Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Laboratory Tests
2.3. Assessment of Liver Elasticity (Fibroscan Elastography)
2.4. Serum Cytokine Levels
2.5. Statistical Analysis
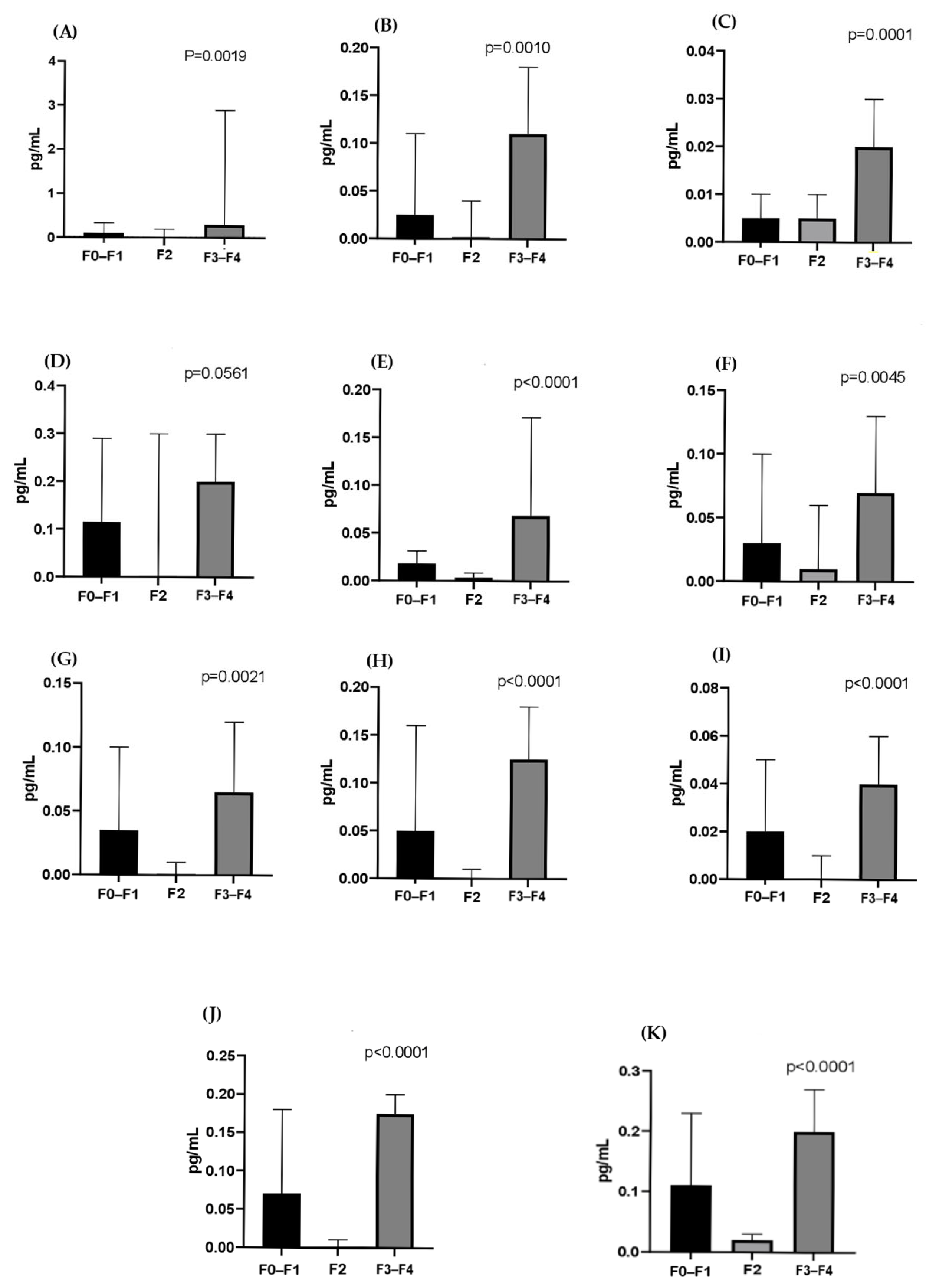
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. 2024. Available online: https://www.who.int/news (accessed on 20 July 2024).
- Nascimento, S.M.L.; Fabris, M.E.M.; Barros, J.M.; Ribeiro, L.M.; Frizanco, A.B.; Santiago, A.L.P.; Hoffmann-Santos, H.D.; Nogueira, P.L.B. Transplante de fígado no Brasil entre 2010 e 2021: Sobrevida de 30 dias. Braz. J. Transplant. 2023, 26, e3823. [Google Scholar] [CrossRef]
- Gonçalves, N.V.; Vieira, D.C.; Miranda, C.d.S.C.; Palácios, V.R.d.C.M.; Costa, S.B.N.; Guedes, J.A.; Santos, B.d.O.; Costa, R.J.F.; Silva, S.C.M.; Oliveira, R.A.C. Análise Espacial e Epidemiológica de Hepatites B e C e Índice de Desenvolvimento Humano Municipal, no Estado Do Pará. Hygeia 2019, 15, 29–42. [Google Scholar] [CrossRef]
- Konstantinou, D.; Deutsch, M. The spectrum of HBV/HCV coinfection: Epidemiology, clinical characteristics, viral interactions and management. Ann. Gastroenterol. 2015, 28, 221–228. [Google Scholar] [PubMed]
- Yan, L.B.; Rao, H.Y.; Ma, Y.J.; Bai, L.; Chen, E.Q.; Du, L.Y.; Yang, R.F.; Wei, L.; Tang, H.; CCgenos Study Group. Hepatitis B virus infection in Chinese patients with hepatitis C virus infection: Prevalence, clinical characteristics, viral interactions and host genotypes: A nationwide cross-sectional study. BMJ Open 2016, 6, e012016. [Google Scholar] [CrossRef]
- Sampaio, R.M.A.; Dantas, P.E.F.; da Silva, M.I.C.; da Silva, J.R.; Nunes, P.F.; Gomes, A.C.; Martins, L.C. Comparison of Patients Monoinfected with Hepatitis C Virus and Coinfected with Hepatitis B/C in the Amazon Region of Brazil. Viruses 2022, 14, 856. [Google Scholar] [CrossRef]
- Alberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; Lesi, O.A.; Hutin, Y.J.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chen, P.J. Changing epidemiology of liver disease in Asia: Dual infection of HBV and HCV. Liver Int. 2022, 42, 1945–1954. [Google Scholar] [CrossRef]
- Pamarthy, R.; Ali, H.; Kapuria, D. Comparison of inpatient outcomes in patients with Hepatitis B, Hepatitis C, and Hepatitis B and C co-infection with Cirrhosis. Ir. J. Med. Sci. 2024, 193, 157–163. [Google Scholar] [CrossRef]
- Marot, A.; Belaid, A.; Orlent, H.; Sersté, T.; Michielsen, P.; Colle, I.; Laleman, W.; de Galocsy, C.; Reynaert, H.; D’Heygere, F.; et al. Characteristics of patients with hepatitis B virus and hepatitis C virus dual infection in a Western European country: Comparison with monoinfected patients. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 656–663. [Google Scholar] [CrossRef]
- Tseng, C.W.; Liu, W.C.; Chen, C.Y.; Chang, T.T.; Tseng, K.C. Impact of HCV viremia on HBV biomarkers in patients coinfected with HBV and HCV. BMC Infect. Dis. 2022, 22, 351. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Iqbal, M.; Younas, S.; Hussain, N.; Mahnoor, M.; Wajid, A. Hepatitis C virus/Hepatitis B virus coinfection: Current perspectives. Antivir. Ther. 2023, 28, 13596535231189643. [Google Scholar] [CrossRef]
- Paquissi, F.C. Immunity and Fibrogenesis: The Role of Th17/IL-17 Axis in HBV and HCV-induced Chronic Hepatitis and Progression to Cirrhosis. Front. Immunol. 2017, 8, 1195. [Google Scholar] [CrossRef] [PubMed]
- Amoras, E.S.G.; de Brito, W.B.; Queiroz, M.A.F.; Conde, S.R.S.d.S.; Cayres Vallinoto, I.M.V.; Ishak, R.; Vallinoto, A.C.R. The Genetic Profile and Serum Level of IL-8 Are Associated with Chronic Hepatitis B and C Virus Infection. Biomolecules 2021, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Chen, J.; Kozlowski, M.; Yuan, Z. Innate detection of hepatitis B and C virus and viral inhibition of the response. Cell. Microbiol. 2015, 17, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, L.; Lian, Y.; Gu, L.; Chen, Y.; Bi, Y.; Huang, Z.; Huang, Y.; Hu, B.; Huang, Y. Serum HBV pregenomic RNA is correlated with Th1/Th2 immunity in treatment-naïve chronic hepatitis B patients. J. Med. Virol. 2020, 92, 317–328. [Google Scholar] [CrossRef]
- Hazari, S.; Acharya, S.K.; Panda, S.K. Development and evaluation of a quantitative competitive reverse transcription polymerase chain reaction (RT-PCR) for hepatitis C virus RNA in serum using transcribed thio-RNA as internal control. J. Virol. Met. 2003, 116, 45–54. [Google Scholar] [CrossRef]
- Bonnard, P.; Elsharkawy, A.; Zalata, K.; Delarocque-Astagneau, E.; Biard, L.; Le Fouler, L.; Hassan, A.B.; Abdel-Hamid, M.; El-Daly, M.; Gamal, M.E.; et al. Comparison of liver biopsy and noninvasive techniques for liver fibrosis assessment in patients infected with HCV-genotype 4 in Egypt. J. Viral. Hepat. 2014, 22, 245–253. [Google Scholar] [CrossRef]
- BRASIL. Ministério da Saúde. Hepatites Virais. Boletim Epidemiológico. 2021. Available online: www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2021/boletim-epidemiologico-de-hepatite-2021.pdf (accessed on 16 August 2022).
- BRASIL. Ministério da Saúde. Hepatites Virais: Vigilância Epidemiológica e Desafios. Brasília: Ministério da Saúde. 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/hepatites/hepavi.pdf (accessed on 4 November 2024).
- Desikan, P.; Rangnekar, A.; Khan, Z.; Panwalkar, N.; Bose, P.; Gulwani, H.V.; Kaur, S. Sero-Occurrence of HBV/HCV Coinfection and Levels of Liver Enzymes among Patients at a Tertiary Care Hospital in Central India: A Pilot Study. Cent. Asian J. Glob. Health 2019, 8, 313. [Google Scholar] [CrossRef][Green Version]
- BRASIL; Ministério da Saúde. Hepatites Virais: O Brasil Está Atento, 3rd ed.; Ministério da Saúde: Brasília, Brazil, 2008. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/hepatites_virais_brasil_atento_3ed.pdf (accessed on 5 January 2025).
- Shi, J.; Zhu, L.; Liu, S.; Xie, W.F. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br. J. Cancer 2005, 92, 607–612. [Google Scholar] [CrossRef]
- Murai, K.; Hikita, H.; Kai, Y.; Kondo, Y.; Fukuoka, M.; Fukutomi, K.; Doi, A.; Yamai, T.; Nakabori, T.; Fukuda, R.; et al. Hepatitis C virus infection suppresses hepatitis B virus replication via the RIG-I-like helicase pathway. Sci. Rep. 2020, 10, 941. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, K.; Su, X.; Gao, X.; Yao, Y.; Kong, R.; Wang, Y.; Wu, C.; Lu, M.; Chen, X.; et al. Enhanced host immune responses in presence of HCV facilitate HBV clearance in coinfection. Virol. Sin. 2022, 37, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, J.; Liu, W.; Tang, P.; Xie, T.; Huang, L.; Hu, Y.; Jin, K.; Zhang, P.; Liu, Z.; et al. HBV coinfection with HCV alters circulating Tfh cell distribution and impairs HCV neutralizing antibody responses. J. Viral Hepat. 2019, 26, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Lo, S.J.; Miyamura, T.; Chen, S.Y.; Lee, Y.H. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J. Virol. 1993, 67, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Liaw, Y.F.; Chen, M.H.; Huang, C.Y.; Kuo, G.C. Detection of type 2-like T-helper cells in hepatitis C virus infection: Implications for hepatitis C virus chronicity. Hepatology 1997, 25, 449–458. [Google Scholar] [CrossRef]
- Sobue, S.; Nomura, T.; Ishikawa, T.; Ito, S.; Saso, K.; Ohara, H.; Joh, T.; Itoh, M.; Kakumu, S. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J. Gastroenterol. 2001, 36, 544–551. [Google Scholar] [CrossRef]
- R-Viso, A.; Duarte, M.I.S.; Pagliari, C.; Fernandes, E.A.; Brasil, R.A.; Benard, G.; Romano, C.C.; Ogusuk, S.; Cavalheiro, N.P.; de Melo, C.E.; et al. Tissue and serum immune response in chronic hepatitis c with mild histological lesions. Mem. Ins. Oswaldo Cruz 2010, 105, 25–32. [Google Scholar] [CrossRef]
- Akcam, F.Z.; Tigli, A.; Kaya, O.; Ciris, M.; Vural, H. Cytokine levels and histopathology in chronic hepatitis B and chronic hepatitis C. J. Interferon Cytokine Res. 2012, 32, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.J.; Shen, Y.; Li, X.R.; Wei, Y.N.; Fan, H.; Ren, Q.K. Increased interleukin-32, interleukin-1, and interferon-γ levels in serum from hepatitis B patients and in HBV-stimulated peripheral blood mononuclear cells from healthy volunteers. J. Infect. Public Health 2019, 12, 7–12. [Google Scholar] [CrossRef]
- Kohli, R.; Harris, D.; Whitington, P. Elevações relativas de alanina sérica e aspartato aminotransferase na distrofia muscular. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 121–124. [Google Scholar] [CrossRef]
- Coad, J.; Olsen, S.; Rosenberg, W.; Roderick, P.; Parkes, J. Systematic review of performance of ALT in predicting liver fibrosis: ALT is a sensitive, but not a specific marker of liver fibrosis in populations with/suspected liver disease. Gut 2014, 63 (Suppl. S1), A247.1. [Google Scholar] [CrossRef]
- Falasca, K.; Ucciferri, C.; Dalessandro, M.; Zingariello, P.; Mancino, P.; Petrarca, C.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Cytokine Patterns Correlate with Liver Damage in Patients with Chronic Hepatitis B and C. Ann Clin Lab Sci 2006, 36, 144–150. [Google Scholar] [PubMed]
- de Souza-Cruz, S.; Victória, M.B.; Tarragô, A.M.; da Costa, A.G.; Pimentel, J.P.; Pires, E.F.; Araújo, L.d.P.; Coelho-dos-Reis, J.G.; Gomes, M.d.S.; Amaral, L.R.; et al. Liver and blood cytokine microenvironment in HCV patients is associated to liver fibrosis score: A pro-inflamamatory cytokine ensemble orchestrated by TNF and tuned by IL-10. BMC Microbiol. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.S.; Santos, T.P.S.; Santos, P.L.; Schinoni, M.I.; Oliveira, I.S.; Pereira, A.B.; Atta, A.M.; Sousa-Atta, M.L.B. Immune response of Th17-associated cytokines by peripheral blood mononuclear cells from patients with chronic hepatitis C virus infection. Cytokine 2018, 102, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Nafady, A.; Nafady-Hego, H.; Abdelwahab, N.M.; Eltellawy, R.H.N.; Abu Faddan, N.H. Peripheral lymphocytes analyses in children with chronic hepatitis C virus infection. Eur. J. Clin. Investig. 2018, 48, e13004. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abd El-Rehim, A.S.E.; Ahmed, A.O.; Elsherbiny, N.M.; Abo Elhagag, N.A.E. The Impact of Serum Interleukin-17 on Chronic Hepatitis C and Its Sequelae. J. Liver 2014, 3, 163. [Google Scholar] [CrossRef]
- Yang, C.; Cui, F.; Chen, L.M.; Gong, X.Y.; Qin, B. Correlation between Th17 and nTreg cell frequencies and the stages of progression in chronic hepatitis B. Mol. Med. Rep. 2016, 13, 853–859. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A multifunctional cytokine in viral infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef]
- Yoon, H.; Han, J.; Jang, K.L. Hepatitis B Virus X Protein Stimulates Hepatitis C Virus (HCV) Replication by Protecting HCV Core Protein from E6AP-Mediated Proteasomal Degradation. Microbiol. Spectr. 2022, 10, e01432-22. [Google Scholar] [CrossRef]



| HBV/HCV (n = 34) | HBV (n = 22) | HCV (n = 22) | p | |
|---|---|---|---|---|
| Age * | 47.3 (±9.9) | 42.1 (±9.4) | 59.2 (±11.4) | <0.001 |
| Sex M ** | 17 (50%) | 17 (77.3%) | 15 (68.2%) | 0.098 |
| Fibrosis (%) ** | ||||
| F0/F1 | 16 (47.1) | 6 (27.3) | ||
| F2 | 12 (35.3) | 6 (27.3) | 0.050 | |
| F3/F4 | 6 (17.6) | 10 (45.4) | ||
| Viral genotype HCV (%) *** | ||||
| 1 | 22 (64.7) | 20 (91) | 0.278 | |
| 3 | 6 (17.6) | 2 (9) | ||
| Viral charge (IQR) **** | ||||
| DNA HBV | 1.4 (0.22) | 2.1 (0.77) | 0.001 | |
| RNA HCV | 5.5 (0.45) | 6.1 (0.57) | 0.005 | |
| Liver enzymes (IQR) ***** | ||||
| AST (U/mL) | 65.5 (30.5) | 31 (9.5) | 54.5 (30) | <0.001 |
| ALT (U/mL) | 65.0 (40.5) | 30.0 (8.8) | 79.5 (28.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.R.; Sampaio, R.M.A.; Nunes, P.F.; Guimarães, V.S.; Costa, C.C.d.S.; Coelho, E.d.C.; Souza, M.V.d.; Almeida, L.W.C.; Fuzii, H.T.; Filho, A.B.O.; et al. Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon. Trop. Med. Infect. Dis. 2025, 10, 166. https://doi.org/10.3390/tropicalmed10060166
Silva JR, Sampaio RMA, Nunes PF, Guimarães VS, Costa CCdS, Coelho EdC, Souza MVd, Almeida LWC, Fuzii HT, Filho ABO, et al. Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon. Tropical Medicine and Infectious Disease. 2025; 10(6):166. https://doi.org/10.3390/tropicalmed10060166
Chicago/Turabian StyleSilva, Joseane R., Regiane M. A. Sampaio, Patrícia F. Nunes, Vanessa S. Guimarães, Camila Carla da Silva Costa, Evelen da Cruz Coelho, Micheline Vale de Souza, Luana Wanessa Cruz Almeida, Hellen T. Fuzii, Aldemir Branco Oliveira Filho, and et al. 2025. "Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon" Tropical Medicine and Infectious Disease 10, no. 6: 166. https://doi.org/10.3390/tropicalmed10060166
APA StyleSilva, J. R., Sampaio, R. M. A., Nunes, P. F., Guimarães, V. S., Costa, C. C. d. S., Coelho, E. d. C., Souza, M. V. d., Almeida, L. W. C., Fuzii, H. T., Filho, A. B. O., & Martins, L. C. (2025). Immunological and Virological Responses in Patients with Monoinfection and Coinfection with Hepatitis B and C Viruses in the Brazilian Amazon. Tropical Medicine and Infectious Disease, 10(6), 166. https://doi.org/10.3390/tropicalmed10060166






