Helminth/Protozoan Coinfections in Chronic Fascioliasis Cases in Human Hyperendemic Areas: High Risk of Multiparasitism Linked to Transmission Aspects and Immunological, Environmental and Social Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Study Population
2.4. Sampling Strategy
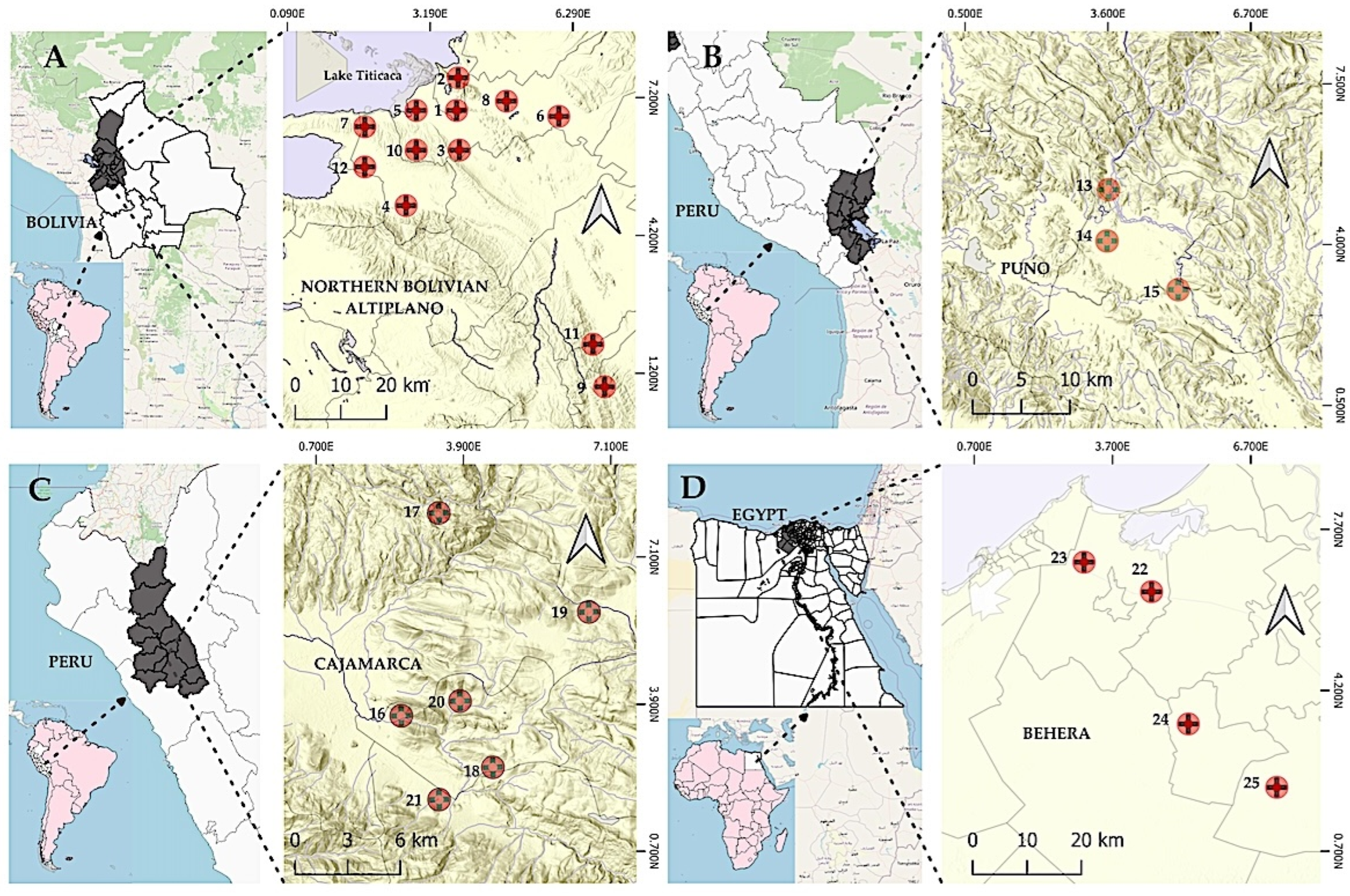
2.5. Study Areas
- One Fasciola species or the two Fasciola species.
- Only one lymnaeid snail vector species or more than one involved in the transmission.
- Seasonal or year-long permanent transmission.
- With or without involvement of pigs, buffaloes and goats as animal reservoirs, in addition to sheep and cows.
- Ranging from very high altitude to lowlands at sea level.
- Ranging from frequently to rarely occurring human infection source by natural water drinking.
- With different influences of behavioral, traditional, social and religious aspects linked to the disease transmission and human infection sources.
2.6. Laboratory Procedures
- Two aliquots of each stool sample were preserved, one in merthiolate–iodine–formalin (MIF) fixative (1:3) and one in 10% formalin solution (1:3) [117].
- Additionally, depending on the stool amount available, up to three Kato–Katz slides [123,124] were made from each stool sample to compensate for the well-known low sensitivity of this technique [125]. Kato–Katz slides were not only used to assess prevalences, but also for the analysis of egg counts.
2.7. Outcomes and Statistical Methods
- Model 1 included the number of the different protozoan and helminth (helminth number) species in each host as independent variables.
- Model 2 included the population characteristics (geographical location, sex and age) and number of protozoan and helminth (helminth number) species as independent variables.
- Model 3 included the population (age) and number of cases of soil-transmitted helminths (STH: infections caused by the three major species of nematodes, including the roundworm Ascaris lumbricoides, the whipworm Trichuris trichiura and the hookworms Ancylostoma duodenale and Necator americanus) as independent variables.
- Model 4 included the population (age), number of cases of Entamoeba coli, number of cases of Entamoeba hartmanni, number of cases of Endolimax nana, number of cases of Iodamoeba buetschlii, number of cases of Giardia intestinalis and number of cases of STH as independent variables.
3. Results
3.1. Socio-Demographic Characteristics and Associated Factors
3.2. Intestinal Parasite Species Among People Living in Fascioliasis Hyperendemic Areas
3.3. Analysis of Parasite Species Prevalences and Coinfections
3.4. Fascioliasis Analysis According to Sex and Age
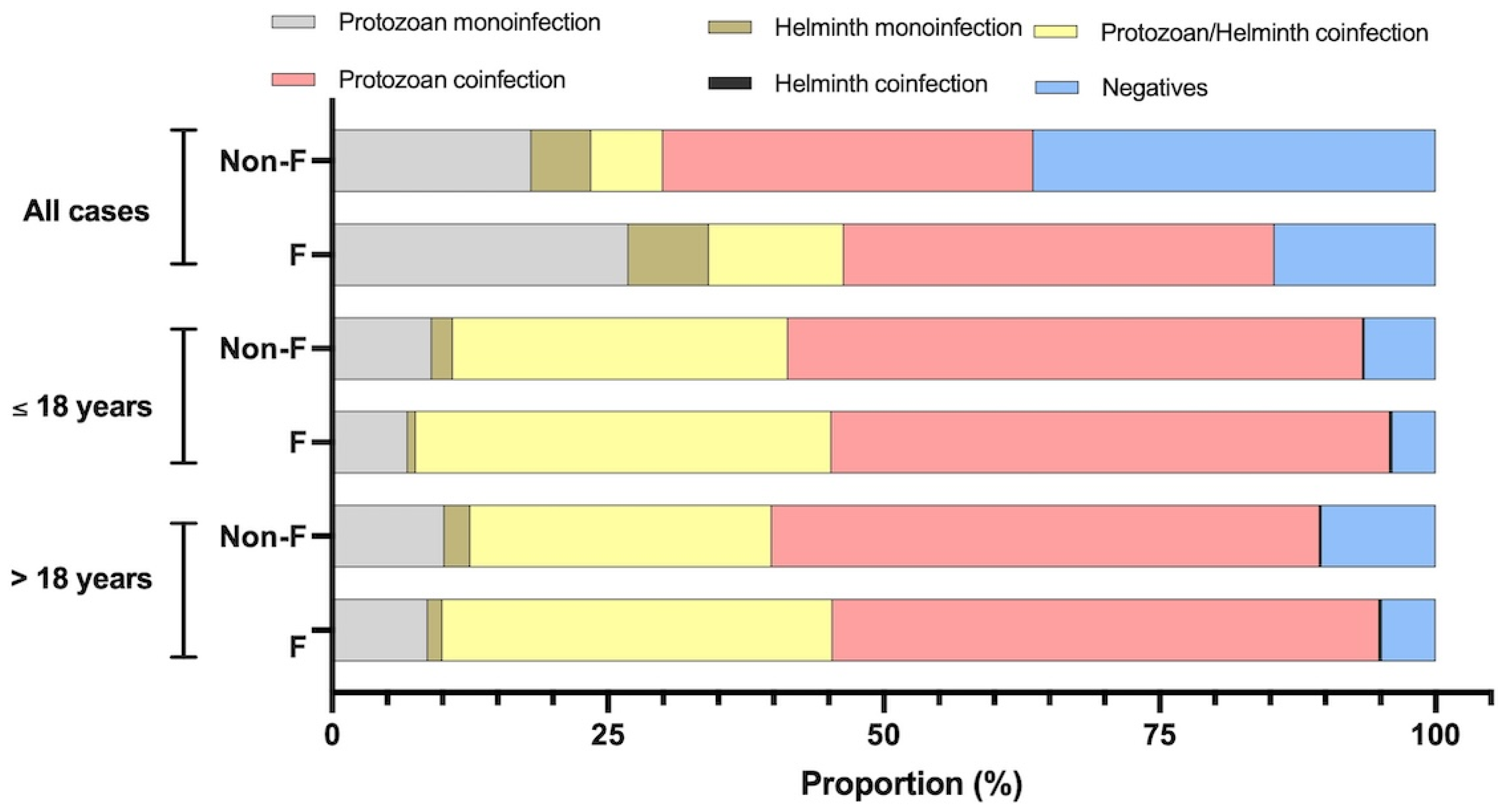
3.5. Fascioliasis and the Number of Coinfecting Parasites
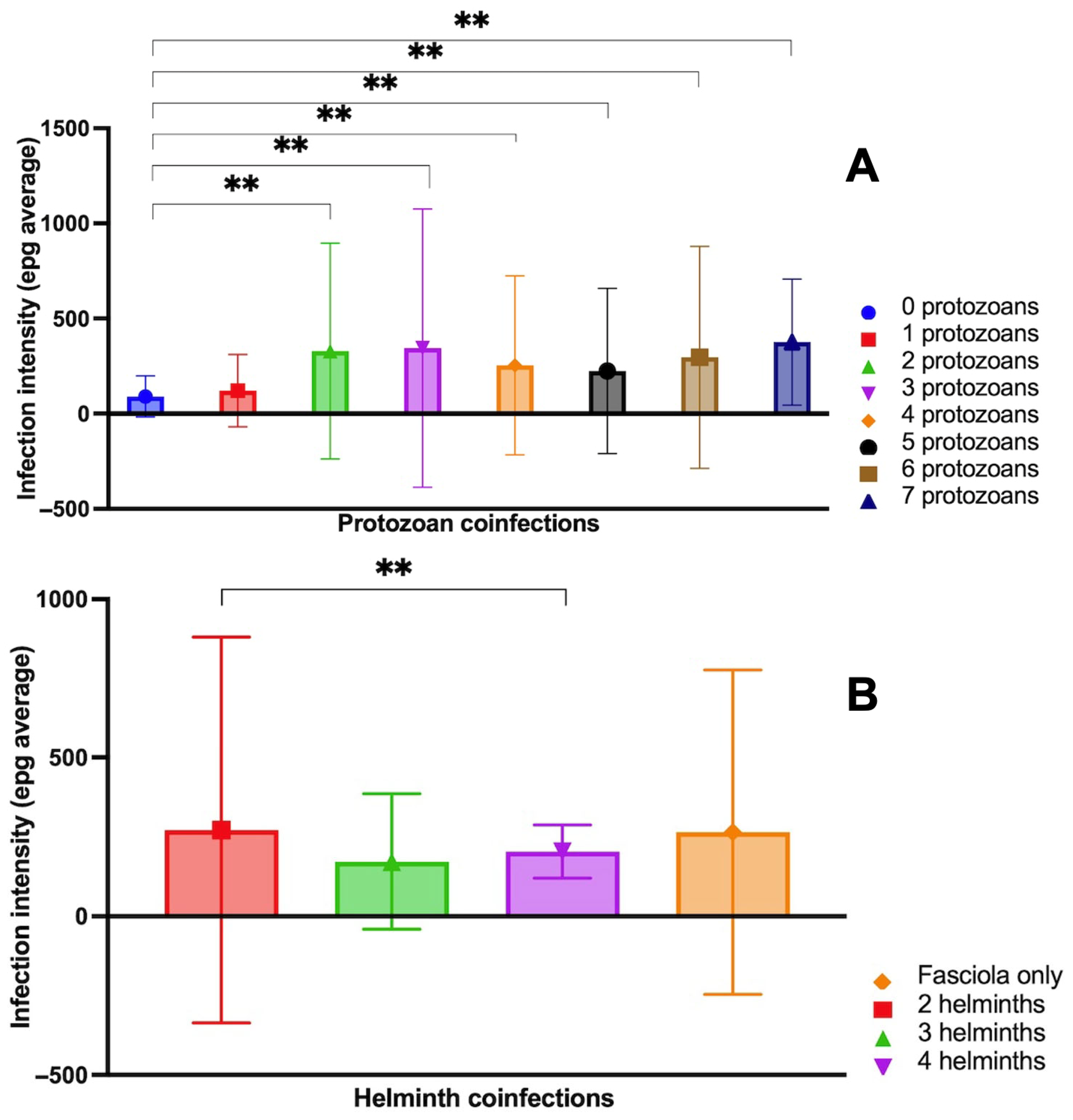
3.6. Analysis of Fasciola Infection Intensity, Age and Coinfection
- The pairwise comparison between Fasciola epg with and without protozoan species coinfection showed significant differences, indicating high levels of Fasciola epg in situations of coinfections with two, three, four and five protozoan species vs. situations with no protozoan coinfection (Figure 5). Summing up, Fasciola epg values increase when the number of protozoan species present in the coinfection increases.
- The pairwise comparison between Fasciola epg with and without helminth species coinfection did not show significant differences, except for the comparison of the Fasciola-only group and the three coinfecting helminth species (including Fasciola) group, indicating lower values of Fasciola epg in situations of coinfections by three helminth species vs. situations with no helminth coinfection (Figure 5B).
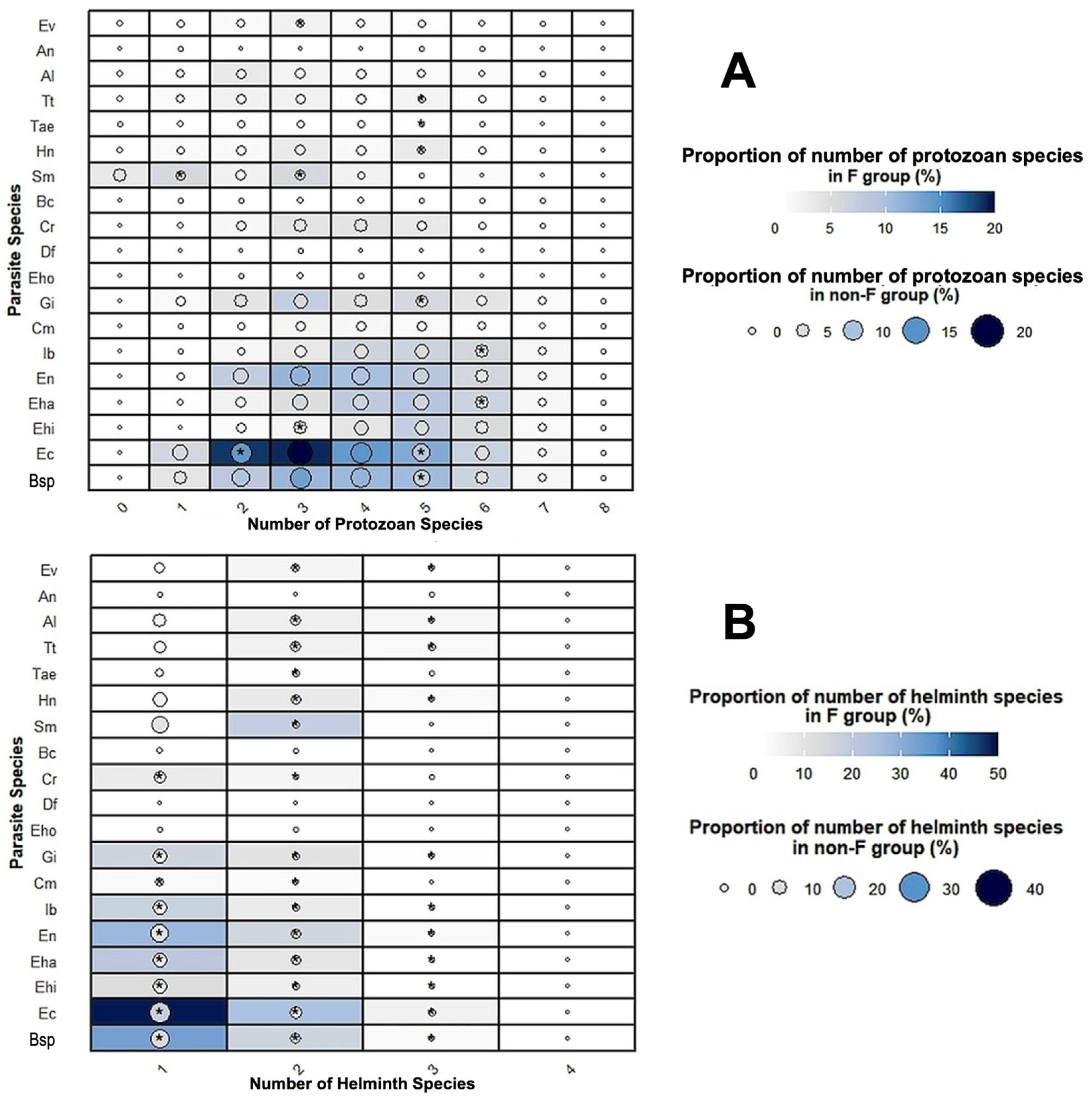
3.7. Parasite Species Associations
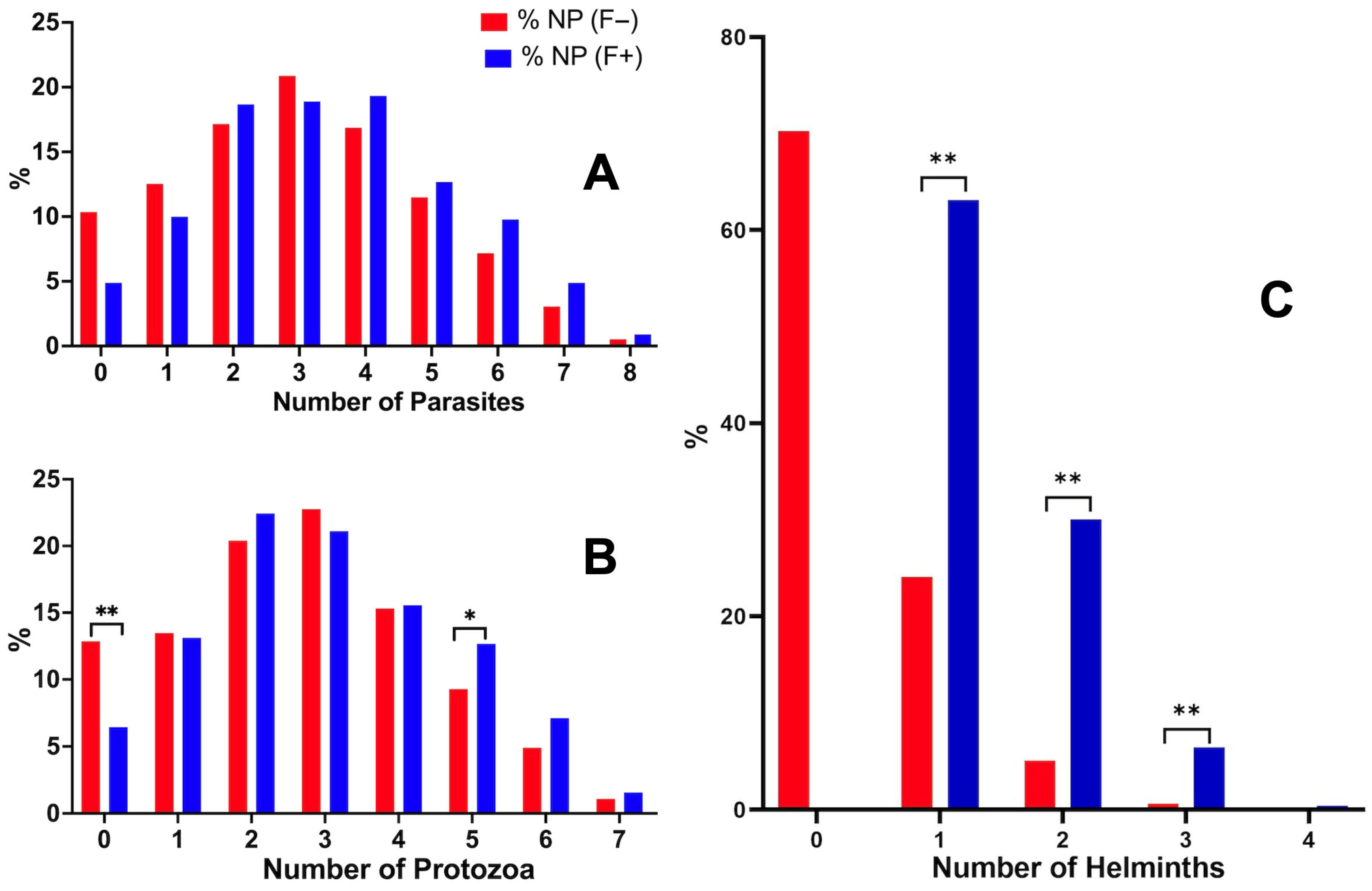
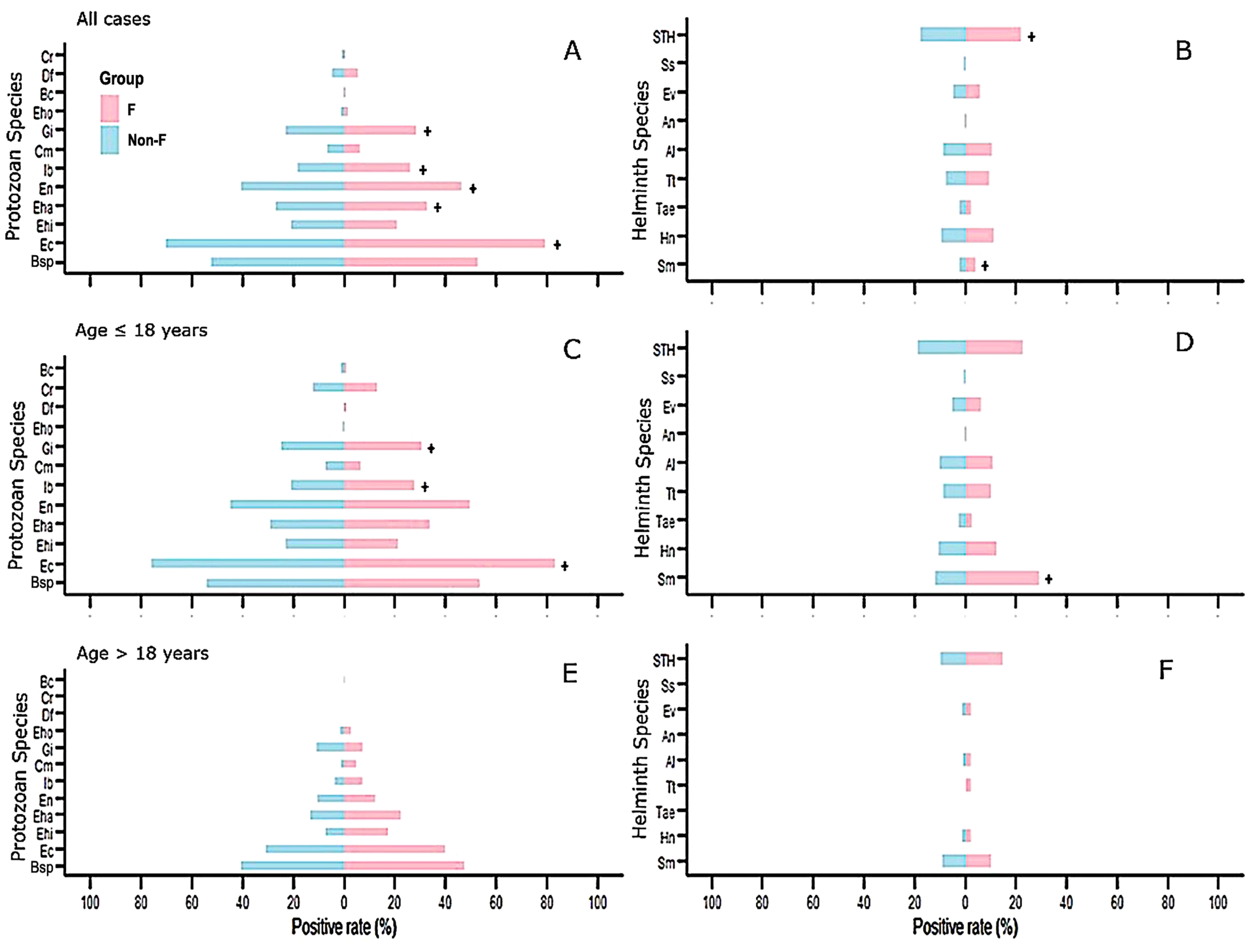
3.8. Comparison of the Positive Rate of Coinfecting Parasites in Fasciola-Infected Individuals and Individuals Not Infected with Fasciola
3.9. Multivariate Logistic Regression Analysis
- Model 1 included the numbers of the different protozoan and helminth species in each host individual as independent variables (Table 7). In this model, the risk of fascioliasis increased when the total number of helminth species per host increased [OR: 7.51 (95%CI 6.25, 9.03), p < 0.001]. Nevertheless, the fascioliasis risk did not increase when the total number of protozoan species was considered [OR: 1.03 (0.96, 1.11), p = 0.37].
- Model 2 included the population characteristics (geographical location, sex and age) and number of protozoan and helminth species as independent variables (Table 7). In this model, the risk of fascioliasis associated with the helminth number increased [OR: 7.78 (6.50–9.46), p < 0.001].
- Model 3 included the population age and number of cases of STH as independent variables (Table 7). In this model, the risk of fascioliasis is associated with STH cases [OR: 1.31 (1.01, 1.67), p = 0.03].
- Model 4 included the population age, in addition to the number of cases of E. coli, of E. hartmanni, of E. nana, of I. buetschlii, of G. intestinalis (in which association with Fasciola was detected, Table 5), and of STH as independent variables (Table 7). In this model, the risk of fascioliasis is associated with the number of cases of E. coli [OR: 1.37 (1.04, 1.80), p < 0.02], of I. buetschlii [OR: 1.32 (1.02, 1.72), p < 0.03], of G. intestinalis [OR: 1.26 (1.00, 1.59), p < 0.04] and of STH [OR: 1.28 (1.00, 1.65), p < 0.04], indicating that these parasitic species also partly explain this process.
4. Discussion
4.1. Species Compositions, Environmental and Social Factors and Infection Sources
- In the Bolivian Altiplano [72,73]: one case with F. hepatica + two protozoans (E. histolytica complex and G. intestinalis) + two helminths (H. nana and T. trichiura); another case with F. hepatica + three protozoans (E. histolytica complex, G. intestinalis and Cryptosporidium sp.) + one helminth (E. vermicularis).
- In the Peruvian Altiplano [83]: 61% of the F. hepatica-infected cases co-infected with pathogenic species such as two protozoans (E. histolytica complex and G. intestinalis) and five–six helminths (H. nana, Taenia spp., T. trichiura, A. lumbricoides and A. duodenale/N. americanus).
- In the Peruvian Cajamarca valley [89]: one case with F. hepatica + six protozoans (E. coli, E. histolytica complex, E. hartmanni, E. nana, Ch. mesnili and Blastocystis sp.) + three helminths (T. trichiura, A. lumbricoides and E. vermicularis).
- In the Egyptian Nile Delta [111]: one case with Fasciola spp. + six protozoans (E. coli, E. histolytica complex, E. nana, G. intestinalis, Ch. mesnili and Blastocystis sp.) + two helminths (S. mansoni, and H. nana).
4.2. The Sex Factor
4.3. Endemic Area Characteristics and Infection Sources
4.4. Parasite Associations and Infection Sources
4.5. Fascioliasis Immune Response
4.6. Fascioliasis Risk Increases as the Number of Helminth Species Increases
4.7. Association Between Fasciola spp. and E. coli, E. hartmanni, E. nana, I. buetschlii and G. intestinalis
4.8. Association Between Fasciola spp., STH and S. mansoni
4.9. Fasciola Infection Intensity, Age and Coinfection
- In protozoan coinfection, a general influence was detected, showing that fascioliasis coinfection with an elevated number of protozoan species presents higher epg levels than in cases of fascioliasis monoinfection.
- In helminth coinfection, a general influence was detected in fascioliasis coinfection with an elevated number of helminth species that presents lower epg levels than in cases of fascioliasis monoinfection.
4.10. Pathology and Coinfections
4.11. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| NTD | Neglected tropical diseases |
| epg | Eggs/gram of feces |
| SD | Standard deviation |
| CI | Confidence interval |
| Bh | Blastocystis hominis |
| Ec | Entamoeba coli |
| Ehi | Entamoeba histolytica complex |
| Eha | Entamoeba hartmanni |
| Ena | Endolimax nana |
| Ib | Iodamoeba buetschlii |
| Cm | Chilomastix mesnili |
| Gi | Giardia intestinalis |
| Eho | Enteromonas hominis |
| Df | Dientamoeba fragilis |
| Cr | Cryptosporidium sp. |
| Bc | Balantidium coli |
| STH | Soil-transmitted helminths |
| Sm | Schistosoma mansoni |
| Hn | Hymenolepis nana |
| Tae | Taenia sp. |
| Tt | Trichuris trichiura |
| Al | Ascaris lumbricoides |
| An | Ancylostoma duodenale and/or Necator americanus |
| Ss | Strongyloides stercolaris |
| Ev | Enterobius vermicularis |
| OR | Odds ratio |
| DALY | Disability-adjusted life year |
References
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Human and animal fascioliasis: Origins and worldwide evolving scenario. Clin. Microbiol. Rev. 2022, 35, e0008819. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Halajian, A.; Artigas, P.; Luus-Powell, W.J.; Valero, M.A.; Mas-Coma, S. Paleobiogeographical origins of Fasciola hepatica and F. gigantica in light of new DNA sequence characteristics of F. nyanzae from hippopotamus. Front. Vet. Sci. 2022, 9, 990872. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Artigas, P.; Valero, M.A.; Bargues, M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: Experimental transmission capacity, field epidemiology, and control within a One Health initiative in Bolivia. Front. Vet. Sci. 2020, 7, 583204. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Donkey fascioliasis within a One Health control action: Transmission capacity, field epidemiology, and reservoir role in a human hyperendemic area. Front. Vet. Sci. 2020, 7, 591384. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Funatsu, I.R.; Angles, R.; Buchon, P.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: Experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. One Health 2021, 13, 100249. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Cafrune, M.M.; Funatsu, I.R.; Mangold, A.J.; Angles, R.; Buchon, P.; Fantozzi, M.C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Fascioliasis in llama, Lama glama, in Andean endemic areas: Experimental transmission capacity by the high altitude snail vector Galba truncatula and epidemiological analysis of Its reservoir role. Animals 2021, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.D.; Skuce, P.J.; McNeill, T.N. The influence of liver fluke infection on production in sheep and cattle: A meta-analysis. Int. J. Parasitol. 2021, 51, 913–924. [Google Scholar] [CrossRef]
- Bargues, M.D.; Valero, M.A.; Trueba, G.A.; Fornasini, M.; Villavicencio, A.F.; Guaman, R.; De Elias-Escribano, A.; Perez-Crespo, I.; Artigas, P.; Mas-Coma, S. DNA multi-marker genotyping and CIAS morphometric phenotyping of Fasciola gigantica-sized flukes from Ecuador, with an analysis of the Radix absence in the New World and the evolutionary lymnaeid snail vector filter. Animals 2021, 11, 2495. [Google Scholar] [CrossRef]
- Mas-Coma, S. Epidemiology of fascioliasis in human endemic areas. J. Helminthol. 2005, 79, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fascioliasis. Adv. Exp. Med. Biol. 2024, 1154, 157–201. [Google Scholar]
- Afshan, K.; Fortes-Lima, C.A.; Artigas, P.; Valero, M.A.; Qayyum, M.; Mas-Coma, S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health 2014, 8, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.W.; Tanveer, A.; Mas-Coma, S. Epidemiological analysis of human fascioliasis in northeastern Punjab, Pakistan. Acta Trop. 2016, 156, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.W.; Zeb, A.; Mansoor, A.; Hayat, A.; Mas-Coma, S. Fasciola hepatica infection in children actively detected in a survey in rural areas of Mardan district, Khyber Pakhtunkhawa province, northern Pakistan. Parasitol. Int. 2019, 69, 39–46. [Google Scholar] [CrossRef]
- Sunita, K.; Mas-Coma, S.; Bargues, M.D.; Sadaf Khan, M.A.; Habib, M.; Mustafa, S.; Husain, S.A. Buffalo infection by Fasciola gigantica transmitted by Radix acuminata in Uttar Pradesh, India: A molecular tool to improve snail vector epidemiology assessments and control surveillance. Acta Parasitol. 2021, 66, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Varghese, G.M.; John, T.J.; Ajjampur, S.S.R.; Ahasan, S.A.; Chowdhury, E.H.; Gabrielli, A.F.; Mas-Coma, S. Human fascioliasis emergence in southern Asia: Complete nuclear rDNA spacer and mtDNA gene sequences prove Indian patient infection related to fluke hybridization in northeastern India and Bangladesh. One Health 2024, 18, 100675. [Google Scholar] [CrossRef]
- De, N.V.; Minh, P.N.; Le, T.H.; Dung, D.T.; Duon, T.T.; Tuan, B.V.; Dong, L.T.; Chau, N.V.V.; Cuervo, P.F.; Bargues, M.D.; et al. A multidisciplinary analysis of over 53,000 fascioliasis patients along the 1995–2019 countrywide spread in Vietnam defines a new epidemiological baseline for One Health approaches. One Health 2024, 19, 100869. [Google Scholar] [CrossRef]
- Mas-Coma, S. Human fascioliasis emergence risks in developed countries: From individual patients and small epidemics to climate and global change impacts. Enf. Infec. Microbiol.Clin. 2020, 38, 253–256. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 2005, 35, 1255–1278. [Google Scholar] [CrossRef]
- WHO. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases, Department of Control of Neglected Tropical Diseases; WHO: Geneva, Switzerland, 2013; pp. 1–128. [Google Scholar]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020; pp. 1–47. [Google Scholar]
- Valero, M.A.; Perez-Crespo, I.; Chillón-Marinas, C.; Khoubbane, M.; Quesada, C.; Reguera-Gomez, M.; Mas-Coma, S.; Fresno, M.; Gironès, N. Fasciola hepatica reinfection potentiates a mixed Th1/Th2/Th17/Treg response and correlates with the clinical phenotypes of anemia. PLoS ONE 2017, 12, e0173456. [Google Scholar] [CrossRef]
- Valero, M.A.; Gironès, N.; Reguera-Gomez, M.; Pérez-Crespo, I.; López-García, M.P.; Quesada, C.; Bargues, M.D.; Fresno, M.; Mas-Coma, S. Impact of fascioliasis reinfection on Fasciola hepatica egg shedding: Relationship with the immune-regulatory response. Acta Trop. 2020, 209, 105518. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. One Health for fascioliasis control in human endemic areas. Trends Parasitol. 2023, 39, 650–667. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Bargues, M.D.; Khoubbane, M.; Artigas, P.; Quesada, C.; Berinde, L.; Ubeira, F.M.; Mezo, M.; Hernandez, J.L.; Agramunt, V.H.; et al. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: Experimental long-term follow-up of biochemical markers. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Rondelaud, D.; Dreyfuss, G.; Vignoles, P. Clinical and biological abnormalities in patients after fasciolosis treatment. Med. Mal. Infect. 2006, 36, 466–508. [Google Scholar] [CrossRef]
- Chen, M.G.; Mott, K.E. Progress in assessment of morbidity due to Fasciola hepatica infection: A review of recent literature. Trop. Dis. Bull. 1990, 87, R1–R38. [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Marty, A.M.; Neafie, R.C. Hepatic Trematodiases. In Pathology of Infectious Diseases, Helminthiases; Meyers, W.M., Neafie, R.C., Marty, A.M., Wear, D.J., Eds.; Armed Forces Institute of Pathology and American Registry of Pathology: Washington, DC, USA, 2000; Volume 1, pp. 69–92. [Google Scholar]
- Mas-Coma, S.; Agramunt, V.H.; Valero, M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014, 84, 27–149. [Google Scholar]
- González-Miguel, J.; Valero, M.A.; Reguera-Gómez, M.; Mas-Bargues, C.; Bargues, M.D.; Simón, F.; Mas-Coma, S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology 2019, 146, 284–298. [Google Scholar] [CrossRef]
- Serrat, J.; Becerro-Recio, D.; Torres-Valle, M.; Simón, F.; Valero, M.A.; Bargues, M.D.; Mas-Coma, S.; Siles-Lucas, M.; González-Miguel, J. Fasciola hepatica juveniles interact with the host fibrinolytic system as a potential early-stage invasion mechanism. PLoS Negl. Trop. Dis. 2023, 17, e0010936. [Google Scholar] [CrossRef]
- Steinmann, P.; Du, Z.W.; Utzinger, J.; Zhou, X.N. Multiparasitism a neglected reality on global, regional and local scale. Adv. Parasitol. 2010, 73, 21–50. [Google Scholar]
- Garza-Cuartero, L.; Garcia-Campos, A.; Zintl, A.; Chryssafidis, A.; O’Sullivan, J.; Sekiya, M.; Mulcahy, G. The worm turns: Trematodes steering the course of co-infections. Vet. Pathol. 2014, 51, 385. [Google Scholar] [CrossRef]
- Vaumourin, E.; Vourch, G.; Gasqui, P.; Vayssier-Taussat, M. The importance of multiparasitism: Examining the consequences of co-infections for human and animal health. Parasites Vectors 2015, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.J.H.; Turner, L.; Morgan, E.R. Inappropriate measures of population health for parasitic disease? Trends Parasitol. 2009, 25, 393–394. [Google Scholar] [CrossRef]
- Rousseau, D.; Le Fichoux, Y.; Stien, X.; Suffia, I.; Ferrua, B.; Kubar, J. Progression of visceral leishmaniasis due to Leishmania infantum. BALB/c mice is markedly slowed by prior infection with Trichinella spiralis. Infect. Immun. 1997, 65, 4978–4983. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Inuo, G.; Akao, N.; Tsukidate, S.; Fujita, K. Down-regulation of murine susceptibility to cerebral malaria by inoculation with third-stage larvae of the filarial nematode Brugia pahangi. Parasitology 1997, 114, 333–338. [Google Scholar] [CrossRef]
- Thomas, F.P.J.F.; Guegan, Y.; Michalakis, Y.; Renaud, F. Are there pros as well as cons to being parasitized. Parasitol. Today 2000, 16, 533–536. [Google Scholar] [CrossRef]
- Elelu, N.; Ambali, A.; Coles, G.C.; Eisler, M.C. Cross-sectional study of Fasciola gigantica and other trematode infections of cattle in Edu Local Government Area, Kwara State, north-central Nigeria. Parasites Vectors 2016, 9, 470. [Google Scholar] [CrossRef]
- Che-Kamaruddin, N.; Isa, N.M.M. Assessment of Fasciola and paramphistomes co-infection in large ruminants through faecal egg counts around Taiping, Malaysia. Trop. Biomed. 2023, 40, 344–350. [Google Scholar] [CrossRef]
- Vogel, D.W. Experimental studies on mixed infection of cattle with hydatid cyst and Fasciola. Abst. Vet. J. 1971, 33, 190–195. [Google Scholar]
- Hidalgo, C.; Stoore, C.; Hernández, M.; Paredes, R. Fasciola hepatica coinfection modifies the morphological and immunological features of Echinococcus granulosus cysts in cattle. Vet. Res. 2020, 51, 76. [Google Scholar] [CrossRef]
- Stoore, C.; Andrade, C.; Hidalgo, C.; Corrêa, F.; Jiménez, M.; Hernandez, M.; Paredes, R. Echinococcus granulosus hydatid cyst location is modified by Fasciola hepatica infection in cattle. Parasites Vectors 2018, 11, 542. [Google Scholar] [CrossRef]
- Petraglia, A.A. Parasitosis humana por Fasciola hepatica: Primer caso que se describe en el Noreste Argentino. Act. Trab. Asoc. Arg. Est. Enf. Transm. 1954, 3, 47–48. [Google Scholar]
- Froyd, G. The incidence of liver flukes (Fasciola gigantica) and hydatid cysts (Echinococcus granulosus) in Kenya cattle. J. Parasitol. 1960, 46, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Strada, L. Fascioliasis hepática humana. Prensa Méd. Arg. 1961, 48, 2985–2992. [Google Scholar]
- Dawes, B. Hyperplasia of the bile duct in fascioliasis and its relation to the problem of mixed infection of Fasciola and hydatid cysts. Parasitology 1963, 53, 128–133. [Google Scholar] [CrossRef]
- Peiretti, J.A. Distomatosis hepática, contribución al diagnóstico precoz. Día. Médico. 1969, 10, 248. [Google Scholar]
- Debray, C.; Paolaggi, J.A.; Cerf, M.; Benhamou, G.; Morin, T.; Gosset, F. Association of hepatic hidatidosis (3 cysts) and choledochal distomiasis. Sem. Hôp. Paris 1975, 51, 2735–2737. [Google Scholar]
- Duron, J.J.; Benhamou, G.; Nardi, C. Association of a hydatid cyst and distomatosis of the liver. Nouv. Presse Méd. 1975, 4, 1364. [Google Scholar]
- Miguel, C.M.; Mallea Gil, M.S.; Basile, M.A.; Mauro, E.L. Distomatosis por Fasciola hepatica. Prensa Méd. Arg. 1985, 72, 192–195. [Google Scholar]
- Mera y Sierra, R.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasites Vectors 2011, 4, 104. [Google Scholar] [CrossRef]
- Şakru, N.; Korkmaz, M.; Demirci, M.; Kuman, A.; Ok, U.Z. Fasciola hepatica infection in echinococcosis suspected cases. Turk. J. Parasitol. 2011, 35, 77–80. [Google Scholar] [CrossRef]
- Kaya, M.; Bestas, R.; Girgin, S.; Cicek, M.; Kaplan, M.A. Increased anti-Echinococcus granulosus antibody positivity in Fasciola hepatica infection. Turk. J. Gastroenterol. 2012, 23, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Lee, Y.S.; Yun, J.H.; Kim, J.J.; Choi, W.H.; Oh, I.H.; Song, H.O.; Chu, J.P. A case of probable mixed-infection with Clonorchis sinensis and Fasciola sp.: CT and parasitological findings. Kor. J. Parasitol. 2010, 48, 157–160. [Google Scholar] [CrossRef]
- Wong, R.K.; Peura, D.A.; Mutter, M.L.; Heit, H.A.; Birns, M.T.; Johnson, L.F. Hemobilia and liver flukes in a patient from Thailand. Gastroenterology 1985, 88, 1958–1963. [Google Scholar] [CrossRef]
- Doğan, N.; Koçman, N.U. Uzun süreli karın ağrısı sikayeti olan hastada poliparazitizm olgusu [Case of polyparasitism with long-term abdominal pain in a patient]. Turk. J. Parasitol. 2013, 37, 157–160. (In Turkish) [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Xiong, Z.; Li, Z.; Li, J.; Xu, X.; Zhang, M.; Xing, M.; Ning, Q.; Wu, D.; et al. Case report: “Area of Focus” atypical trichinellosis and fascioliasis coinfection. Front. Med. 2022, 9, 88. [Google Scholar] [CrossRef]
- Torrus-Tendero, D.; Ramos-Rincón, J.M.; Salvador, F.; Oliveira, I.; Llenas-García, J.; Arsuaga, M.; Crespillo-Andújar, C.; Pérez-Molina, J.A. Imported fascioliasis in Spain: Report of 12 cases from the +REDIVI collaborative network (2009–2019). Travel Med. Infect. Dis. 2022, 47, 102286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jang, B.K. Toxocara canis and Fasciola hepatica co-infection leading to hepatic abscess: A case report. J. Kor. Med. Sci. 2023, 38, e323. [Google Scholar] [CrossRef]
- Oujamaa, L.; Sibon, I.; Vital, A.; Menegon, P. Vasculite cérébrale secondaire à une co-infestation par Toxocara canis et Fasciola hepatica. Rev. Neurol. 2023, 159, 447–450. [Google Scholar]
- Zumaquero-Ríos, J.L.; Sarracent-Pérez, J.; Rojas-García, R.; Rojas-Rivero, L.; Martínez-Tovilla, Y.; Valero, M.A.; Mas-Coma, S. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: Epidemiology and treatment with nitazoxanide. PLoS Negl. Trop. Dis. 2013, 7, e2553. [Google Scholar] [CrossRef]
- Graham, C.S.; Brodie, S.B.; Weller, P.F. Imported Fasciola hepatica infection in the United States and treatment with triclabendazole. Clin. Infect. Dis. 2001, 33, 1–5. [Google Scholar] [CrossRef]
- Miller, C.M.; Smith, N.C.; Ikin, R.J.; Boulter, N.R.; Dalton, J.P.; Donnelly, S. Immunological interactions between 2 common pathogens, Th1-inducing protozoan Toxoplasma gondii and the Th2-inducing helminth Fasciola hepatica. PLoS ONE 2009, 4, e5692. [Google Scholar] [CrossRef][Green Version]
- Deveci, Ö.; Aslan, E.; Tekin, A.; Toka Özer, T.; Tekin, R.; Bozkurt, F.; Çetinçakmak, M.G. Ayni hastada fascioliazis ve bruselloz [fascioliasis and brucellosis in same patient]. Turk. J. Parasitol. 2014, 38, 197–200. (In Turkish) [Google Scholar] [CrossRef]
- Önal, U.; Yamazhan, T.; Pullukçu, H.; Tasbakan, M.; Tamsel, S.; Erdogan, D.D.; Korkmaz, M.; Sipahi, O.R. Two rare causes of hepatitis: Fascioliasis and brucellosis. J. Viral Hepat. 2017, 23, 76–79. [Google Scholar] [CrossRef]
- Garza-Cuartero, L.; O’Sullivan, J.; Blanco, A.; McNair, J.; Welsh, M.; Flynn, R.J.; Williams, D.; Diggle, P.; Cassidy, J.; Mulcahy, G. Fasciola hepatica infection reduces Mycobacterium bovis burden and mycobacterial uptake and suppresses the pro-inflammatory response. Parasite Immunol. 2016, 38, 387–402. [Google Scholar] [CrossRef]
- Demirci, M.; Isler, M.; Cicioglu Aridogan, B.; Senoi, A.; Korkma, M. Coinfection of chronic hepatitis B and fasciolosis. Infection 2004, 32, 54–56. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Esteban, J.G. Human fasciolosis. In Fasciolosis; Dalton, J.P., Ed.; CAB International Publishing: Wallingford, Oxon, UK, 1999; pp. 411–434. [Google Scholar][Green Version]
- Bjorland, J.; Bryan, R.T.; Strauss, W.; Hillyer, G.V.; McAuley, J.B. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin. Infect. Dis. 1995, 11, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Angles, R.; Esteban, J.G.; Bargues, M.D.; Buchon, P.; Franken, M.; Strauss, W. The human fascioliasis high endemic region of the Northern Bolivian Altiplano. Trop. Med. Int. Health 1999, 4, 454–467. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Aguirre, C.; Strauss, W.; Angles, R.; Mas-Coma, S. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the Northern Bolivian Altiplano. Acta Trop. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Angles, R.; Strauss, W.; Aguirre, C.; Mas-Coma, S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop. Med. Int. Health 1997, 2, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.G.; Flores, A.; Angles, R.; Mas-Coma, S. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Angles, R.; Strauss, W.; Ramirez, S.; Esteban, J.G.; Mas-Coma, S. Human fascioliasis in Bolivia: Coprological surveys in different provinces of the Department of La Paz. Res. Rev. Parasitol. 1997, 57, 33–37. [Google Scholar]
- Hillyer, G.V.; Soler de Galanes, M.; Rodriguez-Perez, J.; Bjorland, J.; Silva de Lagrava, M.; Ramirez Guzman, S.; Bryan, R.T. Use of the Falcon Assay Screening Test—Enzyme-Linked Immunosorbent Assay (FAST-ELISA) and the Enzyme-Linked Immunoelectrotransfer Blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1992, 46, 603–609. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Parkinson, M.; Strauss, W.; Anglés, R.; Dalton, J.P. Immunodiagnosis of Fasciola hepatica (Fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am. J. Trop. Med. Hyg. 1998, 58, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Periago, M.V.; Perez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Funatsu, I.R.; Bargues, M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 2001, 123, S115–S127. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Angles, R.; Osca, D.; Duran, P.; Buchon, P.; Gonzales-Pomar, R.K.; Pinto-Mendieta, J.; Mas-Coma, S. Genetic uniformity, geographical spread and anthropogenic habitat modifications of lymnaeid vectors found in a One Health initiative in the highest human fascioliasis hyperendemic of the Bolivian Altiplano. Parasit. Vectors 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Angles, R.; Coello, J.; Artigas, P.; Funatsu, I.R.; Cuervo, P.F.; Buchon, P.; Mas-Coma, S. One Health initiative in the Bolivian Altiplano human fascioliasis hyperendemic area: Lymnaeid biology, population dynamics, microecology and climatic factor influences. Braz. J. Vet. Parasitol. 2021, 30, e025620. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.; Angles, R.; Barrientos, R.; Barrios, G.; Valero, M.A.; Hamed, K.; Grueningr, H.; Ault, S.K.; Montresor, A.; Engels, D.; et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl. Trop. Dis. 2012, 6, e1720. [Google Scholar] [CrossRef]
- Esteban, J.G.; González, C.; Bargues, M.D.; Angles, R.; Sánchez, C.; Náquira, C.; Mas-Coma, S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health 2002, 7, 339–348. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef]
- Angles, R.; Buchon, P.; Valero, M.A.; Bargues, M.D.; Mas-Coma, S. One Health action against human fascioliasis in the Bolivian Altiplano: Food, water, housing, behavioural traditions, social aspects, and livestock management linked to disease transmission and infection sources. Int. J. Environ. Res. Public Health 2022, 19, 1120. [Google Scholar] [CrossRef]
- Marcos, L.A.; Maco, V.; Terashima, M.A.; Samalvides, F.; Gotuzzo, E. Características clínicas de la infección crónica por Fasciola hepatica en niños. Rev. Gastroenterol Peru 2002, 22, 228–233. [Google Scholar]
- Marcos, L.A.; Maco, V.; Terashima, A.; Samalvides, F.; Espinoza, J.R.; Gotuzzo, E. Fascioliasis in relatives of patients with Fasciola hepatica infection in Peru. Rev. Inst. Med. Trop. Sâo Paulo 2005, 47, 219–222. [Google Scholar] [CrossRef]
- Marcos, L.; Maco, V.; Samalvides, F.; Terashima, A.; Espinoza, J.R.; Gotuzzo, E. Risk factors for Fasciola hepatica infection in children: A case-control study. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 158–166. [Google Scholar] [CrossRef]
- Gonzalez, L.C.; Esteban, J.G.; Bargues, M.D.; Valero, M.A.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Hyperendemic human fascioliasis in Andean valleys: An altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 2011, 120, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.R.; Maco, V.; Marcos, L.; Saez, S.; Neyra, V.; Terashima, A.; Samalvides, F.; Gotuzzo, E.; Chavarry, E.; Huaman, M.C.; et al. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am. J. Trop. Med. Hyg. 2007, 76, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Contreras, J.; Burga-Hernandez, A.; Geldres-Moreno, L.; Bazan-Vasquez, C. Estudio clínico y epidemiológico de la distomatosis hepática en escolares de la zona rural de Cajamarca. Rev. Peru Pediatría 1971, 29, 165–171. [Google Scholar]
- Storck, M.G.; Venables, G.S.; Jennings, S.M.F.; Beesley, J.R.; Bendezu, P.; Capron, A. An investigation of endemic fascioliasis in Peruvian village children. J. Trop. Med. Hyg. 1973, 76, 231–235. [Google Scholar]
- Knobloch, J. Human fascioliasis in Cajamarca/Peru. II. Humoral antibody response and antigenaemia. Trop. Med. Parasitol. 1985, 36, 91–93. [Google Scholar] [PubMed]
- Knobloch, J.; Delgado, E.; Alvarez, A.G.; Reymann, U.; Bialek, R. Human fascioliasis in Cajamarca/Peru. I. Diagnostic methods and treatment with praziquantel. Trop. Med. Parasitol. 1985, 36, 88–90. [Google Scholar]
- Ortiz, P.; Cabrera, M.; Jave, J.; Claxton, J.; Williams, D. Human fascioliasis: Prevalence and treatment in a rural area of Peru. Inf. Dis. Rev. 2000, 2, 42–46. [Google Scholar]
- Hillyer, G.V.; Soler de Galanes, M.; Delgado Azañero, E. Immunodiagnosis of human fasciolosis in children from Cajamarca, Peru. Parasitol. Día. 2001, 25, 82–84. [Google Scholar] [CrossRef]
- Alban Olaya, M.; Ortiz, J.J.; Quispe Lazo, T. Fasciolasis en Cajamarca. Rev. Gastroenterol Perú. 2002, 22, 28–32. [Google Scholar]
- Favennec, L.; Jave Ortiz, J.; Gargala, G.; Lopez Chegne, N.; Ayoub, A.; Rossignol, J.F. Double blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Alim. Pharmacol. Ther. 2003, 17, 265–270. [Google Scholar] [CrossRef]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Flores, R.; Glöer, P.; Rojas-Garcia, R.; Ashrafi, K.; Falkner, G.; Mas-Coma, S. Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: Genotype, phenotype, ecology, worldwide spread, susceptibility, applicability. PLoS ONE 2011, 6, e24567. [Google Scholar] [CrossRef]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Molecular characterisation of Galba truncatula, Lymnaea neotropica and L. schirazensis from Cajamarca, Peru and their potential role in transmission of human and animal fascioliasis. Parasites Vectors 2012, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Bardales-Valdivia, J.N.; Bargues, M.D.; Hoban-Vergara, C.; Bardales-Bardales, C.; Goicoechea-Portal, C.; Bazan-Zurita, H.; Del Valle-Mendoza, J.; Ortiz, P.; Mas-Coma, S. Spread of the fascioliasis endemic area assessed by seasonal follow-up of rRNA ITS-2 sequenced lymnaeid populations in Cajamarca, Peru. One Health 2021, 13, 100265. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Gayo, V.; Sanchis, J.; Artigas, P.; Khoubbane, M.; Birriel, S.; Mas-Coma, S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005352. [Google Scholar] [CrossRef]
- Bargues, M.D.; Malandrinni, J.B.; Artigas, P.; Soria, C.C.; Velasquez, J.N.; Carnevale, S.; Mateo, L.; Khoubbane, M.; Mas-Coma, S. Human fascioliasis endemic areas in Argentina: Multigene characterisation of the lymnaeid vectors and climatic-environmental assessment of the transmission pattern. Parasites Vectors 2016, 9, 306. [Google Scholar] [CrossRef]
- Claxton, J.R.; Zambrano, H.; Ortiz, P.; Amoros, C.; Delgado, E.; Escurra, E.; Clarkson, M.J. The epidemiology of fasciolosis in the inter-Andean valley of Cajamarca, Peru. Parasitol. Int. 1997, 46, 281–288. [Google Scholar] [CrossRef]
- Claxton, J.R.; Zambrano, H.; Ortiz, P.; Delgado, E.; Escurra, E.; Clarkson, M.J. Strategic control of fasciolosis in the inter-Andean valley of Cajamarca, Peru. Vet. Rec. 1998, 143, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Claxton, J.R.; Sutherst, J.; Ortiz, P.; Clarkson, M.J. The effect of cyclic temperatures on the growth of Fasciola hepatica and Lymnaea viatrix. Vet. J. 1999, 157, 166–171. [Google Scholar] [CrossRef]
- Rivera-Jacinto, M.; Rodriguez-Ulloa, C.; Rojas-Huaman, Y.; Valdivia-Melendez, Y.; Saucedo-Duran, T. Conocimientos, actitudes y prácticas sobre fascioliasis en madres de una zona rural andina del Norte peruano. Rev. Peru Med. Exp. Salud Públ. 2010, 27, 59–62. [Google Scholar]
- Soliman, M.S. Control of veterinary fascioliasis. In Infectious Diseases and Public Health; Angelico, M., Rocchi, G., Eds.; A Research and Clinical Update; Balaban Publishers: Philadelphia, PA, USA, 1998; pp. 334–346. [Google Scholar]
- Curtale, F.; Nabil, M.; El Wakeel, A.; Shamy, M.Y. Behera Survey Team. Anaemia and intestinal parasitic infections among school age children in Behera Governorate, Egypt. J. Trop. Pediatr. 1998, 44, 323–328. [Google Scholar] [CrossRef]
- Curtale, F.; Hammoud, E.S.; El Wakeel, A.; Mas-Coma, S.; Savioli, L. Human fascioliasis, an emerging public health problem in the Nile Delta, Egypt. Res. Rev. Parasitol. 2000, 60, 129–134. [Google Scholar]
- Esteban, J.G.; González, C.; Curtale, F.; Muñoz-Antoli, C.; Valero, M.A.; Bargues, M.D.; El Sayed, M.; El Wakeel, A.; Abdel-Wahab, Y.; Montresor, A.; et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am. J. Trop. Med. Hyg. 2003, 69, 429–437. [Google Scholar] [CrossRef]
- Curtale, F.; Moursy, B.E.M.; El Deen, M.S.S.; Nabil, M.; El Wakeel, A. Operational Research on Health, Nutrition and Environmental Needs in Behera Governorate. Final Report. Strengthening Rural Health Services in Behera, Dakalhia and Qena Governorates; Egyptian-Italian Cooperation Cooperation, Editing and Printing Center, Extension of the Medical Research Institute of Alexandria University: Alexandria, Egypt, 1997; pp. 1–52, 1–7 Annexes. [Google Scholar]
- Periago, M.V.; Valero, M.A.; Artigas, P.; Agramunt, V.H.; Bargues, M.D.; Curtale, F.; Mas-Coma, S. Very high fascioliasis intensities in schoolchildren of Nile Delta governorates: The Old World highest burdens found in lowlands. Pathogens 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Farag, H.F.; Salem, A.I.; Khalil, S.S.; Farahat, A. Studies on human fascioliasis in Egypt. 1-Seasonality of transmission. J. Egypt. Soc. Parasitol. 1993, 23, 331–340. [Google Scholar] [PubMed]
- Curtale, F.; Mas-Coma, S.; Hassanein, Y.A.E.W.; Barduagni, P.; Pezzotti, P.; Savioli, L. Clinical signs and household characteristics associated with human fascioliasis among rural population in Egypt: A case-control study. Parassitologia 2003, 45, 5–11. [Google Scholar]
- Curtale, F.; Hassanein, Y.A.W.; Savioli, L. Control of human fascioliasis by selective chemotherapy: Design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 599–609. [Google Scholar] [CrossRef]
- Ash, L.R.; Orihel, T.C. Parasites: A Guide to Laboratory Procedures and Identification; American Society of Clinical Pathologists: Chicago, IL, USA, 1987. [Google Scholar]
- Sapero, J.J.; Lawless, D. The “MIF“ stain-preservation technic for the identification of intestinal protozoa. Am. J. Trop. Med. Hyg. 1953, 2, 613–619. [Google Scholar] [CrossRef]
- Blagg, W.; Schloegel, E.L.; Mansour, N.S.; Khalaf, G.I. A new concentration technic for the demonstration of protozoa and helminth eggs in feces. Am. J. Trop. Med. Hyg. 1955, 4, 23–28. [Google Scholar] [CrossRef]
- Knight, W.B.; Hiatt, R.A.; Cline, B.L.; Ritchie, L.S. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1976, 55, 818–823. [Google Scholar] [CrossRef]
- Henriksen, S.A.; Pohlenz, J.F.L. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 1981, 22, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.G.; Aguirre, C.; Flores, A.; Strauss, W.; Angles, R.; Mas-Coma, S. High Cryptosporidium prevalences in healthy Aymara children from the Northern Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1998, 58, 50–55. [Google Scholar] [CrossRef]
- Kato, K.; Miura, M. Comparative examinations. Jap. J. Parasitol. 1954, 3, 35. [Google Scholar]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop Sao Paulo 1972, 14, 397–402. [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Diagnosis of human fascioliasis by stool and blood techniques: Update for the present global scenario. Parasitology 2014, 141, 1918–1946. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Funatsu, I.R.; Oviedo, J.A.; Mas-Coma, S. Natural water, an additional source for human infection by Fasciola hepatica in the Northern Bolivian Altiplano. Parassitologia 1996, 38, 252. [Google Scholar]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef]
- Curtale, F.; Hassanein, Y.A.W.; Barduagni, P.; Yousef, M.M.; El Wakeel, A.; Hallaj, Z.; Mas-Coma, S. Human fascioliasis infection: Gender difference within school-age children from endemic areas of the Nile Delta, Egypt. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 155–160. [Google Scholar] [CrossRef]
- De, N.V.; Le, T.H.; Agramunt, V.H.; Mas-Coma, S. Early postnatal and preschool age infection by Fasciola spp.: Report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020, 103, 1578–1589. [Google Scholar] [CrossRef]
- WHO. Soil-Transmitted Helminthiases. World Health Organization. Available online: https://www.who.int/health-topics/soil-transmitted-helminthiases#tab=tab_1 (accessed on 22 March 2024).
- Flores, A.; Esteban, J.G.; Angles, R.; Mas-Coma, S. Soil-transmitted helminth infections at very high altitude in Bolivia. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 272–277. [Google Scholar] [CrossRef]
- De, N.V.; Minh, P.N.; Duyet, L.V.; Mas-Coma, S. Strongyloidiasis in northern Vietnam: Epidemiology, clinical characteristics and molecular diagnosis of the causal agent. Parasit. Vectors 2019, 12, 515. [Google Scholar]
- Lebu, S.; Kibone, W.; Muoghalu, C.C.; Ochaya, S.; Salzberg, A.; Bongomin, F.; Manga, M. Soil-transmitted helminths: A critical review of the impact of co-infections and implications for control and elimination. PLoS Negl. Trop. Dis. 2023, 17, e0011496. [Google Scholar] [CrossRef]
- Rudan, I.; Lawn, J.; Cousens, S.; Rowe, A.K.; Boschi-Pint, C.; Tomaskovic, L.; Mendoza, W.; Lanata, C.F.; Roca-Feltrer, A.; Carneiro, I.; et al. Gaps in policy-relevant information on burden of disease in children: A systematic review. Lancet 2005, 365, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, G.V.; Apt, W. Food-borne trematode infections in the Americas. Parasitol. Today 1997, 13, 87–88. [Google Scholar] [CrossRef]
- Brady, M.T.; O’Neill, S.M.; Dalton, J.P.; Mills, K.H. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 1999, 67, 5372–5378. [Google Scholar] [CrossRef]
- Dowling, D.J.; Hamilton, C.M.; Donnelly, S.; La Course, J.; Brophy, P.M.; Dalton, J.; O’Neill, S.M. Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect. Immun. 2010, 78, 793–801. [Google Scholar] [CrossRef]
- Vukman, K.V.; Adams, P.N.; Met, M.; Maurer, M.; O’Neill, S.M. Fasciola hepatica tegumental coat impairs mast cells’ ability to drive Th1 immune responses. J. Immunol. 2013, 190, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.; O’Neill, S.M.; Sekiya, M.; Mulcahy, G.; Dalton, J.P. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 2005, 73, 166–173. [Google Scholar] [CrossRef]
- Flynn, R.J.; Irwin, J.A.; Olivier, M.; Sekiya, M.; Dalton, J.P.; Mulcahy, G. Alternative activation of ruminant macrophages by Fasciola hepatica. Vet. Immunol. Immunopathol. 2007, 120, 31–40. [Google Scholar] [CrossRef]
- Flynn, R.; Mannion, C.; Golden, O.; Hacariz, O.; Mulcahy, G. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immun. 2007, 75, 1373–1381. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Brady, M.T.; Callanan, J.J.; Mulcahy, G.; Joyce, P.; Mills, K.H.; Dalton, J.P. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 2000, 22, 147–155. [Google Scholar] [CrossRef]
- Walsh, K.P.; Brady, M.T.; Finlay, C.M.; Boon, L.; Mills, K.H.G. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J. Immunol. 2009, 183, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Rojas, C.A.; Scheerlinck, J.P.; Ansell, B.R.; Hall, R.S.; Gasser, R.B.; Jex, A.R. Time-Course study of the transcriptome of peripheral blood mononuclear cells (PBMCs) from sheep Infected with Fasciola hepatica. PLoS ONE 2016, 11, e0159194. [Google Scholar] [CrossRef]
- Fu, Y.; Chryssafidis, A.L.; Browne, J.A.; O’Sullivan, J.; McGettigan, P.A.; Mulcahy, G. Transcriptomic Study on Ovine Immune Responses to Fasciola hepatica infection. PloS Negl. Trop. Dis. 2016, 10, e0005015. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J.; Mulcahy, G. The roles of IL-10 and TGF-β in controlling IL-4 and IFN-γ production during experimental Fasciola hepatica infection. Int. J. Parasitol. 2008, 38, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.P.; Robinson, M.W.; Mulcahy, G.; O’Neill, S.M.; Donnelly, S. Immunomodulatory molecules of Fasciola hepatica: Candidates for both vaccine and immunotherapeutic development. Vet. Parasitol. 2013, 195, 272–285. [Google Scholar] [CrossRef]
- Sachdev, D.; Gough, K.C.; Flynn, R.J. The chronic stages of bovine Fasciola hepatica are dominated by CD4 T-cell exhaustion. Front. Immunol. 2017, 8, 1002. [Google Scholar] [CrossRef]
- Cwiklinski, K.; Donnelly, S.; Drysdale, O.; Jewhurst, H.; Smith, D.; De Marco Verissimo, C.; Pritsch, I.C.; O’Neill, S.; Dalton, J.P.; Robinson, M.W. The cathepsin-like cysteine peptidases of trematodes of the genus Fasciola. Adv. Parasitol. 2019, 104, 113–164. [Google Scholar]
- Ryan, S.; Shiels, J.; Taggart, C.C.; Dalton, J.P.; Weldon, S. Fasciola hepatica-derived molecules as regulators of the host immune response. Front. Immunol. 2020, 11, 2182. [Google Scholar] [CrossRef] [PubMed]
- Aron-Said, C.; Montes, M.; White, A.C.; Cabada, M.M. Plasma cytokines during acute human fascioliasis. Parasitol. Res. 2021, 120, 2965–2968. [Google Scholar] [CrossRef]
- Jankovic, D.; Liu, Z.; Gause, W.C. Th1- and Th2-cell commitment during infectious disease: Asymmetry in divergent pathways. Trends Immunol. 2001, 22, 450–457. [Google Scholar] [CrossRef]
- Cortes, A.; Peachey, L.; Scotti, R.; Jenkins, T.P.; Cantacessi, C. Helminth-microbiota cross-talk—A journey through the vertebrate digestive system. Mol. Biochem. Parasitol. 2019, 233, 111222. [Google Scholar] [CrossRef]
- Homan, E.J.; Bremel, R.D. A role for epitope networking in immunomodulation by helminths. Front. Immunol. 2018, 9, 1763. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.; van den Biggelaar, A.; Maizels, R.M. Th2 responses without atopy: Immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001, 22, 372–377. [Google Scholar] [CrossRef]
- Wohlfert, E.; Belkaid, Y. Role of endogenous and induced regulatory cells during infections. J. Clin. Immunol. 2008, 28, 707–715. [Google Scholar] [CrossRef]
- McKee, A.S.; Pearce, E.J. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 2004, 173, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Cools, N.; Ponsaerts, P.; Van Tendeloo, V.F.; Berneman, Z.N. Regulatory T cells and human disease. Clin. Dev. Immunol. 2007, 89195. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; LeGoff, L.; Harris, A.; Malone, E.; Allen, J.E.; Maizels, R.M. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 2005, 174, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Osorio, M.; Gomez Garcia, V.; Rojas Gonzalez, J.; Ramajo Martin, V.; Manga González, M.Y.; Gonzalez Lanza, C. Resistance to Schistosoma bovis in sheep induced by an experimental Fasciola hepatica infection. J. Parasitol. 1993, 79, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Abruzzi, A.; Fried, B. Coinfection of Schistosoma (Trematoda) with bacteria, protozoa and helminths. Adv. Parasitol. 2011, 77, 1–85. [Google Scholar] [CrossRef]
- Ricafrente, A.; Nguyen, H.; Tran, N.; Donnelly, S. An evaluation of the Fasciola hepatica miRnome predicts a targeted regulation of mammalian innate immune responses. Front. Immunol. 2021, 11, 608686. [Google Scholar] [CrossRef]
- Girones, N.; Valero, M.A.; García-Bodelón, M.A.; Chico-Calero, I.; Punzón, C.; Fresno, M.; Mas-Coma, S. Immune suppression in advanced chronic fascioliasis: An experimental study in a rat model. J. Infect. Dis. 2007, 195, 1504–1512. [Google Scholar] [CrossRef]
- Rodriguez-Sosa, M.; Satoskar, A.R.; Calderon, R.; Gomez-Garcia, L.; Saavedra, R.; Bojalil, R.; Terrazas, L.I. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2 biasing ability. Infect. Immunol. 2002, 70, 3656–3664. [Google Scholar] [CrossRef]
- Valero, M.A.; Navarro, M.; Garcia-Bodelon, M.A.; Marcilla, A.; Morales, M.; Hernandez, J.L.; Mengual, P.; Mas-Coma, S. High risk of bacterobilia in advanced experimental chronic fasciolosis. Acta Trop. 2006, 100, 17–23. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Adam, R.D. Giardia duodenalis: Biology and pathogenesis. Clin. Microbiol. Rev. 2021, 34, e0002419. [Google Scholar] [CrossRef]
- Wojciech, L.; Png, C.W.; Koh, E.Y.; Kioh, D.Y.Q.; Deng, L.; Wang, Z.; Wu, L.Z.; Hamidinia, M.; Tung, D.W.; Zhang, W.; et al. A tryptophan metabolite made by a gut microbiome eukaryote induces pro-inflammatory T cells. EMBO J. 2023, 42, e112963. [Google Scholar] [CrossRef]
- Veas, F.; Rey, J.P. Infection à VIH et parasitoses en zone tropicale. Cahiers Santé 1991, 1, 189–201. [Google Scholar]
- Mengist, H.M.; Taye, B.; Tsegaye, A. Intestinal parasitosis in relation to CD4+ T cells levels and anemia among HAART initiated and HAART naive pediatric HIV patients in a model ART center in Addis Ababa, Ethiopia. PLoS ONE. 2015, 10, e0117715. [Google Scholar] [CrossRef]
- Mülayim, S.; Dalkılıç, S.; Akbulut, H.H.; Aksoy, A.; Kaplan, M. Investigation of the relationship between lymphocyte subsets and intestinal parasites. Acta Trop. 2022, 225, 106221. [Google Scholar] [CrossRef]
- Caner, A.; Zorbozan, O.; Tunalı, V.; Kantar, M.; Aydoğdu, S.; Aksoylar, S.; Gürüz, Y.; Turgay, N. Intestinal protozoan parasitic infections in immunocompromised child patients with diarrhea. Jpn. J. Infect. Dis. 2020, 73, 187–192. [Google Scholar] [CrossRef]
- Laksemi, D.A.; Suwanti, L.T.; Mufasirin, M.; Suastika, K.; Sudarmaja, M. Opportunistic parasitic infections in patients with human immunodeficiency virus/acquired immunodeficiency syndrome: A review. Vet. World. 2019, 13, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, G.; Quintero, J.; Astiazarán-García, H.; Velazquez, C. Host defenses against Giardia lamblia. Parasite Immunol. 2015, 37, 394–406. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Mills, K.H.G.; Dalton, J.P. Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferongamma production in vivo. Parasite Immunol. 2001, 23, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J.; Mulcahy, G.; Elsheikha, H.M. Coordinating innate and adaptive immunity in Fasciola hepatica infection: Implications for control. Vet. Parasitol. 2010, 169, 235–240. [Google Scholar] [CrossRef]
- Flynn, R.J.; Mulcahy, G.; Welsh, M.; Cassidy, J.P.; Corbett, D.; Milligan, C.; Andersen, P.; Strain, S.; McNair, J. Co-infection of cattle with Fasciola hepatica and Mycobacterium bovis—Immunological consequences. Transb. Emerg. Dis. 2009, 56, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.K.; McCann, C.M.; Wickstead, F.; Williams, D.J.L. Co-infection of cattle with Fasciola hepatica or F. gigantica and Mycobacterium bovis: A systematic review. PLoS ONE 2019, 14, e0226300. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, D.Y.; Kerimoğlu, U.; Oto, A.; Ergüven, S.; Arslan, S.; Unal, S.; Batman, F.; Bayraktar, Y. Fasciola hepatica infection: Clinical and computerized tomographic findings of ten patients. Turk. J. Gastroenterol. 2006, 17, 40–45. [Google Scholar]
- Howell, A.K.; Tongue, S.C.; Currie, C.; Evans, J.; Williams, D.J.L.; McNeilly, T.N. Co-infection with Fasciola hepatica may increase the risk of Escherichia coli O157 shedding in British cattle destined for the food chain. Prev. Vet. Med. 2018, 150, 70–76. [Google Scholar] [CrossRef]
- Kelly, R.F.; Callaby, R.; Egbe, N.F.; Williams, D.J.L.; Victor, N.N.; Tanya, V.N.; Sander, M.; Ndip, L.; Ngandolo, R.; Morgan, K.L.; et al. Association of Fasciola gigantica co-infection with bovine tuberculosis infection and diagnosis in a naturally infected cattle population in Africa. Front. Vet. Sci. 2018, 5, 214. [Google Scholar] [CrossRef]
- Harris, N.L.; Loke, P. Recent advances in type-2-cell-mediated Immunity: Insights from helminth infection. Immunity 2017, 47, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Palma, M.; Bleich, D.; Loke, P.; Gause, W.C. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014, 7, 753–762. [Google Scholar] [CrossRef]
- Segura, M.; Su, Z.; Piccirillo, C.; Stevenson, M.M. Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 2007, 37, 1887–1904. [Google Scholar] [CrossRef]
- Leroux, L.P.; Nasr, M.; Valanparambil, R.; Tam, M.; Rosa, B.A.; Siciliani, E.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Sci. Rep. 2018, 8, 15921. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.A.; Barreto, M.L.; Rodrigues, L.C.; Cooper, P.J.; Silva, N.B.; Amorim, L.D.; Alcantara-Neves, N.M. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun. 2010, 78, 3160–3167. [Google Scholar] [CrossRef]
- Wammes, L.J.; Hamid, F.; Wiria, A.E.; de Gier, B.; Sartono, E.; Maizels, R.M.; Luty, A.J.F.; Fillie, Y.; Brice, G.T.; Supali, T.; et al. Regulatory T cell in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 2010, 40, 437–442. [Google Scholar] [CrossRef]
- Elias, D.; Britton, S.; Aseffa, A.; Engers, H.; Akuffo, H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-b production. Vaccine 2008, 26, 3897–3902. [Google Scholar] [CrossRef]
- Toulza, F.; Tsang, L.; Ottenhoff, T.H.; Brown, M.; Dockrell, H.M. Mycobacterium tuberculosis-specific CD41 T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur. J. Immunol. 2016, 46, 752–761. [Google Scholar] [CrossRef]
- Needham, C.; Kim, H.T.; Hoa, N.V.; Cong, L.D.; Michael, E.; Drake, L.; Hall, A.; Bundy, D.A. Epidemiology of soil-transmitted nematode infections in Ha Nam Province, Vietnam. Trop. Med. Int. Health. 1998, 3, 904–912. [Google Scholar] [CrossRef]
- Brooker, S.; Miguel, E.; Moulin, S.; Luoba, A.; Bundy, D.; Kremer, M. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East Afr. Med. J. 2000, 77, 157–161. [Google Scholar] [CrossRef]
- Keiser, J.; N’Goran, E.K.; Singer, B.H.; Lengeler, C.; Tanner, M.; Utzinger, J. Association between Schistosoma mansoni and hookworm infections among schoolchildren in Cote d’Ivoire. Acta Trop. 2002, 84, 31–41. [Google Scholar] [CrossRef]
- Keiser, J.; N’Goran, E.K.; Traore, M.; Lohourignon, K.L.; Singer, B.H.; Lengeler, C.; Tanner, M.; Utzinger, J. Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Cote d’Ivoire. J. Parasitol. 2002, 88, 461–466. [Google Scholar]
- Tchuem Tchuente, L.A.; Behnke, J.M.; Gilbert, F.S.; Southgate, V.R.; Vercruysse, J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop. Med. Int. Health 2003, 8, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.M.; Brooker, S.; Geiger, S.M.; Caldas, I.R.; Correa-Oliveira, R.; Hotez, P.J.; Bethony, J.M. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 2006, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Albonico, M.; Allen, H.; Chitsulo, L.; Engels, D.; Gabrielli, A.F.; Savioli, L. Controlling Soil-Transmitted Helminthiasis in Pre-School-Age Children through Preventive Chemotherapy. PLoS Negl. Trop. Dis. 2008, 2, e126. [Google Scholar] [CrossRef] [PubMed]
- Bhengu, K.N.; Singh, R.; Naidoo, P.; Mpaka-Mbatha, M.N.; Nembe-Mafa, N.; Mkhize-Kwitshana, Z.L. Cytokine Responses during Mycobacterium tuberculosis H37Rv and Ascaris lumbricoides costimulation using human THP-1 and Jurkat Cells, and a pilot human tuberculosis and helminth coinfection study. Microorganisms 2023, 11, 1846. [Google Scholar] [CrossRef]
- Abou Holw, S.A.; El-Taweel, H.; El-Abd, E.; Osman, M.M. The serum gastrin level patients with schistosomiasis and fascioliasis. J. Egypt. Soc. Parasitol. 2007, 37, 299–312. [Google Scholar]
- Abou-Basha, L.M.; Salem, A.; Osman, M.; el-Hefni, S.; Zaki, A. Hepatic fibrosis due to fascioliasis and/or schistosomiasis in Abis 1 village, Egypt. East Mediterr. Health J. 2000, 6, 870–878. [Google Scholar] [CrossRef]
- Shousha, S.A.; Khalil, S.S.; Rashwan, E.A. Oxygen free radical and nitric oxide production in single or combined human schistosorniasis and fascioliasis. J. Egypt. Soc. Parasitol. 1999, 29, 149–156. [Google Scholar]
- Salem, A.l.; Basha, L.M.A.; Farag, H.F. Immunoglobulin levels and intensity of infection in patients with fascioliasis single or combined with schistosomiasis. J. Egypt. Soc. Parasitol. 1987, 17, 33–40. [Google Scholar]
- Yabe, J.; Phiri, I.K.; Phiri, A.M.; Chembensofu, M.; Dorny, P.; Vercruysse, J. Concurrent infections of Fasciola, Schistosoma and Amphistomum spp. in cattle from Kafue and Zambezi river basins of Zambia. J. Helminthol. 2008, 82, 373–376. [Google Scholar] [CrossRef]
- Bundy, D.A.; Medley, G.F. Immunoepidemiology of human geohelminthiasis: Ecological and immunological determinants of worm burden. Parasitology 1992, 104, S105–S119. [Google Scholar] [CrossRef]
- Pullan, R.; Brooker, S. The health impact of polyparasitism in humans: Are we under-estimating the burden of parasitic diseases? Parasitology 2008, 135, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Haswell-Elkins, M.R.; Elkins, D.B.; Anderson, R.M. Evidence for predisposition in humans to infection with Ascaris, hookworm, Enterobius and Trichuris in a South Indian fishing community. Parasitology 1987, 95, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.V.; Asaolu, S.O.; Crompton, D.W.; Stoddart, R.C.; MacDonald, R.; Torimiro, S.E. The epidemiology of Ascaris lumbricoides and other soil-transmitted helminths in primary school children from Ile-Ife, Nigeria. Parasitology 1989, 99, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Chamone, M.; Marques, C.A.; Atuncar, G.S.; Pereira, A.L.; Pereira, L.H. Are there interactions between schistosomes and intestinal nematodes? Trans. Roy. Soc. Trop. Med. Hyg. 1990, 84, 557–558. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Ferreira, M.U.; Nogueira, M.R. The prevalence of infection by intestinal parasites in an urban slum in Sao Paulo, Brazil. J. Trop. Med. Hyg. 1994, 97, 121–127. [Google Scholar] [PubMed]
- Kightlinger, L.K.; Seed, J.R.; Kightlinger, M.B. The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. J. Parasitol. 1995, 81, 159–169. [Google Scholar] [CrossRef]
- Booth, M.; Bundy, D.A.; Albonico, M.; Chwaya, H.M.; Alawi, K.S.; Savioli, L. Associations among multiple geohelminth species infections in schoolchildren from Pemba Island. Parasitology 1998, 116, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, H.; Turner, J.; Behnke, J.; Kamgno, J.; Rowlinson, M.C.; Bradley, J.E.; Boussinesq, M. Associations between filarial and gastrointestinal nematodes. Trans. Roy. Soc. Trop. Med. Hyg. 2005, 99, 301–312. [Google Scholar] [CrossRef]
- Nacher, M. Interactions between worm infections and malaria. Clin. Rev. Allergy Immunol. 2004, 26, 85–92. [Google Scholar] [CrossRef]
- Druilhe, P.; Tall, A.; Sokhna, C. Worms can worsen malaria: Towards a new means to roll back malaria? Trends Parasitol. 2005, 21, 359–362. [Google Scholar] [CrossRef]
- Mwangi, T.W.; Bethony, J.M.; Brooker, S. Malaria and helminth interactions in humans: An epidemiological viewpoint. Ann. Trop. Med. Parasitol. 2006, 100, 551–570. [Google Scholar] [CrossRef]
- Valero, M.A.; Santana, M.; Morales, M.; Hernandez, J.L.; Mas-Coma, S. Risk of gallstone disease in advanced chronic phase of fascioliasis: An experimental study in a rat model. J. Infect. Dis. 2003, 188, 787–793. [Google Scholar] [CrossRef]
- Valero, M.A.; Gironès, N.; Garcia-Bodelon, M.A.; Periago, M.V.; Chico-Calero, I.; Khoubbane, M.; Fresno, M.; Mas-Coma, S. Anaemia in advanced chronic fasciolosis. Acta Trop. 2008, 108, 35–43. [Google Scholar] [CrossRef]
- Powrie, F.; Leach, M.W.; Mauze, S.; Menon, S.; Caddle, L.B.; Coffman, R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1994, 1, 553–562. [Google Scholar] [CrossRef]
- McKinley, L.; Logar, A.J.; McAllister, F.; Zheng, M.; Steele, C.; Kolls, J.K. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of Pneumocystis pneumonia. J. Immunol. 2006, 177, 6215–6226. [Google Scholar] [CrossRef]
- Hesse, M.; Piccirillo, C.A.; Belkaid, Y.; Prufer, J.; Mentink-Kane, M.; Leusink, M.; Cheever, A.W.; Shevach, E.M.; Wynn, T.A. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 2004, 172, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S.; Azkur, A.K.; Kim, B.S.; Kumaraguru, U.; Rouse, B.T. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 2004, 172, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Ezeamama, A.E.; Friedman, J.F.; Olveda, R.M.; Acosta, L.P.; Kurtis, J.D.; Mor, V.; McGarvey, S.T. Functional significance of low-intensity polyparasite helminth infections in anemia. J. Infect. Dis. 2005, 192, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Antoli, C.; Pérez, P.; Pavón, A.; Toledo, R.; Esteba, J.G. Soil-transmitted helminth infections and anemia in schoolchildren from Corn Island Archipelago (RAAS, Nicaragua). Am. J. Trop. Med. Hyg. 2018, 99, 1591–1597. [Google Scholar] [CrossRef]
- Sardinha-Silva, A.; Alves-Ferreira, E.V.C.; Grigg, M.E. Intestinal immune responses to commensal and pathogenic protozoa. Front. Immunol. 2022, 13, 963723. [Google Scholar] [CrossRef]


| Country | Bolivia (n = 1195) | Peru (n = 701) | Egypt (n = 679) | Total (n = 2575) | |
|---|---|---|---|---|---|
| Geographical area | Northern Bolivian Altiplano—between Lake Titicaca and La Paz | Peruvian Altiplano—Asillo zone, Puno n = 339 | Cajamarca valley n = 362 | Behera Governorate, Nile Delta | |
| Transmission pattern | Altiplanic | Altiplanic | Valley | Eastern Mediterranean | |
| Altitude m a.s.l. | 3820–4100 m | 3910 m | 2627–3061 m | Lowlands at 4–20 m | |
| Study population | % | % | % | % | % |
| Male sex | 56.1% | 58.4% | 51.4% | 33.4% | 49.7% |
| Age (years) | |||||
| <7 | 9.5% | 10.9% | 14.4% | 18.7% | 12.8% |
| 7–9 | 34.8% | 50.1% | 43.7% | 7.2% | 30.9% |
| 10–12 | 37.1% | 30.7% | 30.2% | 11.9% | 28.6% |
| 13–15 | 15.3% | 8.3% | 8.9% | 9.8% | 12.0% |
| 16–18 | 3.1% | 0% | 1.1% | 6.8% | 3.4% |
| >18 | 0.2% | 0% | 1.7% | 45.4% | 12.3% |
| Age mean ± IQR (Range) | 10.0 ± 4 (2–19) | 9.0 ± 4 (5–15) | 9.0 ± 4 (2–72) | 16 ± 4 (1–80) | 10.00 ± 5 (1–80) |
| Geographical Area | Bolivia (n = 1195) | Peru (n = 701) | Egypt (n = 679) | Total (n = 2575) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Puno (n = 339) | Cajamarca (n = 362) | |||||||||
| Species | % | % | % | % | % | |||||
| Protozoans * | ||||||||||
| Blastocystis sp. | 43.2 | 84.0 | 73.4 | 41.5 | 52.4 | |||||
| Entamoeba coli | 86.7 | 91.1 | 75.3 | 34.0 | 71.9 | |||||
| Entamoeba histolytica a | 19.9 | 39.6 | 33.0 | 7.1 | 21.0 | |||||
| Entamoeba hartmanni | 13.3 | 91.1 | 41.2 | 15.5 | 28.1 | |||||
| Endolimax nana | 54.1 | 47.0 | 52.5 | 10.6 | 41.5 | |||||
| Iodamoeba buetschlii | 12.6 | 54.7 | 37.6 | 5.9 | 19.9 | |||||
| Chilomastix mesnili | 8.9 | 7.1 | 6.0 | 2.5 | 6.6 | |||||
| Giardia intestinalis | 23.9 | 30.2 | 27.2 | 19.4 | 24.1 | |||||
| Enteromonas hominis | 1.2 | 0.6 | 0.5 | 1.3 | 1.0 | |||||
| Dientamoeba fragilis | 0.3 | 0 | 0 | 0.0 | 0.1 | |||||
| Cryptosporidium sp. | 12.6 | 0 | 0 | 0 | 5.8 | |||||
| Balantidium coli | 1.5 | 0.9 | 0.3 | 0.3 | 0.9 | |||||
| Total number of protozoa * species | 12 | 10 | 10 | 10 | 12 | |||||
| Helminths | ||||||||||
| Fasciola spp. | 16.8 ** | 201 | 24.3 | 78 | 21.4 | 83 | 13.0 | 88 | 17.5 | 450 |
| Schistosoma mansoni | 0.0 | 0 | 0 | 12.1 | 3.2 | |||||
| Hymenolepis nana | 8.6 | 16.9 | 15.7 | 4.9 | 9.7 | |||||
| Taenia ssp. | 4.7 | 0.3 | 0.8 | 0.0 | 2.3 | |||||
| Trichuris trichiura | 8.8 | 18.3 | 7.1 | 1.9 | 8.0 | |||||
| Ascaris lumbricoides | 10.8 | 8.0 | 14.3 | 4.0 | 9.1 | |||||
| Ancylostomatidae spp. b | 0.2 | 0.6 | 0 | 0.1 | 0.2 | |||||
| Strongyloides stercolaris c | 0.0 | 0.3 | 0 | 0.0 | 0.1 | |||||
| Enterobius vermicularis c | 8.5 | 1.5 | 1.8 | 2.8 | 6.0 | |||||
| STH | 17.15 | 25.36 | 18.23 | 17.37 | 18.44 | |||||
| Total number of helminth species | 7 | 8 | 6 | 7 | 8 | |||||
| Total number of parasite species | 19 | 18 | 16 | 17 | 20 | |||||
| % population infected with at least one species | 97.9 | 100 | 97.3 | 72.7 | ||||||
| % individuals with fascioliasis and coinfections | 96.5 | 100 | 98.7 | 84.1 | ||||||
| % individuals with fascioliasis and without coinfections | 3.5 | 0.0 | 1.3 | 15.9 | ||||||
| Total | Females | Males | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | PC | Prevalence (95% CI) | PC | Prevalence (95% CI) | PC | Prevalence (95% CI) | |||
| Bolivia (n = 1195) | <7 | 23 | 9.5% (6.3, 13.7) | 12 | 10.6% (5.8, 18.2) | 11 | 8.5% (4.5, 15.1) | ||
| 7–9 | 38 | 13.2% (9.6, 17.5) | 22 | 15.5% (10.2, 22.7) | 16 | 10.9% (6.6, 17.4) | |||
| 10–12 | 97 | 21.9% (18.3, 25.9) | 44 | 22.2% (16.8, 28.8) | 53 | 21.7% (16.8, 27.5) | |||
| 13–15 | 37 | 20.2% (14.9, 26.5) | 7 | 10.9% (4.9, 21.8) | 30 | 25.2% (17.9, 34.1) | |||
| 16–18 | 6 | 16.2 % (6.8, 30.7) | 2 | 25.0% (4.4, 64.4) | 4 | 13.8% (4.5, 32.6) | |||
| >18 | - | - | - | - | - | - | |||
| Total | 201 | 16.8% (14.8, 19.0) | 87 | 16.6% (13.5, 20.1) | 114 | 17.0% (14.3, 20.1) | |||
| p-value * | <0.001 | <0.001 | |||||||
| Peru (n = 701) | Puno (n = 339) | <7 | 26 | 25.2% (17.4, 34.9) | 11 | 28.2% (15.8, 43.7) | 15 | 23.4% (14.3, 34.9) | |
| 7–9 | 28 | 27.2% (19.1, 37.0) | 13 | 25.5% (15.0, 38.7) | 15 | 28.9% (17.8, 42.2) | |||
| 10–12 | 25 | 24.0% (16.4, 33.6) | 11 | 28.2% (15.8, 43.7) | 14 | 21.5% (12.8, 32.7) | |||
| 13–15 | 4 | 14.3% (4.7, 33.6) | 1 | 8.3% (0.5, 34.7) | 3 | 18.7% (5.0, 43.0) | |||
| 16–18 | - | - | - | - | - | - | |||
| >18 | - | - | - | - | - | - | |||
| Total | 83 | 24.5% (20.1, 29.6) | 36 | 25.5% (18.8, 33.2) | 47 | 23.9% (18.3, 30.2) | |||
| p-value * | <0.001 | <0.001 | |||||||
| Cajamaca (n = 362) | <7 | 21 | 20.8% (13.6, 30.2) | 10 | 22.2% (11.9, 36.0) | 11 | 20.7% (11.4, 33.2) | ||
| 7–9 | 21 | 19.1% (12.5, 27.9) | 12 | 20.3% (11.5, 32.0) | 9 | 17.3% (8.8, 29.4) | |||
| 10–12 | 26 | 23.8% (16.4, 33.1) | 13 | 26.5% (15.6, 40.1) | 13 | 21.7% (12.6, 33.4) | |||
| 13–15 | 8 | 25.0% (12.1, 43.7) | 2 | 14.3% (2.5, 39.7) | 6 | 33.3% (14.8, 56.9) | |||
| 16–18 | 1 | 25.0% (1.3, 78.0) | - | - | 1 | 50.0% (2.5, 97.5) | |||
| >18 | 1 | 16.7% (0.9, 66.5) | 1 | 20.0% (1.0, 66.6) | - | ||||
| Total | 78 | 21.5% (17.5, 26.2) | 38 | 21.8% (16.2, 28.4) | 40 | 21.3% (15.9, 27.6) | |||
| p-value * | <0.05 | <0.001 | |||||||
| Egypt (n = 679) | <7 | 13 | 8.5% (4.8, 13.8) | 10 | 10.4% (5.4, 17.8) | 3 | 5.4% (1.4, 13.9) | ||
| 7–9 | 8 | 30.7% (15.4, 50.2) | 6 | 35.3% (15.7, 59.5) | 2 | 22.2% (3.9, 56.2) | |||
| 10–12 | 12 | 14.8% (8.3, 23.8) | 8 | 17.8% (8.6, 31.0) | 4 | 11.1% (3.6, 24.7) | |||
| 13–15 | 11 | 16.7% (9.1, 27.1) | 8 | 17.4% (8.4, 30.4) | 3 | 15.0% (4.0, 35.6) | |||
| 16–18 | 4 | 8.7% (2.8, 19.6) | 4 | 11.8% (3.8, 26.0) | - | - | |||
| >18 | 40 | 13.0% (9.5, 17.4) | 31 | 9.8% (6.3, 14.8) | 9 | 9.6% (4.7, 17.8) | |||
| Total | 88 | 13.0% (10.6, 15.7) | 67 | 14.8% (11.7, 18.5) | 21 | 6.7% (6.2, 14.6) | |||
| p-value * | <0.001 | <0.001 | |||||||
| Fasciola-Infected Subjects | Subjects Not Infected by Fasciola | p-Value b | |||||
|---|---|---|---|---|---|---|---|
| N | Prevalence (%) | (95% CI) | n | Prevalence (%) | (95% CI) | ||
| Parasite species (protozoans * and helminths) | |||||||
| 0 | 22 | 4.89 | (3.25, 7.29) | 220 | 10.36 | (9.13, 11.72) | 0.0002 |
| 1 | 45 | 10.00 | (7.56, 13.12) | 266 | 12.53 | (11.18, 13.99) | 0.1517 |
| 2 | 84 | 18.67 | (15.34, 22.53) | 364 | 17.15 | (15.59, 18.79) | 0.4517 |
| 3 | 85 | 18.89 | (15.54, 22.76 | 443 | 20.87 | (19.17, 22.63) | 0.3686 |
| 4 | 87 | 19.33 | (15.95, 23.24 | 358 | 16.86 | (15.32, 18.50) | 0.2168 |
| 5 | 57 | 12.67 | (9.91, 16.06) | 244 | 11.49 | (10.20, 12.91) | 0.4684 |
| 6 | 44 | 9.78 | (7.37, 12.87) | 152 | 7.16 | (6.14, 8.33) | 0.0629 |
| 7 | 22 | 4.89 | (3.25, 7.28) | 65 | 3.06 | (2.41, 3.88) | 0.061 |
| 8 | 4 | 0.89 | (0.35, 2.26) | 11 | 0.52 | (0.29, 0.93) | 0.3151 |
| 9 | 0 | 0.00 | (0.00, 0.85) | 2 | 0.09 | (0.02, 0.34) | - |
| Total | 450 | 2125 | |||||
| Protozoan * species | |||||||
| 0 | 29 | 6.44 | (4.53, 9.11) | 273 | 12.86 | (11.49, 13.34) | <0.0001 |
| 1 | 59 | 13.11 | (10.30, 16.54) | 286 | 13.47 | (12.07, 14.98) | 0.8791 |
| 2 | 101 | 22.44 | (18.83, 26.52) | 433 | 20.40 | (18.72, 22.14) | 0.3373 |
| 3 | 95 | 21.11 | (17.59, 25.12) | 483 | 22.75 | (20.99, 24.56) | 0.494 |
| 4 | 70 | 15.56 | (12.50, 19.19) | 325 | 15.31 | (13.83, 16.89) | 0.8857 |
| 5 | 57 | 12.67 | (9.90, 16.06) | 197 | 9.28 | (8.11, 10.58) | 0.0364 |
| 6 | 32 | 7.11 | (5.08, 9.87) | 104 | 4.90 | (4.06, 5.90) | 0.0632 |
| 7 | 7 | 1.56 | (0.76, 3.18) | 23 | 1.08 | (0.72, 1.62) | 0.4652 |
| 8 | 0 | 0.00 | (0.00, 0.85) | 1 | 0.05 | (0.00, 0.27) | - |
| Total | 450 | 2125 | |||||
| Helminth species a | |||||||
| 0 | 0 | 0.00 | (0.00, 0.845) | 1492 | 70.28 | (68.23, 72.12) | <0.0001 |
| 1 | 284 | 63.11 | (58.56, 67.44) | 511 | 24.07 | (22.28, 25.91) | <0.0001 |
| 2 | 135 | 30.00 | (29.95, 34.40) | 108 | 5.09 | (4.23, 6.10) | <0.0001 |
| 3 | 29 | 6.44 | (4.52, 9.10) | 14 | 0.66 | (0.39, 1.10) | <0.0001 |
| 4 | 2 | 0.44 | (0.08, 1.61) | 0 | 0.00 | (0.00, 0.18) | - |
| Total | 450 | 2125 | |||||
| Parasite Species | Fasciola-Infected Subjects | Subjects Not Infected by Fasciola | All Cases | ≤18 Years | >18 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Cases | ≤18 Years | >18 Years | All Cases | ≤18 Years | >18 Years | p- Value g | p- Value h | p- Value i | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Protozoa * | |||||||||||||||
| Blastocystis sp. | 238 | 52.89 | 219 | 53.55 | 19 | 47.50 | 1110 | 52.24 | 999 | 54.00 | 112 | 40.43 | 0.835 | 0.870 | 0.397 |
| Entamoeba coli | 356 | 79.11 | 340 | 83.13 | 16 | 40.00 | 1492 | 70.21 | 1408 | 76.11 | 86 | 31.05 | 0.0001 | 0.002 | 0.279 |
| Entamoeba histolytica a | 94 | 20.89 | 87 | 21.27 | 7 | 17.50 | 446 | 20.99 | 426 | 23.03 | 20 | 7.22 | >0.999 | 0.473 | 0.061 |
| Entamoeba hartmanni | 147 | 32.67 | 138 | 33.74 | 9 | 22.50 | 575 | 27.06 | 539 | 29.14 | 37 | 13.36 | 0.017 | 0.074 | 0.148 |
| Endolimax nana | 208 | 46.22 | 203 | 49.63 | 5 | 12.50 | 861 | 40.52 | 833 | 45.03 | 29 | 10.47 | 0.027 | 0.090 | 0.784 |
| Iodamoeba buetschlii | 117 | 26.00 | 114 | 27.87 | 3 | 7.50 | 395 | 18.59 | 386 | 20.86 | 10 | 3.61 | 0.0006 | 0.002 | 0.217 |
| Chilomastix mesnili | 29 | 6.44 | 27 | 6.60 | 2 | 5.00 | 140 | 6.59 | 137 | 7.41 | 3 | 1.08 | >0.999 | 0.674 | 0.121 |
| Giardia intestinalis | 128 | 28.44 | 125 | 30.56 | 3 | 7.50 | 491 | 23.11 | 461 | 24.92 | 30 | 10.83 | 0.017 | 0.021 | 0.781 |
| Enteromonas hominis | 7 | 1.56 | 0 | 0.00 | 1 | 2.50 | 23 | 1.08 | 8 | 0.43 | 4 | 1.44 | 0.465 | 0.383 | 0.493 |
| Dientamoeba fragilis b | 2 | 0.99 | 2 | 1.00 | 0 | 0.00 | 1 | 0.10 | 1 | 0.10 | 0 | 0.00 | 0.081 | 0.075 | |
| Cryptosporidium sp. b | 26 | 12.94 | 26 | 12.94 | 0 | 0.00 | 124 | 5.83 | 124 | 12.50 | 0 | 0.00 | 0.387 | 0.908 | |
| Balantidium coli | 3 | 0.67 | 3 | 0.73 | 0 | 0.00 | 21 | 0.98 | 20 | 1.08 | 1 | 0.36 | 0.786 | 0.785 | 0.260 |
| Helminths | |||||||||||||||
| Schistosoma mansoni c | 18 | 20.45 | 14 | 29.17 | 4 | 10.26 | 64 | 10.82 | 39 | 12.07 | 25 | 9.33 | 0.043 | 0.004 | 0.774 |
| Hymenolepis nana | 51 | 11.33 | 50 | 12.22 | 1 | 2.50 | 199 | 9.36 | 196 | 10.59 | 3 | 1.08 | 0.219 | 0.335 | 0.419 |
| Taenia ssp | 11 | 2.44 | 11 | 2.69 | 0 | 0.00 | 49 | 2.31 | 49 | 2.65 | 0 | 0.00 | 0.863 | >0.999 | |
| Trichuris trichiura | 43 | 9.56 | 42 | 10.27 | 1 | 2.50 | 163 | 7.67 | 163 | 8.81 | 0 | 0.00 | 0.181 | 0.343 | 0.126 |
| Ascaris lumbricoides | 47 | 10.44 | 45 | 11.00 | 1 | 2.50 | 188 | 8.85 | 186 | 10.05 | 2 | 0.72 | 0.280 | 0.588 | 0.334 |
| Ancylostomatidae spp. d | 2 | 0.54 | 2 | 0.49 | 0 | 0.00 | 3 | 0.16 | 3 | 0.16 | 0 | 0.00 | 0.212 | 0.169 | |
| Strongyloides stercolaris e | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.39 | 1 | 0.39 | 0 | 1.08 | >0.999 | 0.552 | |
| Enterobius vermicularis | 27 | 6.00 | 26 | 6.36 | 1 | 2.50 | 102 | 4.80 | 99 | 5.35 | 3 | 0.00 | 0.285 | 0.445 | 0.419 |
| STH f | 350 | 77.78 | 93 | 22.74 | 6 | 15.00 | 375 | 17.65 | 348 | 18.81 | 27 | 9.75 | <0.0001 | 0.073 | 0.280 |
| Parasite Species | Fasciola-Infected Subjects | Subjects Not Infected by Fasciola | All Cases | ≤18 Years | >18 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Cases | ≤18 Years | >18 Years | All Cases | ≤18 Years | >18 Years | p- Value a | p- Value b | p- Value c | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Monoinfection by protozoan * parasites | 39 | 8.67 | 28 | 6.85 | 11 | 26.83 | 216 | 10.2 | 167 | 9.03 | 50 | 18.05 | 0.385 | 0.173 | 0.203 |
| Monoinfection by helminth parasites | 6 | 1.33 | 3 | 0.73 | 3 | 7.32 | 50 | 2.4 | 35 | 1.89 | 15 | 5.42 | 0.214 | 0.134 | 0.714 |
| Coinfection by protozoan *–helminth parasites | 159 | 35.33 | 154 | 37.65 | 5 | 12.20 | 580 | 27.3 | 562 | 30.38 | 18 | 6.50 | 0.001 * | 0.005 * | 0.196 |
| Coinfection by protozoan *–protozoan parasites | 223 | 49.56 | 207 | 50.61 | 16 | 39.02 | 1056 | 49.7 | 964 | 52.11 | 93 | 33.57 | 0.959 | 0.585 | 0.496 |
| Coinfection by helminth–helminth parasites | 1 | 0.22 | 1 | 0.24 | 0 | 0.00 | 3 | 0.1 | 3 | 0.16 | 0 | 0.00 | 0.536 | 0.556 | - |
| Negative | 22 | 4.89 | 16 | 3.91 | 6 | 14.63 | 220 | 10.4 | 119 | 6.43 | 101 | 36.46 | 0.0001 * | 0.051 * | 0.005 * |
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | |
| Geographical location | 1.02 | (0.87, 1.16) | 0.98 | |||||||||
| Sex | 0.92 | (0.71, 1.18) | 0.51 | |||||||||
| Age | 1.81 | (1.17, 2.80) | 0.007 | 0.69 | (0.48, 0.97) | 0.03 | 0.91 | (0.63, 1.32) | 0.63 | |||
| Protozoans * | 1.03 | (0.96, 1.11) | 0.37 | 1.07 | (0.99, 1.16) | 0.10 | ||||||
| Helminths | 7.51 | (6.25, 9.03) | <0.001 | 7.78 | (6.50, 9.46) | <0.001 | ||||||
| E. coli | 1.37 | (1.04, 1.80) | 0.02 | |||||||||
| E. hartmanni | 1.09 | (0.86, 1.39) | 0.43 | |||||||||
| E. nana | 1.08 | (0.86, 1.34) | 0.48 | |||||||||
| I. buetschlii | 1.32 | (1.02, 1.72) | 0.03 | |||||||||
| G. intestinalis | 1.26 | (1.00, 1.59) | 0.04 | |||||||||
| STH | 1.31 | (1.01, 1.67) | 0.03 | 1.28 | (1.00, 1.65) | 0.04 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, M.A.; Morales-Suarez-Varela, M.M.; Marquez-Guzman, D.J.; Angles, R.; Espinoza, J.R.; Ortiz, P.; Curtale, F.; Bargues, M.D.; Mas-Coma, S. Helminth/Protozoan Coinfections in Chronic Fascioliasis Cases in Human Hyperendemic Areas: High Risk of Multiparasitism Linked to Transmission Aspects and Immunological, Environmental and Social Factors. Trop. Med. Infect. Dis. 2025, 10, 224. https://doi.org/10.3390/tropicalmed10080224
Valero MA, Morales-Suarez-Varela MM, Marquez-Guzman DJ, Angles R, Espinoza JR, Ortiz P, Curtale F, Bargues MD, Mas-Coma S. Helminth/Protozoan Coinfections in Chronic Fascioliasis Cases in Human Hyperendemic Areas: High Risk of Multiparasitism Linked to Transmission Aspects and Immunological, Environmental and Social Factors. Tropical Medicine and Infectious Disease. 2025; 10(8):224. https://doi.org/10.3390/tropicalmed10080224
Chicago/Turabian StyleValero, M. Adela, M. Manuela Morales-Suarez-Varela, Davis J. Marquez-Guzman, Rene Angles, Jose R. Espinoza, Pedro Ortiz, Filippo Curtale, M. Dolores Bargues, and Santiago Mas-Coma. 2025. "Helminth/Protozoan Coinfections in Chronic Fascioliasis Cases in Human Hyperendemic Areas: High Risk of Multiparasitism Linked to Transmission Aspects and Immunological, Environmental and Social Factors" Tropical Medicine and Infectious Disease 10, no. 8: 224. https://doi.org/10.3390/tropicalmed10080224
APA StyleValero, M. A., Morales-Suarez-Varela, M. M., Marquez-Guzman, D. J., Angles, R., Espinoza, J. R., Ortiz, P., Curtale, F., Bargues, M. D., & Mas-Coma, S. (2025). Helminth/Protozoan Coinfections in Chronic Fascioliasis Cases in Human Hyperendemic Areas: High Risk of Multiparasitism Linked to Transmission Aspects and Immunological, Environmental and Social Factors. Tropical Medicine and Infectious Disease, 10(8), 224. https://doi.org/10.3390/tropicalmed10080224









