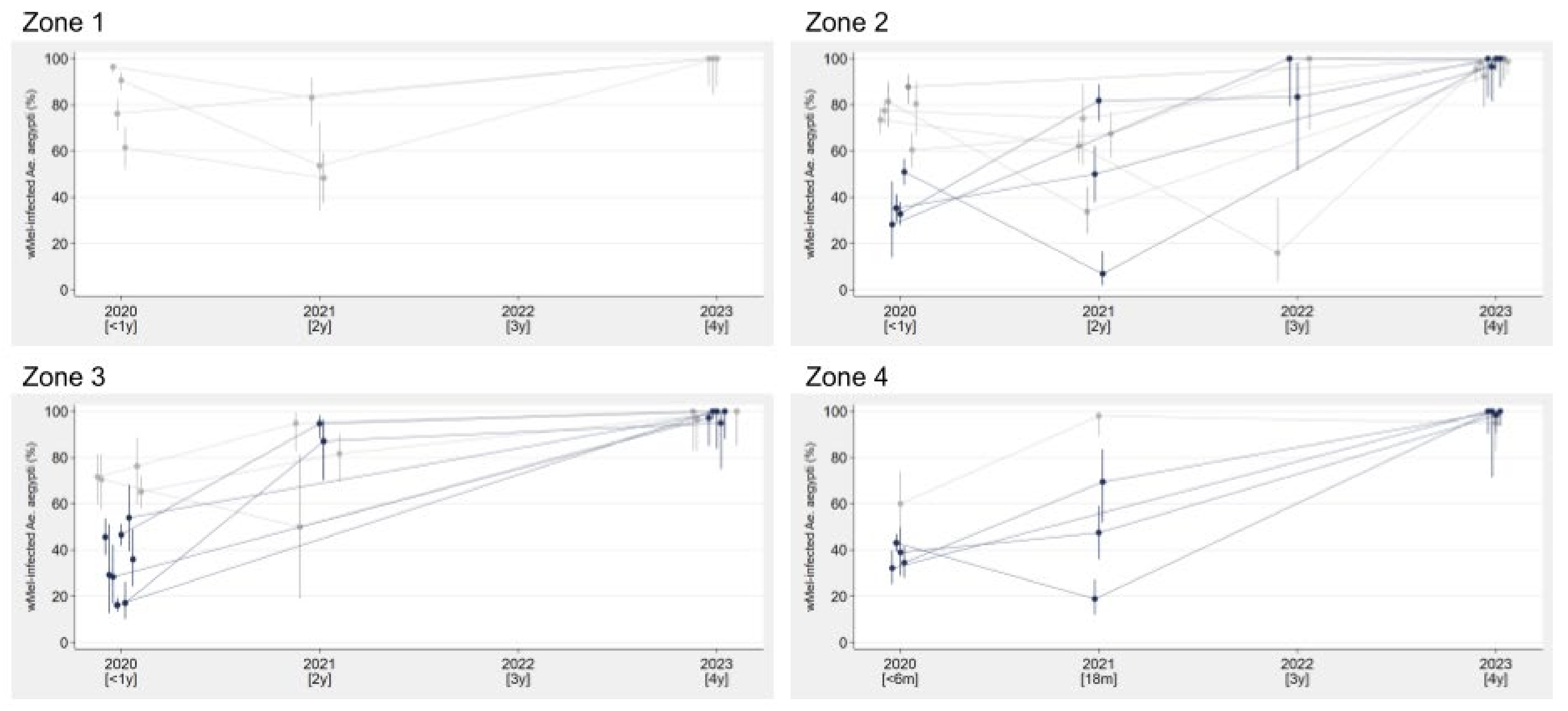
3.1. City-Wide Coverage and Long-Term Stability of Wolbachia in the Niterói Ae. aegypti Mosquito Population
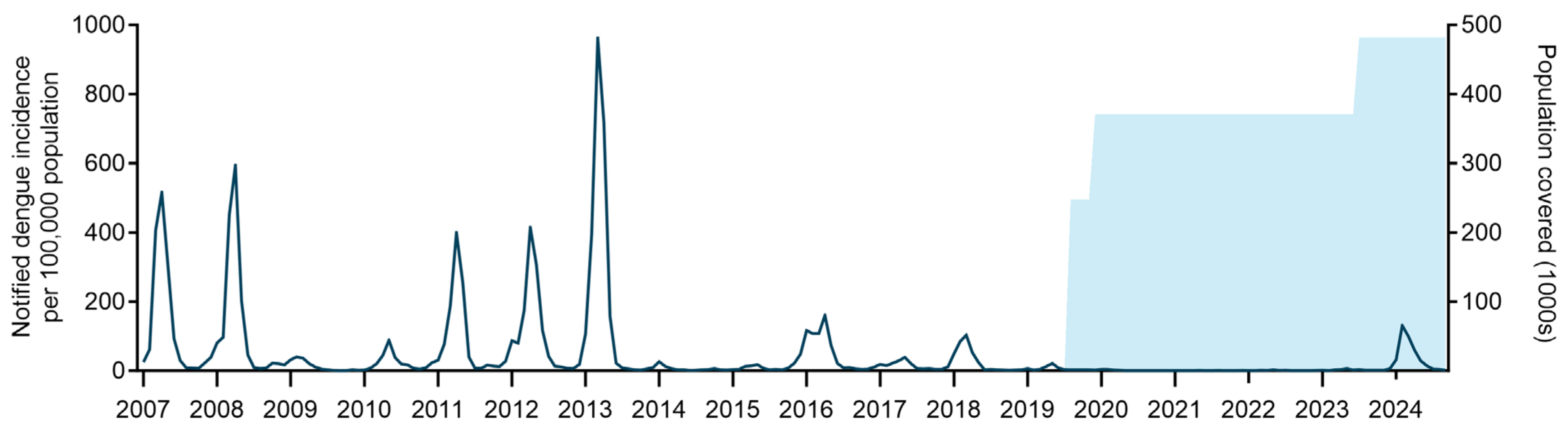
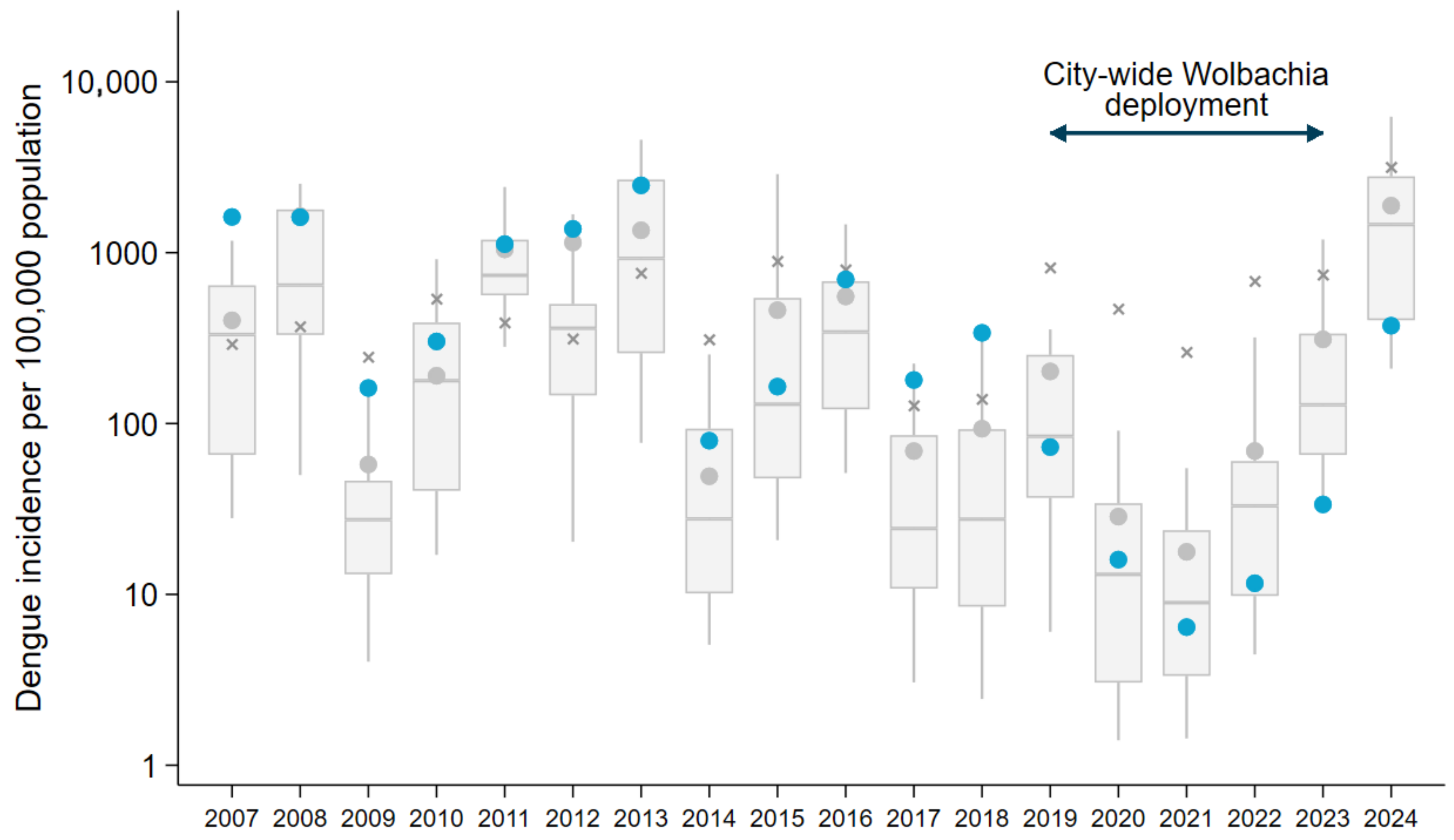
3.2. Sustained Suppression of Dengue Transmission in Niterói
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SINAN | Sistema de Informação de Agravos de Notificação |
| PCR | Polymerase chain reaction |
| DENV | Dengue virus |
| ITS | Interrupted time series |
References
- World Health Organization. WHO Global Dengue Dashboard. Available online: https://worldhealthorg.shinyapps.io/dengue_global/ (accessed on 17 February 2025).
- Ministry of Health Brazil. Arbovirus Monitoring Panel. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses (accessed on 17 February 2025).
- Dengue, Zika, and Chikungunya Expected to Deduct $ 3 Billion from Brazil’s Economy in 2024. Folha de SPaulo. 2024. Available online: https://www1.folha.uol.com.br/internacional/en/scienceandhealth/2024/03/dengue-zika-and-chikungunya-expected-to-deduct-3-billion-from-brazils-economy-in-2024.shtml (accessed on 17 February 2025).
- Pan American Health Organization. Integrated Management Strategy for Dengue Prevention and Control in the Region of the Americas. 2020. Available online: https://iris.paho.org/handle/10665.2/34860 (accessed on 17 February 2025).
- Triunfol, M. Brazil’s dengue vaccine campaign falters. Lancet Infect. Dis. 2024, 24, e358. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbeormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef]
- Rocha, M.N.; Duarte, M.M.; Mansur, S.B.; e Silva, B.D.M.; Pereira, T.N.; Adelino, T.É.R.; Giovanetti, M.; Alcantara, L.C.J.; Santos, F.M.; Costa, V.R.d.M.; et al. Pluripotency of Wolbachia against Arboviruses: The case of yellow fever. Gates Open Res. 2019, 3, 161. [Google Scholar] [CrossRef]
- Garcia, G.D.A.; Sylvestre, G.; Aguiar, R.; Da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-De-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Dos Santos, G.; Durovni, B.; Saraceni, V.; Souza Riback, T.I.; Pinto, S.B.; Anders, K.L.; Moreira, L.A.; Salje, H. Estimating the effect of the wMel release programme on the incidence of dengue and chikungunya in Rio de Janeiro, Brazil: A spatiotemporal modelling study. Lancet Infect. Dis. 2022, 22, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2019, 2, 36. [Google Scholar] [CrossRef]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2020, 3, 1547. [Google Scholar] [CrossRef]
- Indriani, C.; Tantowijoyo, W.; Rancès, E.; Andari, B.; Prabowo, E.; Yusdi, D.; Ansari, M.R.; Wardana, D.S.; Supriyati, E.; Nurhayati, I.; et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: A quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Indriani, C.; Tanamas, S.K.; Khasanah, U.; Ansari, M.R.; Rubangi; Tantowijoyo, W.; Ahmad, R.A.; Dufault, S.M.; Jewell, N.P.; Utarini, A.; et al. Impact of randomised w mel Wolbachia deployments on notified dengue cases and insecticide fogging for dengue control in Yogyakarta City. Glob. Health Action 2023, 16, 2166650. [Google Scholar] [CrossRef] [PubMed]
- Velez, I.D.; Tanamas, S.K.; Arbelaez, M.P.; Kutcher, S.C.; Duque, S.L.; Uribe, A.; Zuluaga, L.; Martínez, L.; Patiño, A.C.; Barajas, J.; et al. Reduced dengue incidence following city-wide wMel Wolbachia mosquito releases throughout three Colombian cities: Interrupted time series analysis and a prospective case-control study. PLoS Neglected Trop. Dis. 2023, 17, e0011713. [Google Scholar] [CrossRef]
- Zimmermann, I.R.; Fernandes, R.R.A.; da Costa, M.G.S.; Pinto, M.; Peixoto, H.M. Simulation-based economic evaluation of the Wolbachia method in Brazil: A cost-effective strategy for dengue control. Lancet Reg. Health Am. 2024, 35, 100783. [Google Scholar] [CrossRef]
- Brady, O.J.; Kharisma, D.D.; Wilastonegoro, N.N.; O’Reilly, K.M.; Hendrickx, E.; Bastos, L.S.; Yakob, L.; Shepard, D.S. The cost-effectiveness of controlling dengue in Indonesia using wMel Wolbachia released at scale: A modelling study. BMC Med. 2020, 18, 186. [Google Scholar] [CrossRef]
- Turner, H.C.; Le Quyen, D.; Dias, R.; Huong, P.T.; Simmons, C.P.; Anders, K.L.; Christofferson, R.C. An economic evaluation of Wolbachia deployments for dengue control in Vietnam. PLoS Neglected Trop. Dis. 2023, 17, e0011356. [Google Scholar] [CrossRef]
- Dainty, K.R.; Hawkey, J.; Judd, L.M.; Pacidônio, E.C.; Duyvestyn, J.M.; Gonçalves, D.S.; Lin, S.Y.; O’Donnell, T.B.; O’Neill, S.L.; Simmons, C.P.; et al. wMel Wolbachia genome remains stable after 7 years in Australian Aedes aegypti field populations. Microb. Genom. 2021, 7, e000641. [Google Scholar] [CrossRef]
- Ross, P.A.; Robinson, K.L.; Yang, Q.; Callahan, A.G.; Schmidt, T.L.; Axford, J.K.; Coquilleau, M.P.; Staunton, K.M.; Townsend, M.; Ritchie, S.A.; et al. A decade of stability for wMel Wolbachia in natural Aedes aegypti populations. PLoS Pathog. 2022, 18, e1010256. [Google Scholar] [CrossRef]
- Velez, I.D.; Uribe, A.; Barajas, J.; Uribe, S.; Ángel, S.; Suaza-Vasco, J.D.; Ahumada, J.S.D.; Torres, M.C.M.; Arbeláez, M.P.; Santacruz-Sanmartin, E.; et al. Large-scale releases and establishment of wMel Wolbachia in Aedes aegypti mosquitoes throughout the cities of Bello, Medellin and Itagui, Colombia. PLoS Neglected Trop. Dis. 2023, 17, e0011642. [Google Scholar] [CrossRef]
- Hien, N.T.; Anh, D.D.; Le, N.H.; Yen, N.T.; Phong, T.V.; Nam, V.S.; Duong, T.N.; Nguyen, N.B.; Huong, D.T.T.; Hung, L.Q.; et al. Environmental factors influence the local establishment of Wolbachia in Aedes aegypti mosquitoes in two small communities in central Vietnam. Gates Open Res. 2022, 5, 147. [Google Scholar] [CrossRef]
- Pavan, M.G.; Gnonhoue, F.J.; Corrêa-Antônio, J.; Padilha, K.P.; Garcia, G.A.; de Oliveira, F.; Brito, L.P.; Dias, L.; Martins, A.J.; Corbel, V.; et al. The long-term persistence of the wMel strain in Rio de Janeiro is threatened by poor integrated vector management and bacterium fitness cost on Aedes aegypti. PLoS Neglected Trop. Dis. 2025, 19, e0013372. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Brazil (Health Surveillance Secretariat). Guia de Vigilância em Saúde: Volume único. 2014. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_4ed.pdf (accessed on 20 February 2025).
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, C.P.; Dias, F.B.S.; Villela, D.M.A.; Maciel-de-Freitas, R. Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population. Insects 2020, 11, 92. [Google Scholar] [CrossRef]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White, V.L.; Endersby-Harshman, N.M.; Hoffmann, A.A.; McGraw, E.A. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog. 2017, 13, e1006006. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Ritchie, S.A.; Axford, J.K.; Hoffmann, A.A.; Rasgon, J.L. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Neglected Trop. Dis. 2019, 13, e0007357. [Google Scholar] [CrossRef]
- Ross, P.A.; Axford, J.K.; Yang, Q.; Staunton, K.M.; Ritchie, S.A.; Richardson, K.M.; Hoffmann, A.A.; Kohl, A. Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Neglected Trop. Dis. 2020, 14, e0007958. [Google Scholar]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, 361–366. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Kien, D.T.H.; Clapham, H.; Aguas, R.; Trung, V.T.; Chau, T.N.B.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015, 7, 279ra37. [Google Scholar] [CrossRef]
- Dorigatti, I.; McCormack, C.; Nedjati-Gilani, G.; Ferguson, N.M. Using Wolbachia for dengue control: Insights from modelling. Trends Parasitol. 2018, 34, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Cattarino, L.; Rodriguez-Barraquer, I.; Imai, N.; Cummings, D.A.T.; Ferguson, N.M. Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 2020, 12, eaax4144. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Difference in difference, controlled interrupted time series and synthetic controls. Int. J. Epidemiol. 2019, 48, 2062–2063. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.M.; Siqueira, J.B., Jr.; Parente, M.P.; Zara, A.L.; Oliveira, C.S.; Braga, C.; Pimenta, F.G., Jr.; Cortes, F.; Lopez, J.G.; Bahia, L.R.; et al. Economic Impact of Dengue: Multicenter Study across Four Brazilian Regions. PLoS Neglected Trop. Dis. 2015, 9, e0004042. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Junior, J.B.S.; Massad, E.; Lobao-Neto, A.; Kastner, R.; Oliver, L.; Gallagher, E. Epidemiology and costs of dengue in Brazil: A systematic literature review. Int. J. Infect. Dis. 2022, 122, 521–528. [Google Scholar] [CrossRef]
- Ministry of Health Brazil. Plano de Contingência Nacional para Dengue, Chikungunya e Zika. 2025. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/guias-e-manuais/2025/plano-de-contingencia-nacional-para-dengue-chikungunya-e-zika.pdf/view (accessed on 20 February 2025).
- Ministry of Health Brazil. National Guidelines for the Prevention and Control of Urban Arboviral Diseases: Entomological Surveillance and Control Strategies. 2025. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_nacionais_arboviroses_urbanas.pdf (accessed on 8 May 2025).
- Pan American Health Organisation. Evaluation of Innovative Strategies for Aedes Aegypti Control: Challenges for Their Introduction and Impact Assessment. 2019. Available online: https://iris.paho.org/bitstream/handle/10665.2/51375/9789275120965_eng.pdf (accessed on 23 July 2025).
- Collins, M.H.; Potter, G.E.; Hitchings, M.D.T.; Butler, E.; Wiles, M.; Kennedy, J.K.; Pinto, S.B.; Teixeira, A.B.M.; Casanovas-Massana, A.; Rouphael, N.G.; et al. EVITA Dengue: A cluster-randomized controlled trial to EValuate the efficacy of Wolbachia-InfecTed Aedes aegypti mosquitoes in reducing the incidence of Arboviral infection in Brazil. Trials 2022, 23, 185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, K.L.; Ribeiro, G.S.; Lopes, R.d.S.; Amadeu, P.; da Costa, T.R.; Riback, T.I.S.; Chalegre, K.D.d.M.; de Oliveira, W.P.; da Silva, C.C.; Blanco, M.V.F.M.; et al. Long-Term Durability and Public Health Impact of City-Wide wMel Wolbachia Mosquito Releases in Niterói, Brazil, During a Dengue Epidemic Surge. Trop. Med. Infect. Dis. 2025, 10, 237. https://doi.org/10.3390/tropicalmed10090237
Anders KL, Ribeiro GS, Lopes RdS, Amadeu P, da Costa TR, Riback TIS, Chalegre KDdM, de Oliveira WP, da Silva CC, Blanco MVFM, et al. Long-Term Durability and Public Health Impact of City-Wide wMel Wolbachia Mosquito Releases in Niterói, Brazil, During a Dengue Epidemic Surge. Tropical Medicine and Infectious Disease. 2025; 10(9):237. https://doi.org/10.3390/tropicalmed10090237
Chicago/Turabian StyleAnders, Katherine L., Gabriel Sylvestre Ribeiro, Renato da Silva Lopes, Pilar Amadeu, Thiago Rodrigues da Costa, Thais Irene Souza Riback, Karlos Diogo de Melo Chalegre, Wesley Pimentel de Oliveira, Cátia Cabral da Silva, Marcos Vinicius Ferreira Mendes Blanco, and et al. 2025. "Long-Term Durability and Public Health Impact of City-Wide wMel Wolbachia Mosquito Releases in Niterói, Brazil, During a Dengue Epidemic Surge" Tropical Medicine and Infectious Disease 10, no. 9: 237. https://doi.org/10.3390/tropicalmed10090237
APA StyleAnders, K. L., Ribeiro, G. S., Lopes, R. d. S., Amadeu, P., da Costa, T. R., Riback, T. I. S., Chalegre, K. D. d. M., de Oliveira, W. P., da Silva, C. C., Blanco, M. V. F. M., Eppinghaus, A. L. F., Boas, F. V., Frossard, T., Green, B. R., O’Neill, S. L., Ryan, P. A., Simmons, C. P., & Moreira, L. A. (2025). Long-Term Durability and Public Health Impact of City-Wide wMel Wolbachia Mosquito Releases in Niterói, Brazil, During a Dengue Epidemic Surge. Tropical Medicine and Infectious Disease, 10(9), 237. https://doi.org/10.3390/tropicalmed10090237





