Cutaneous Larva Migrans Refractory to Therapy with Ivermectin: Case Report and Review of Implicated Zoonotic Pathogens, Epidemiology, Anthelmintic Drug Resistance and Therapy
Abstract
1. Introduction
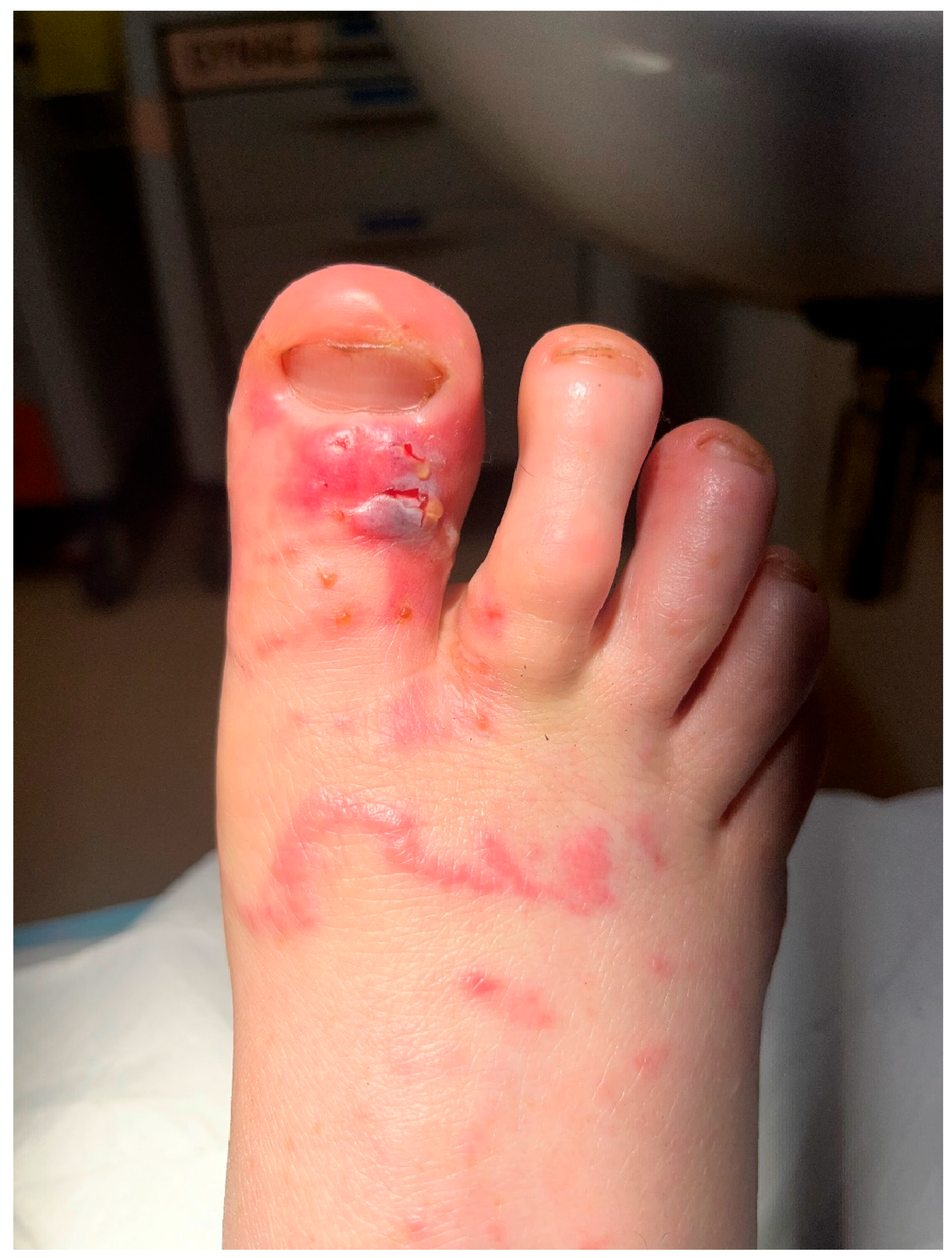
2. Patient Clinical History
3. Exposure History
4. History of Animal Management Programs in the Region Where Infection Occurred
5. Parasites That Could Be Potentially Causing the Cutaneous Larva Migrans and Other Differential Diagnoses
6. Wildlife and Cutaneous Larva Migrans
7. Ivermectin and Other Anthelmintic Resistance in Australia and Globally
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouchaud, O.; Houze, S.; Schiemann, R.; Durand, R.; Ralaimazava, P.; Ruggeri, C.; Coulaud, J.P. Cutaneous larva migrans in travelers: A prospective study, with assessment of therapy with ivermectin. Clin. Infect. Dis. 2000, 31, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kuna, A.; Olszanski, R.; Wroczynska, A.; Biernat, B.; Sikorska, K. Beach volleyball and Cutaneous Larva Migrans. J. Travel. Med. 2024, 31, taad087. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Zendejas-Heredia, P.A.; Massetti, L.; Colella, V. Zoonotic hookworms of dogs and cats—Lessons from the past to inform current knowledge and future directions of research. Int. J. Parasitol. 2021, 51, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Van den Enden, E.; Stevens, A.; Van Gompel, A. Treatment of cutaneous larva migrans. N. Engl. J. Med. 1998, 339, 1246–1247. [Google Scholar]
- Caumes, E. Treatment of cutaneous larva migrans. Clin. Infect. Dis. 2000, 30, 811–814. [Google Scholar] [CrossRef]
- Smith, B.P.; Litchfield, C.A. A review of the relationship between indigenous Australians, dingoes (Canis dingo) and domestic dogs (Canis familiaris). Anthrozoös 2009, 22, 111–128. [Google Scholar] [CrossRef]
- Constable, S.E.; Brown, G.; Dixon, R.; Dixon, R. Healing the hand that feeds you: Exploring solutions for dog and community health and welfare in Australian Indigenous Cultures. Int. J. Interdiscip. Soc. Sci. Annu. Rev. 2008, 3, 219–230. [Google Scholar] [CrossRef]
- Ma, G.C.; Ford, J.; Lucas, L.; Norris, J.M.; Spencer, J.; Withers, A.-M.; Ward, M.P. “They Reckon They’re Man’s Best Friend and I Believe That.” Understanding relationships with dogs in Australian Aboriginal communities to inform effective dog population management. Animals 2020, 10, 810. [Google Scholar] [CrossRef]
- Raw, L. Human health in relation to pets in urban and Indigenous communities. In Rural and Remote Environmental Health I; Australasian College of Tropical Medicine: Brisbane, Australia, 2001; p. 23. [Google Scholar]
- Gaskin, S.; Bentham, R.; Cromar, N.; Fallowfield, H. The zoonotic potential of dogs in Aboriginal communities in central Australia. Environ. Health 2007, 7, 36–45. [Google Scholar]
- Bradbury, L.; Corlette, S. Dog health program in Numbulwar, a remote aboriginal community in east Arnhem Land. Aust. Vet. J. 2006, 84, 317–320. [Google Scholar] [CrossRef]
- Kennedy, B.; Brown, W.Y.; Vernes, K.; Körtner, G.; Butler, J.R.A. Dog and cat interactions in a remote Aboriginal community. Animals 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Dobson, C.; Freeman, C. Distribution and diagnosis of dirofilariasis and toxocariasis in Australia. Aust. Vet. J. 1979, 55, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Andrew, P.L. Intestinal parasites in dogs from an aboriginal community in New South Wales. Aust. Vet. J. 1993, 70, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Thompson, R.C.A.; Hopkins, R.M.; Reynoldson, J.A.; Gracey, M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med. J. Aust. 1993, 158, 157–159. [Google Scholar] [CrossRef]
- Thompson, R.C.; Meloni, B.P.; Hopkins, R.M.; Deplazes, P.; Reynoldson, J.A. Observations on the endo- and ectoparasites affecting dogs and cats in aboriginal communities in the north-west of Western Australia. Aust. Vet. J. 1993, 70, 268–270. [Google Scholar] [CrossRef]
- Palmer, C.S.; Traub, R.J.; Robertson, I.D.; Hobbs, R.P.; Elliot, A.; While, L.; Rees, R.; Thompson, R.C. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet. Parasitol. 2007, 145, 304–313. [Google Scholar] [CrossRef]
- King, J.S.; Brown, G.K.; Jenkins, D.J.; Ellis, J.T.; Fleming, P.J.S.; Windsor, P.A.; Šlapeta, J. Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia. Vet. Parasitol. 2012, 187, 85–92. [Google Scholar] [CrossRef]
- Šlapeta, J.; Dowd, S.E.; Alanazi, A.D.; Westman, M.E.; Brown, G.K. Differences in the faecal microbiome of non-diarrhoeic clinically healthy dogs and cats associated with Giardia duodenalis infection: Impact of hookworms and coccidia. Int. J. Parasitol. 2015, 45, 585–594. [Google Scholar] [CrossRef]
- Smout, F.A.; Skerratt, L.F.; Butler, J.R.A.; Johnson, C.N.; Congdon, B.C.; Thompson, R.C.A. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health 2017, 3, 66–69. [Google Scholar] [CrossRef]
- Beknazarova, M.; Barratt, J.L.N.; Bradbury, R.S.; Lane, M.; Whiley, H.; Ross, K. Detection of classic and cryptic Strongyloides genotypes by deep amplicon sequencing: A preliminary survey of dog and human specimens collected from remote Australian communities. PLoS Neglected Trop. Dis. 2019, 13, e0007241. [Google Scholar] [CrossRef]
- Beknazarova, M.; Whiley, H.; Traub, R.; Ross, K. Opportunistic Mapping of Strongyloides stercoralis and Hookworm in Dogs in Remote Australian Communities. Pathogens 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Raw, C.; Traub, R.J.; Wiethoelter, A. A comparative field efficacy trial of three treatment programs against endo-and ectoparasites in naturally infected dogs. Front. Vet. Sci. 2024, 11, 1460452. [Google Scholar] [CrossRef]
- Barton, M.A.; McEwan, D.R. Spirurid nematodes in dogs and cats from central Australia. Aust. Vet. J. 1993, 70, 270. [Google Scholar] [CrossRef]
- Setasuban, P.; Waddell, A.H. Hookworms in cats and dogs in Queensland. Aust. Vet. J. 1973, 49, 110. [Google Scholar] [CrossRef]
- Traub, R.J.; Hobbs, R.; Adams, P.; Behnke, J.M.; Harris, P.D.; Thompson, R. A case of mistaken identity–reappraisal of the species of canid and felid hookworms (Ancylostoma) present in Australia and India. Parasitology 2007, 134, 113–119. [Google Scholar] [CrossRef]
- Smout, F.A.; Skerratt, L.F.; Johnson, C.N.; Butler, J.R.; Congdon, B.C. Zoonotic helminth diseases in dogs and dingoes utilising shared resources in an Australian aboriginal community. Trop. Med. Infect. Dis. 2018, 3, 110. [Google Scholar] [CrossRef]
- Nolan, T.J.; Lok, J.B. Macrocyclic lactones in the treatment and control of parasitism in small companion animals. Curr. Pharm. Biotechnol. 2012, 13, 1078–1094. [Google Scholar] [CrossRef]
- Heydon, G.M. Creeping eruption or larva migrans in north Queensland and a note on the worm Gnathostoma spinergum (Owen). Med. J. Aust. 1929, 1, 583–591. [Google Scholar] [CrossRef]
- Prociv, P.; Croese, J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 1990, 335, 1299–1302. [Google Scholar] [CrossRef]
- Walker, N.I.; Croese, J.; Clouston, A.D.; Parry, M.; Loukas, A.; Prociv, P. Eosinophilic enteritis in northeastern Australia. Pathology, association with Ancylostoma caninum, and implications. Am. J. Surg. Pathol. 1995, 19, 328–337. [Google Scholar] [CrossRef]
- Currie, B.; Anstey, N. Eosinophilic enteritis in the Northern Territory. Med. J. Aust. 1991, 154, 71. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, R.S.; Hii, S.F.; Harrington, H.; Speare, R.; Traub, R. Ancylostoma ceylanicum Hookworm in the Solomon Islands. Emerg. Infect. Dis. 2017, 23, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Colella, V.; Bradbury, R.; Traub, R. Ancylostoma ceylanicum. Trends Parasitol. 2021, 37, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Haydon, G.A.M.; Bearup, A.J. Ancylostoma braziliense and A. ceylanicum. Trans. R. Soc. Trop. Med. Hyg. 1962, 57, 76. [Google Scholar] [CrossRef]
- Inpankaew, T.; Schar, F.; Dalsgaard, A.; Khieu, V.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; Odermatt, P.; et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis. 2014, 20, 976–982. [Google Scholar] [CrossRef]
- Ngui, R.; Lim, Y.A.; Traub, R.; Mahmud, R.; Mistam, M.S. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl. Trop. Dis. 2012, 6, e1522. [Google Scholar] [CrossRef]
- Traub, R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int. J. Parasitol. 2013, 43, 1009–1015. [Google Scholar] [CrossRef]
- Puerta-Pena, M.; Calleja Algarra, A. Larva Currens in Strongyloides Hyperinfection Syndrome. N. Engl. J. Med. 2022, 386, 1559. [Google Scholar] [CrossRef]
- Tian, Y.; Monsel, G.; Paris, L.; Danis, M.; Caumes, E. Larva Currens: Report of Seven Cases and Literature Review. Am. J. Trop. Med. Hyg. 2023, 108, 340–345. [Google Scholar] [CrossRef]
- Buonfrate, D.; Tamarozzi, F.; Paradies, P.; Watts, M.R.; Bradbury, R.S.; Bisoffi, Z. The diagnosis of human and companion animal Strongyloides stercoralis infection: Challenges and solutions. A scoping review. Adv. Parasitol. 2022, 118, 1–84. [Google Scholar]
- Wulcan, J.M.; Dennis, M.M.; Ketzis, J.K.; Bevelock, T.J.; Verocai, G.G. Strongyloides spp. in cats: A review of the literature and the first report of zoonotic Strongyloides stercoralis in colonic epithelial nodular hyperplasia in cats. Parasites Vectors 2019, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.P.; Suzuki, K.; Canales-Ramos, M.; Htwe, M.P.P.T.H.; Htike, W.W.; Yoshida, A.; Montes, M.; Morishita, K.; Gotuzzo, E.; Maruyama, H. Phylogenetic relationships of Strongyloides species in carnivore hosts. Parasitol. Int. 2020, 78, 102151. [Google Scholar] [CrossRef]
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A. The global prevalence of Strongyloides stercoralis infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Thamsborg, S.M.; Ketzis, J.; Horii, Y.; Matthews, J.B. Strongyloides spp. infections of veterinary importance. Parasitology 2017, 144, 274–284. [Google Scholar] [CrossRef]
- Zhao, H.; Bradbury, R.S. Feline strongyloidiasis: An insight into its global prevalence and transmission cycle. One Health 2024, 19, 100842. [Google Scholar] [CrossRef]
- Kuna, A.; Olszanski, R.; Sikorska, K. Cutaneous Larva Migrans as a frequent problem in travellers. Int. Marit. Health 2023, 74, 259–264. [Google Scholar]
- Hamilton, W.L.; Agranoff, D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Loffler’s syndrome. BMJ Case Rep. 2018, 2018, bcr-2017-223132. [Google Scholar] [CrossRef]
- Moorhouse, D.E.; Bhaibulaya, M.; Jones, H.I. Suspected human gnathostomiasis in Queensland. Med. J. Aust. 1970, 2, 250. [Google Scholar]
- Jeremiah, C.J.; Harangozo, C.S.; Fuller, A.J. Gnathostomiasis in remote northern Western Australia: The first confirmed cases acquired in Australia. Med. J. Aust. 2011, 195, 42–44. [Google Scholar] [CrossRef]
- Singh, S.; Banerjee, D.P. Role of Wildlife in Parasitic Diseases of Man and Animals. ZOO’S PRINT 1997, 12, 14–16. [Google Scholar]
- Smout, F.A.; Thompson, R.C.; Skerratt, L.F. First report of Ancylostoma ceylanicum in wild canids. Int. J. Parasitol. Parasites Wildl. 2013, 2, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Dybing, N. Gastro-Intestinal Parasites of Red Foxes (Vulpes vulpes) and Feral Cats (Felis catus) in Southwest Western Australia. Honours Thesis, Murdoch University, Perth, Australia, 2010. [Google Scholar]
- Loos-Frank, B.; Zeyhle, E. The intestinal helminths of the red fox and some other carnivores in southwest Germany. Z. Für Parasitenkd. 1982, 67, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Calvopina, M.; Lozano-Alvarez, K.; Enriquez-Morillo, S.; Cordova-Calisto, I. Vesiculobullous Cutaneous Larva Migrans in the Absence of Domestic Dogs and Cats. Successful Treatment with Oral Ivermectin. Trop. Med. Infect. Dis. 2024, 9, 106. [Google Scholar] [CrossRef]
- Albonico, M.; Wright, V.; Bickle, Q. Molecular analysis of the beta-tubulin gene of human hookworms as a basis for possible benzimidazole resistance on Pemba Island. Mol. Biochem. Parasitol. 2004, 134, 281–284. [Google Scholar] [CrossRef]
- Kotze, A.C.; Coleman, G.T.; Mai, A.; McCarthy, J.S. Field evaluation of anthelmintic drug sensitivity using in vitro egg hatch and larval motility assays with Necator americanus recovered from human clinical isolates. Int. J. Parasitol. 2005, 35, 445–453. [Google Scholar] [CrossRef]
- Vercruysse, J.; Levecke, B.; Prichard, R. Human soil-transmitted helminths: Implications of mass drug administration. Curr. Opin. Infect. Dis. 2012, 25, 703–708. [Google Scholar] [CrossRef]
- Kitchen, S.; Ratnappan, R.; Han, S.; Leasure, C.; Grill, E.; Iqbal, Z.; Granger, O.; O’Halloran, D.M.; Hawdon, J.M. Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum. Int. J. Parasitol. 2019, 49, 397–406. [Google Scholar] [CrossRef]
- Kotze, A.C.; Clifford, S.; O’Grady, J.; Behnke, J.M.; McCarthy, J.S. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am. J. Trop. Med. Hyg. 2004, 71, 608–616. [Google Scholar] [CrossRef]
- Jimenez Castro, P.D.; Howell, S.B.; Schaefer, J.J.; Avramenko, R.W.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasit. Vectors 2019, 12, 576. [Google Scholar] [CrossRef]
- Jackson, R.; Lance, D.; Townsend, K.; Stewart, K. Isolation of anthelmintic resistant Ancylostoma caninum. N. Z. Vet. J. 1987, 35, 215–216. [Google Scholar] [CrossRef]
- Hopkins, T.; Gyr, P. Synergism of a combination of febantel and pyrantel embonate against Ancylostoma caninum on dogs. Vet. Med. Rev. 1991, 61, 3–9. [Google Scholar]
- Kopp, S.R.; Kotze, A.C.; McCarthy, J.S.; Coleman, G.T. High-level pyrantel resistance in the hookworm Ancylostoma caninum. Vet. Parasitol. 2007, 143, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.; Xu, G.; Kopp, S.R.; Jones, M.K.; Kotze, A.C.; Abdullah, S. Pyrantel resistance in canine hookworms in Queensland, Australia. Vet. Parasitol. Reg. Stud. Rep. 2024, 48, 100985. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Stocker, T.; Kang, H.; Scott, I.; Hayward, D.; Jaensch, S.; Ward, M.P.; Jones, M.K.; Kotze, A.C.; Slapeta, J. Widespread occurrence of benzimidazole resistance single nucleotide polymorphisms in the canine hookworm, Ancylostoma caninum, in Australia. Int. J. Parasitol. 2025, 55, 173–182. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N.; Howell, S.B.; Neiss, J.M.; Williamson, L.H.; Terrill, T.H. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in gastrointestinal nematodes of goats. Int. J. Parasitol. 2007, 37, 795–804. [Google Scholar] [CrossRef]
- Condi, G.K.; Soutello, R.G.; Amarante, A.F. Moxidectin-resistant nematodes in cattle in Brazil. Vet. Parasitol. 2009, 161, 213–217. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Kotze, A.C.; Hunt, P.W. The current status and outlook for insecticide, acaricide and anthelmintic resistances across the Australian ruminant livestock industries: Assessing the threat these resistances pose to the livestock sector. Aust. Vet. J. 2023, 101, 321–333. [Google Scholar] [CrossRef]
- Balk, J.D.; Mitchell, N.D.; Hughes, J.; Soto Nauto, P.; Rossi, J.; Ramirez-Barrios, R. Multiple anthelmintic drug resistant Ancylostoma caninum in foxhounds. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 102–106. [Google Scholar] [CrossRef]
- Venkatesan, A.; Jimenez Castro, P.D.; Morosetti, A.; Horvath, H.; Chen, R.; Redman, E.; Dunn, K.; Collins, J.B.; Fraser, J.S.; Andersen, E.C.; et al. Molecular evidence of widespread benzimidazole drug resistance in Ancylostoma caninum from domestic dogs throughout the USA and discovery of a novel beta-tubulin benzimidazole resistance mutation. PLoS Pathog. 2023, 19, e1011146. [Google Scholar] [CrossRef]
- McKean, E.L.; Grill, E.; Choi, Y.J.; Mitreva, M.; O’Halloran, D.M.; Hawdon, J.M. Altered larval activation response associated with multidrug resistance in the canine hookworm Ancylostoma caninum. Parasitology 2024, 151, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Caumes, E.; Carriere, J.; Datry, A.; Gaxotte, P.; Danis, M.; Gentilini, M. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am. J. Trop. Med. Hyg. 1993, 49, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, C.; Perignon, A.; Monsel, G.; Regnier, S.; Bricaire, F.; Caumes, E. The efficacy of single dose ivermectin in the treatment of hookworm related cutaneous larva migrans varies depending on the clinical presentation. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; McCarthy, J.S. Permethrin and ivermectin for scabies. N. Engl. J. Med. 2010, 362, 717–725. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M.; DeClementi, C.; Gupta, R.C. Macrocyclic lactone endectocides. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 539–550. [Google Scholar]
- Butler, J.R.A.; Brown, W.Y.; du Toit, J.T. Anthropogenic Food Subsidy to a Commensal Carnivore: The Value and Supply of Human Faeces in the Diet of Free-Ranging Dogs. Animals 2018, 8, 67. [Google Scholar] [CrossRef]
- Shamad, M.; Al-Mutairi, N. Successful Treatment of Cutaneous Larva Migrans With Combined Albendazole and Ivermectin Therapy: A Report of Two Cases From Sudan. Cureus 2024, 16, e64665. [Google Scholar] [CrossRef]
- Clarke, N.E.; Doi, S.A.R.; Wangdi, K.; Chen, Y.; Clements, A.C.A.; Nery, S.V. Efficacy of Anthelminthic Drugs and Drug Combinations Against Soil-transmitted Helminths: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2019, 68, 96–105. [Google Scholar] [CrossRef]
- Gandasegui, J.; Onwuchekwa, C.; Krolewiecki, A.J.; Doyle, S.R.; Pullan, R.L.; Enbiale, W.; Kepha, S.; Hatherell, H.A.; van Lieshout, L.; Cambra-Pelleja, M.; et al. Ivermectin and albendazole coadministration: Opportunities for strongyloidiasis control. Lancet Infect. Dis. 2022, 22, e341–e347. [Google Scholar] [CrossRef]
- Krolewiecki, A.; Kepha, S.; Fleitas, P.E.; van Lieshout, L.; Gelaye, W.; Messa, A., Jr.; Gandasegui, J.; Algorta, J.; Novela, V.; de Jesus, A.; et al. Albendazole-ivermectin co-formulation for the treatment of Trichuris trichiura and other soil-transmitted helminths: A randomised phase 2/3 trial. Lancet Infect. Dis. 2025, 25, 548–559. [Google Scholar] [CrossRef]
- Buonfrate, D. Albendazole and ivermectin co-formulation for soil-transmitted helminthiases. Lancet Infect. Dis. 2025, 25, 478–479. [Google Scholar] [CrossRef]
- Kincaid, L.; Klowak, M.; Klowak, S.; Boggild, A.K. Management of imported cutaneous larva migrans: A case series and mini-review. Travel. Med. Infect. Dis. 2015, 13, 382–387. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currie, B.J.; Hoopes, J.; Cumming, B. Cutaneous Larva Migrans Refractory to Therapy with Ivermectin: Case Report and Review of Implicated Zoonotic Pathogens, Epidemiology, Anthelmintic Drug Resistance and Therapy. Trop. Med. Infect. Dis. 2025, 10, 163. https://doi.org/10.3390/tropicalmed10060163
Currie BJ, Hoopes J, Cumming B. Cutaneous Larva Migrans Refractory to Therapy with Ivermectin: Case Report and Review of Implicated Zoonotic Pathogens, Epidemiology, Anthelmintic Drug Resistance and Therapy. Tropical Medicine and Infectious Disease. 2025; 10(6):163. https://doi.org/10.3390/tropicalmed10060163
Chicago/Turabian StyleCurrie, Bart J., Jessica Hoopes, and Bonny Cumming. 2025. "Cutaneous Larva Migrans Refractory to Therapy with Ivermectin: Case Report and Review of Implicated Zoonotic Pathogens, Epidemiology, Anthelmintic Drug Resistance and Therapy" Tropical Medicine and Infectious Disease 10, no. 6: 163. https://doi.org/10.3390/tropicalmed10060163
APA StyleCurrie, B. J., Hoopes, J., & Cumming, B. (2025). Cutaneous Larva Migrans Refractory to Therapy with Ivermectin: Case Report and Review of Implicated Zoonotic Pathogens, Epidemiology, Anthelmintic Drug Resistance and Therapy. Tropical Medicine and Infectious Disease, 10(6), 163. https://doi.org/10.3390/tropicalmed10060163





