Abstract
Candida auris possesses distinctive features that facilitate its persistence and transmission in healthcare settings, causing outbreaks of infection that are difficult to treat. So, emphasis has been placed on implementing measures for controlling, eliminating, and preventing fungal transmission, such as environmental disinfection and patient decolonization. This review aimed to understand and analyze the agents for environmental disinfection and patient decolonization reported in the last 5 years. The PubMed database was reviewed, using the terms “Candida auris”, “disinfection”, and “decolonization”. Only original papers, published between 2020–2025, in English or Spanish, that included relevant information on the topic, were selected. After the selection process, 52 articles were chosen to analyze the agents for environmental disinfection and decolonization of C. auris. Natural and synthetic disinfectants and ultraviolet radiation were reported for the environmental disinfection, with variable efficacy, depending on factors such as concentration and exposure time. Natural and synthetic antiseptics were also reported for decolonization, with varying efficacy. For example, 2% chlorhexidine shows a 0.5 log reduction, while at concentrations >10% it is >4 log. However, most have only been tested in animal models. Based on the review, Far-UV-C radiation (222 nm) is safe and appropriate to mitigate (up to 1 log reduction) the spread of C. auris in the hospital setting. However, it is important to consider that the cost and limited availability of the device present a barrier to its implementation. Patient decolonization is still challenging nowadays due to the absence of agents with proven high efficacy in humans.
1. Introduction
The incidence of invasive fungal infections (IFIs) is increasing worldwide due to the growth of populations with typical risk characteristics for IFIs, which include prolonged intensive care unit (ICU) stays, use of mechanical ventilation or catheters, prolonged antibiotic treatment, and presence of underlying diseases (chronic kidney disease, diabetes mellitus, HIV infection, among others) [1]. In addition, new populations at risk have emerged, such as patients with COVID-19 who present frequent IFIs, particularly invasive candidiasis (IC) [1]. Several Candida species can cause IC, mainly Candida albicans, C. parapsilosis, C. glabrata (Nakaseomyces glabratus), and C. auris (Candidozyma auris) [2]. These yeasts were classified as high or critical priority fungal pathogens by the World Health Organization (WHO) [3], with C. auris standing out for causing severe IFIs with a high mortality rate worldwide (40–60%) [4]. Unlike the other species, C. auris is a non-commensal yeast, which is usually resistant to at least two of the main classes of systemic antifungals (93% are resistant to fluconazole, 35% to amphotericin B, and 7% to echinocandins) [5], limiting treatment options for patients [6]. In addition, this yeast has a halotolerant feature, which facilitates its transmission and causes outbreaks of infection that are difficult to treat in healthcare environments [7]. Due to its distinctive characteristics, C. auris represents a threat to public health worldwide.
Outbreaks of hospital-acquired infection have been associated with the ability of C. auris to colonize the skin, particularly areas exposed to high salinity and temperature, such as the armpit and groin [8], showing no signs of infection, and persisting for weeks [9]. Patients colonized by C. auris can develop invasive infections in 5–10% of cases, with high mortality rates [10]. Skin colonization facilitates the spread of this yeast to biotic surfaces (skin of healthcare workers and family members) and abiotic surfaces (mattresses, bed rails, thermometers, catheters, medical equipment, even to surfaces that are not in direct contact, such as floors, chairs, etc.) [11] of the hospital environment. They also persist for prolonged periods, resisting the action of disinfectants commonly used in hospitals [12]. It has been shown that the fungus can persist for up to 4 weeks on abiotic surfaces, remaining metabolically active, but not culturable, which makes detection difficult [13]. This explains how easily other patients can contract C. auris through contact with contaminated surfaces. For this reason, emphasis has been placed on implementing measures to control, eliminate, and prevent fungal transmission in the hospital environment. Some recommended measures are hygiene and disinfection of the patient’s environment. In the case of hygiene, it is critical to comply with the five moments for handwashing recommended by the WHO. In addition, it is necessary to follow some precautions, such as ensuring that infected and colonized patients are accommodated in isolated rooms, as well as cleaning these rooms daily. Regarding disinfection, it is crucial to thoroughly disinfect rooms at the time of patient discharge, using effective agents against microorganisms such as Clostridium difficile [9]. Another way to prevent and control outbreaks is by detecting patients colonized with C. auris, followed by their decolonization with topical antiseptics, such as chlorhexidine, since they can spread the fungus to other non-colonized patients. Decolonization and disinfection of areas during an outbreak has been shown to reduce the frequency of C. auris isolation in both clinical and colonization samples [14]. The rate of reduction in transmission risk associated with decolonization is rarely clearly known due to the influence of various factors, such as recolonization and the effectiveness of different interventions [14]. However, screening for this yeast at the time of patient admission or during their hospital stay is not routinely performed [15]. However, evidence justifies the need for screening, as patients with chronic respiratory diseases are at significant risk of persistent colonization [9]. It has even been shown that patients discharged from the hospital and reintegrated into the community usually take about 8 months to test negative for colonization [16]. Thus, controlling the spread of C. auris is still a challenge [17].
Due to the increasing incidence of C. auris infections and the various outbreaks that have occurred in several parts of the world [18], as well as the difficulty in controlling the spread of C. auris, it is essential to identify the current prevention and control strategies. Therefore, this study aimed to understand and analyze the agents for environmental disinfection and patient decolonization, which have been reported in the last 5 years.
2. Materials and Methods
A systematic literature search was conducted in four databases: PubMed, Scopus, SciELO, and EBSCO using the terms “Candida auris”, “disinfection”, and “decolonization”. For the systematic review, the PRISMA 2020 guidelines were used [19] (Figure 1). A protocol for this review was registered in PROSPERO’s international prospective register of systematic reviews (crd.york.ac.uk/prospero) with the ID CRD420251034737. The search was limited to articles published in English or Spanish from January 2020 to January 2025. After the automated database search and duplication were performed, three independent authors (M.G.F.D.-L., P.B.-C., and P.B.Z.-S.) determined the eligibility of articles based on title and abstract. The inclusion criteria were original articles, in English, on environmental disinfection strategies and decolonization of C. auris. The quality assessment for the risk of bias was performed in duplicate (E.M.-H., P.B.Z.-S., C.A.C.-F., and E.G.-S.) using two tools, the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses, which indicates in the general assessment that an article meets the necessary quality for publication, and the Critical Appraisal Skills Program (CASP), which establishes that a study has a logical development that makes it feasible for publication.
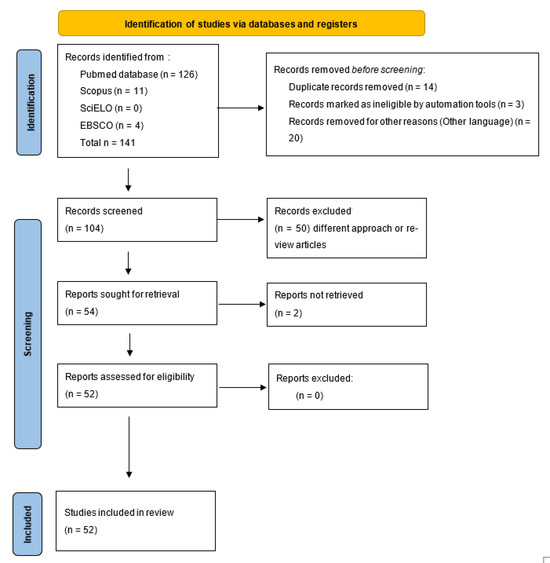
Figure 1.
Prisma 2020 flowchart of the data extracted for the systematic review from bibliographic search.
3. Results
After the selection process, 52 articles were chosen to analyze the strategies of environmental disinfection and decolonization of C. auris. Of these, 40 corresponded exclusively to strategies for disinfecting environments contaminated by C. auris, 11 to decolonization strategies, and one addressed both strategies.
The quality assessment of the studies using the JBI Critical Appraisal Checklist and CASP showed that 22 studies received a score of 10, 13 of nine, and 17 of eight. Based on the scores, 35 studies were classified as high quality and the remaining 17 as moderate methodological quality.
3.1. Disinfection of Environments Contaminated by C. auris
In the last 5 years, North America has been the region that has focused the most on improving and developing strategies for disinfecting the healthcare environment, followed by Europe, Asia, Africa, and South America (Table 1).

Table 1.
Disinfection agents for the hospital environment contaminated with Candida auris.
Various disinfectant agents for environmental disinfection, mainly for hard surfaces, have been reported in recent literature, including everything from natural and synthetic disinfectants to ultraviolet radiation (Table 1).
Among the natural disinfectants, lavender essential oil (Lavandula angustifolia), in free form or encapsulated in liposomes, has shown adequate results in eradicating C. auris in the form of primary and persistent biofilms on various surfaces [27]. In the same way that has been reported in other Candida species, the action of essential oils against C. auris entails producing reactive oxygen species (ROS) and affecting the expression of some biofilm-related genes [27].
Within the group of synthetic disinfectants, there is a wide range of agents, among which hydrogen peroxide, sodium hypochlorite, isopropyl alcohol, quaternary ammonium compounds, chlorhexidine, and benzalkonium chloride stand out due to the frequency of use (Table 1).
Hydrogen peroxide, both in 3.4 and 4.2% solution and aerosol, is effective in removing planktonic cells and C. auris biofilms on steel surfaces and medical equipment, without causing deterioration to surfaces, as it has relatively good compatibility with hard and soft surface materials [22,23,26,28,29,32,34,35,42]. The efficacy of hydrogen peroxide solutions at low concentrations, such as 1.7%, is limited [32], while the effectiveness of disinfection increases with the aerosol form, helping to stop nosocomial transmission of C. auris [29,34].
Sodium hypochlorite has variable efficacy, depending on the concentration. The load of C. auris on high-contact surfaces is considerably reduced with solutions of ≥ 1000 ppm and contact times greater than 1 min [34,46,51]. At concentrations ≥ 4000 ppm, C. auris removal is effective with only 1 min of contact [23]. The main disadvantage of using sodium hypochlorite is the incompatibility with some materials that constitute soft surfaces, such as mattresses, as it can lead to discoloration and deterioration [28].
Quaternary ammonium compounds have shown variable efficacy against C. auris. While some studies report that these compounds significantly limit fungal growth [25,32], others report that the biocidal effect of quaternary ammonium on C. auris is lower than that of other types of compounds, such as hydrogen peroxide, alcohol, or sodium hypochlorite [26,39,46,56].
Alcohol-based environmental disinfectants have shown satisfactory results in significantly reducing the load of C. auris on surfaces [26,32,40]. It has even been observed that C. auris is usually more susceptible to these disinfectants than C. albicans [25].
In recent years, reports on chlorhexidine as an environmental disinfectant have been scarce [45,46,48]. The few reports indicate that its effectiveness in eliminating C. auris on surfaces is variable, as it depends on the concentration and cleanliness of the environment [46]. The use of appropriate chlorhexidine concentrations leads to the elimination of 99.999% of C. auris biofilms [45], and its efficacy increases when coupled with silver sulfadiazine; nevertheless, it is still unknown whether the chlorhexidine–silver sulfadiazine binding has any clinical benefit [48].
Another environmental disinfectant that has been used is benzalkonium chloride; however, the outcome in all cases has been negative, as C. auris survived on surfaces, mainly wet wood [37,46]. Survival occurred due to the yeast’s efflux pump-mediated resistance [37].
Other synthetic disinfectants that have been evaluated for the elimination of C. auris in the hospital environment are peracetic acid, dodecylbenzenesulfonic acid, glutaraldehyde combined with quaternary ammonium and surfactant, potassium peroxymonosulfate, electrolyzed water, a combination of peracetic acid and hydrogen peroxide, furfuryl alcohol; 2-methyl-2-cyclopentenone; guaiac; potassium linoleate and silver nanoparticles, ozone [21,22,24,33,39,42,45,57]. All have shown fungicidal effects on C. auris on surfaces of different materials without causing deterioration. Furthermore, combining some of them, such as furfuryl alcohol, 2-methyl-2-cyclopentenone, and guaiac, increases the efficacy in removing preformed biofilms in stainless steel [57]. Potassium linoleate is a compound that has demonstrated biocidal activity against fungi and bacteria, and it can also be used in skin decolonization [33]. The silver nanoparticles of 1–3 nm in diameter have been evaluated on silicone elastomers and bandage fibers, demonstrating their fungicidal effect by inhibiting the biofilm formation (IC50 of 0.06 ppm) or destroying preformed ones (IC50 of 0.48 ppm). Silver nanoparticles are effective at low concentrations (0.06–0.48 ppm) and remain active even after repeatedly washing surfaces [24]. Another effective compound is ozone, which has eradicated C. auris from bed sheets after a 40-min exposure. The disadvantage of this compound lies in the resistance that some isolates have shown [21]. The use of substances that function as biofilm disruptors has also been reported, with high efficacy even in polymicrobial biofilms resistant to other disinfectants, such as chlorhexidine [45].
In the search for new compounds with fungicidal activity, a study evaluated 240 compounds, included in the Global Health Priority Box®, and found at least two compounds that are candidates for disinfectant development: hydramethylnon (MMV1577471) and flufenerim (MMV1794206). These compounds inhibit the growth of C. auris by more than 58% [55].
Ultraviolet radiation, type A (UV-A), B (UV-B), and C (UV-C), has emerged as a support method to traditional chemical disinfection methods. The least commonly used type of radiation is UV-A, perhaps because, although C. auris is photosensitive to different wavelengths, it is not as sensitive as other yeasts (Saccharomyces cerevisiae) [47]. Short-wave UV-B, at a dose of 51.3 mJ/cm2, has also achieved inactivation of C. auris in solutions [60]; however, no other recent studies support its application. UV-C is the most studied type of radiation for disinfection [49]. Exposure to UV-C (222–280 nm), at distances of 1–3 m and times of 1–45 min, results in reducing the load of C. auris and its biofilms on various surfaces (bed sheets, stainless steel, polystyrene, fabrics, glass) [30,35,36,41,50,53,60]. However, a disadvantage of using UV-C is that the sensitivity of C. auris depends on the clade to which they belong, i.e., isolates from clades I, II, and IV are more sensitive than those from clade III [20], so the efficacy of UV-C can vary between geographical regions.
Far UV-C exposure in the range of 200–230 nm for 45 to 120 min is effective in reducing the load of C. auris on steel surfaces, wheelchairs, portable equipment in clinical areas, etc. [49,54,55]. Disinfection by this method is safe because it cannot penetrate the stratum corneum of the human skin [60]. However, it is important to mention that there are factors that can reduce its effectiveness and, therefore, limit its use. These factors include surface characteristics (porosity, presence of residue or dirt), exposure time, and the distance between the UV-C source and the contaminated surface [50]. One limitation is that it can interact with atmospheric molecules such as oxygen and volatile organic compounds, forming ozone and harmful radicals indoors.
Ultraviolet germicidal radiation (UVGI) is a method that inactivates C. auris in aqueous solutions with an efficacy of 99.999%, using a dose of 192 mJ/cm2 [52].
Notably, antimicrobial surfaces have also been developed to reduce the load of C. auris to the minimum limit of detection [31,44]. These surfaces comprise a self-disinfecting polymer that generates a surface layer of acidic water when hydrated, which causes damage to the microorganisms that come into contact with it [44]. However, more research is required on the mechanism of action of these surfaces to apply them in clinical settings [31].
3.2. Decolonization of C. auris
One of the most challenging issues while controlling the spread of C. auris in healthcare settings has been decolonization, i.e., reducing or eliminating the fungal load in a patient’s body. The evaluation or development of new strategies to decolonize C. auris has been conducted mainly in North America, Asia, and Europe (Table 2).

Table 2.
Decolonization agents for Candida auris.
Chlorhexidine is the most widely used synthetic antiseptic [62,66,67,71]; however, the reduction in fungal load is slight, so it can sometimes present clinical failures [63]. It has been reported that chlorhexidine activity against C. auris in the skin can be enhanced with isopropanol, and the decolonizing effect of chlorhexidine/isopropanol is further enhanced by the combined use of natural antiseptics, such as tea tree and lemongrass oil [63]. In order to increase the effectiveness of chlorhexidine, a formulation based on the proprietary Advanced Performance Technology (APT™) platform was proposed [65]. This formula combines FDA-approved inactive ingredients (ascorbic acid, carbomer, cholecalciferol, citric acid, diazolidinyl urea, dimethyl sulfoxide (DMSO), dipropylene glycol, glycerin, polysorbate 20, sodium dodecylbenzenesulfonate, tetrasodium EDTA, tocopheryl acetate (vitamin E acetate), triethanolamine, water, and aloe barbadensis leaf juice) with an active pharmaceutical ingredient (chlorhexidine) to effectively reduce the burden of C. auris in mouse skin tissue. However, its efficacy in humans needs to be evaluated. Independently, other natural (manuka oil) and synthetic antiseptics (povidone iodine, nystatin, quaternary ammonium iodine tincture, and 75% ethanol, chlorine) have shown fungicidal activity against C. auris, with products containing iodine and benzalkonium chloride being the most effective in vitro [21,68,71]. Some compounds with antimicrobial activity, such as triclosan, boric acid, and zinc oxide, which are primarily used in personal care products, have also been tested. Boric acid triclosan showed antifungal activity against C. auris, but zinc oxide did not [70]. So, further research is needed to determine whether these compounds can reduce the burden of Candida on skin.
4. Discussion
Since its emergence in 2009, C. auris has posed a global health threat, partly due to the ease with which it spreads and persists in the hospital environment. For this reason, prevention and control protocols have been established, in which environmental disinfection and decolonization are essential. There are recommendations by various competent organizations, such as the Centers for Disease Control and Prevention, and WHO, to reduce nosocomial transmission of C. auris [72]. However, the increase in infection outbreaks since the COVID-19 pandemic has prompted the study of the efficacy of commonly used disinfectant agents, as well as the search for new, more effective agents for environmental disinfection and patient decolonization.
In environmental disinfection, the findings of the latest studies highlight the need to choose the right agent since not all commonly used disinfectants are effective against C. auris. For example, benzalkonium chloride, which is widely used in hospitals to disinfect hands and devices, does not have an adequate effect on C. auris, as the fungus appears to develop resistance [37]. Sodium hypochlorite-based disinfectants, which are also frequently used in healthcare settings, have a biocidal effect on C. auris when used at high concentrations, causing oxidative damage to the cell membrane and essential intracellular components [73]. However, at concentrations <4000 ppm, a prolonged contact time is required to achieve the same effect [23]. This can be impractical in hospitals, as it can hinder the dynamics of patient care.
Given the drawbacks of commonly used environmental disinfectants, the need for effective agents against C. auris has led to the evaluation of various compounds, some very simple, such as essential oils, which could be integrated into existing disinfectant formulations to achieve better results due to their effectiveness. Alternatively, there are more complex ones such as silver sulfadiazine combined with chlorhexidine, which has a clear inhibitory effect on C. auris in vitro, but whose clinical benefit has not been confirmed [48]. Hydramethylnon and flufenerim are two compounds in which activity against C. auris has been discovered [55]. However, it still needs to be determined whether they can penetrate the protective layers of biofilms and eliminate viable C. auris cells before using them to develop new disinfectants.
One of the most interesting advances in the attempt to mitigate the persistence of C. auris in the healthcare environment is the development of antimicrobial surfaces, which destroy microorganisms that come into contact with them [44]. It would be ideal if the surfaces in contact with patients were covered by the polymers that constitute these antimicrobial surfaces. However, studies on this topic are still required.
It is worth noting that an innovative and safe approach to air and surface disinfection is far UV-C. This type of radiation can be applied in rooms, even when people are present, since UV-C cannot penetrate even the most superficial layer of the skin [60]. It is worth noting that the studies report that Far UV-C (222-nm) was evaluated rather than 254-nm UV-C due to safety considerations. Far UV-C doses within threshold limit values proposed by the American Conference of Governmental Industrial Hygienists (ACGIH) and the International Commission on Non-Ionizing Radiation Protection (ICNIRP) may be safe in occupied areas. Thus, accidental exposure to far UV-C, but not 254-nm UV-C, would pose minimal risk [49]. However, it is important to consider the limited availability of amplifiers and accessories due to their relatively new technology; the higher initial costs of lamps and accessories compared to mercury vapor lamps; high energy consumption; limited data on potential long-term health effects; and ozone production are some barriers to their implementation.
Patient decolonization continues to represent a challenge in controlling the spread of C. auris since few studies focus on the subject. Chlorhexidine, widely used in hospitals, has limited activity against C. auris, which has led to failure rates ranging from 76.3–81.2% [63,66]. New chlorhexidine formulations have been designed to improve their efficacy. However, they have only been used in animal models, and the efficacy in humans has yet to be evaluated.
Finally, it is necessary to acknowledge that this review has an important limitation, which is the linguistic bias, since only publications in English or Spanish were considered. This exclusion may lead to the omission of relevant data in other languages and alter the conclusions of the review.
5. Conclusions
Recent studies on disinfectant and decolonizing agents for C. auris demonstrate the importance of choosing the right agent, since there may be different susceptibility between isolates of different clades. In addition, not all existing disinfectants show adequate activity against C. auris. One of the most promising strategies to mitigate the spread of C. auris is the use of far-UV-C radiation, as it is safe for the patient and healthcare staff and has the potential to disinfect both surfaces and air in the hospital environment effectively. However, it is important to consider that the cost and limited availability of the device present a barrier to its implementation.
Lastly, patient decolonization remains a challenge nowadays. Some disinfectants have shown promising results in animal models, but the effect on human skin has not been studied yet.
Author Contributions
Conceptualization, E.G.-S. and M.G.F.-D.-L.; Methodology, M.G.F.-D.-L., P.B.-C., E.M.-H., P.B.Z.-S. and C.A.C.-F.; Formal analysis, E.G.-S. and M.G.F.-D.-L.; Investigation, M.G.F.-D.-L., P.B.-C., E.M.-H., E.G.-S., P.B.Z.-S. and C.A.C.-F.; Resources, M.G.F.-D.-L., P.B.-C., E.M.-H. and E.G.-S.; Data curation, E.G.-S., P.B.Z.-S. and C.A.C.-F.; writing—original draft preparation, M.G.F.-D.-L. and P.B.-C.; Writing—review and editing, M.G.F.-D.-L., P.B.-C., E.M.-H., E.G.-S., P.B.Z.-S. and C.A.C.-F.; Visualization, M.G.F.-D.-L., P.B.-C. and E.G.-S.; Supervision, E.G.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., 3rd; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, S.; Midiri, A.; Mancuso, G.; Avola, M.G.; Biondo, C. Candida auris Outbreaks: Current status and future perspectives. Microorganisms 2024, 12, 927. [Google Scholar] [CrossRef]
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An overview of how to screen, detect, test and control this emerging pathogen. Antibiotics 2020, 9, 778. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 67, 134–140. [Google Scholar] [CrossRef]
- Jackson, B.R.; Chow, N.; Forsberg, K.; Litvintseva, A.P.; Lockhart, S.R.; Welsh, R.; Vallabhaneni, S.; Chiller, T. On the origins of a species: What might explain the rise of Candida auris? J. Fungi 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 26 January 2025).
- Tharp, B.; Zheng, R.; Bryak, G.; Litvintseva, A.P.; Hayden, M.K.; Chowdhary, A.; Thangamani, S. Role of microbiota in the skin colonization of Candida auris. mSphere 2023, 8, e0062322. [Google Scholar] [CrossRef]
- Southwick, K.; Ostrowsky, B.; Greenko, J.; Adams, E.; Lutterloh, E.; NYS C. auris Team; Denis, R.J.; Patel, R.; Erazo, R.; Fernandez, R.; et al. A description of the first Candida auris-colonized individuals in New York State, 2016–2017. Am. J. Infect. Control 2022, 50, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef]
- Kean, R.; Sherry, L.; Townsend, E.; McKloud, E.; Short, B.; Akinbobola, A.; Mackay, W.G.; Williams, C.; Jones, B.L.; Ramage, G. Surface disinfection challenges for Candida auris: An in-vitro study. J. Hosp. Infect. 2018, 98, 433–436. [Google Scholar] [CrossRef]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Asadzadeh, M. Strategies to prevent transmission of Candida auris in healthcare settings. Curr. Fungal Infect. Rep. 2023, 17, 36–48. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Herrero Rodríguez, P.; Abril López de Medrano, V.; Ferrer Gómez, C.; Gimeno Cardona, C. Characteristics and management of candidaemia episodes in an established Candida auris outbreak. Antibiotics 2020, 9, 558. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Bergeron, G.; Bloch, D.; Murray, K.; Kratz, M.; Parton, H.; Ackelsberg, J.; Antwi, M.; Del Rosso, P.; Dorsinville, M.; Kubinson, H.; et al. Candida auris Colonization after discharge to a community setting: New York City, 2017–2019. Open Forum Infect. Dis. 2020, 8, ofaa620. [Google Scholar] [CrossRef] [PubMed]
- Salvador-García, P.; Palop, N.T.; Bayona, J.V.M.; García, M.M.; Rodríguez, D.N.; Álvarez, M.B.; Serrano, M.D.R.G.; Cardona, C.G. Candida auris: Report of an outbreak. Enferm. Infecc. Microbiol. Clin. 2020, 38, 39–44. [Google Scholar] [CrossRef]
- Geremia, N.; Brugnaro, P.; Solinas, M.; Scarparo, C.; Panese, S. Candida auris as an emergent public health problem: A current update on European outbreaks and cases. Healthcare 2023, 11, 425. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Choi, H.; Ochoa, B.; Garmon, G.; Coppin, J.D.; Allton, Y.; Lukey, J.; Williams, M.D.; Navarathna, D.; Jinadatha, C. Clade-specific variation in susceptibility of Candida auris to broad-spectrum ultraviolet C light (UV-C). Infect. Control Hosp. Epidemiol. 2020, 41, 1384–1387. [Google Scholar] [CrossRef]
- Fu, L.; Le, T.; Liu, Z.; Wang, L.; Guo, H.; Yang, J.; Chen, Q.; Hu, J. Different efficacies of common disinfection methods against Candida auris and other Candida species. J. Infect. Public Health 2020, 13, 730–736. [Google Scholar] [CrossRef]
- Kumar, J.A.; Cadnum, J.L.; Jencson, A.L.; Donskey, C.J. Efficacy of a multi-purpose high level disinfection cabinet against Candida auris and other health care-associated pathogen. Am. J. Infect. Control 2020, 48, 849–850. [Google Scholar] [CrossRef]
- Kumar, J.A.; Cadnum, J.L.; Jencson, A.L.; Donskey, C.J. Are reduced concentrations of chlorine-based disinfectants effective against Candida auris? Am. J. Infect. Control 2020, 48, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Tan, C.K.; Ißleib, U.; Paßvogel, L.; Eilts, B.; Steinhauer, K. Investigation of the susceptibility of Candida auris and Candida albicans to chemical disinfectants using European Standards EN 13624 and EN 16615. J. Hosp. Infect. 2020, 105, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Sexton, D.J.; Welsh, R.M.; Bentz, M.L.; Forsberg, K.; Jackson, B.; Berkow, E.L.; Litvintseva, A.P. Evaluation of nine surface disinfectants against Candida auris using a quantitative disk carrier method: EPA SOP-MB-35. Infect. Control Hosp. Epidemiol. 2020, 41, 1219–1221. [Google Scholar] [CrossRef]
- De Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of free and liposome-encapsulated essential oil from Lavandula angustifolia against persister-derived biofilm of Candida auris. Antibiotics 2021, 11, 26. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Pearlmutter, B.S.; Haq, M.F.; Jencson, A.L.; Donskey, C.J. Effectiveness and real-world materials compatibility of a novel hydrogen peroxide disinfectant cleaner. Am. J. Infect. Control 2021, 49, 1572–1574. [Google Scholar] [CrossRef]
- Eckbo, E.J.; Wong, T.; Bharat, A.; Cameron-Lane, M.; Hoang, L.; Dawar, M.; Charles, M. First reported outbreak of the emerging pathogen Candida auris in Canada. Am. J. Infect. Control 2021, 49, 804–807. [Google Scholar] [CrossRef]
- Füszl, A.; Zatorska, B.; Van den Nest, M.; Ebner, J.; Presterl, E.; Diab-Elschahawi, M. The use of a UV-C disinfection robot in the routine cleaning process: A field study in an Academic hospital. Antimicrob. Resist. Infect. Control 2021, 10, 84. [Google Scholar] [CrossRef]
- Truong, L.N.; Whitlock, B.D. Efficacy of compressed sodium chloride (CSC) against E. coli and Candida auris in minutes and methods improvement for testing. Sci. Rep. 2021, 11, 149. [Google Scholar] [CrossRef]
- Zatorska, B.; Moser, D.; Diab-Elschahawi, M.; Ebner, J.; Lusignani, L.S.; Presterl, E. The effectiveness of surface disinfectants and a micellic H2O2 based water disinfectant on Candida auris. J. Mycol. Med. 2021, 31, 101178. [Google Scholar] [CrossRef] [PubMed]
- Changaris, D.G.; Carenbauer, A.L. Potassium linoleate (isomerized) satisfies the united states environmental protection agency MB-05-16 for hospital disinfectant on hard, non-porous surfaces. Cureus 2022, 14, e22851. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; Montrucchio, G.; Shbaklo, N.; De Benedetto, I.; Sales, G.; Cedrone, M.; Vita, D.; Costa, C.; Zozzoli, S.; Zaccaria, T.; et al. First Cases of Candida auris in a referral intensive care unit in Piedmont Region, Italy. Microorganisms 2022, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Schnugh, D.; Thomas, T. Effectiveness of ultraviolet-C vs aerosolized hydrogen peroxide in ICU terminal disinfection. J. Hosp. Infect. 2022, 121, 114–119. [Google Scholar] [CrossRef]
- Mariita, R.M.; Davis, J.H.; Lottridge, M.M.; Randive, R.V. Shining light on multi-drug resistant Candida auris: Ultraviolet-C disinfection, wavelength sensitivity, and prevention of biofilm formation of an emerging yeast pathogen. Microbiologyopen 2022, 11, e1261. [Google Scholar] [CrossRef]
- Dire, O.; Ahmad, A.; Duze, S.; Patel, M. Survival of Candida auris on environmental surface materials and low-level resistance to disinfectant. J. Hosp. Infect. 2023, 137, 17–23. [Google Scholar] [CrossRef]
- Haq, M.F.; Cadnum, J.L.; Pearlmutter, B.S.; Jencson, A.L.; Donskey, C.J. Effectiveness of a novel 1-step cleaner and disinfectant against Candida auris. Infect. Control Hosp. Epidemiol. 2023, 44, 837–839. [Google Scholar] [CrossRef]
- Haq, M.F.; Pearlmutter, B.S.; Cadnum, J.L.; Donskey, C.J. Efficacy of 23 commonly used liquid disinfectants against Candida auris isolates from the 4 major clades. Infect. Control Hosp. Epidemiol. 2024, 45, 127–131. [Google Scholar] [CrossRef]
- McDougal, A.N.; DeMaet, M.A.; Garcia, B.; York, T.; Iverson, T.; Ojo, O.; Patel, J. A cluster investigation of Candida auris among hospitalized incarcerated patients. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e244. [Google Scholar] [CrossRef]
- Różańska, A.; Walkowicz, M.; Bulanda, M.; Kasperski, T.; Synowiec, E.; Osuch, P.; Chmielarczyk, A. Evaluation of the efficacy of UV-C radiation in eliminating microorganisms of special epidemiological importance from touch surfaces under laboratory conditions and in the hospital environment. Healthcare 2023, 11, 3096. [Google Scholar] [CrossRef]
- Solomon, S.; Stachel, A.; Kelly, A.; Mraz, J.; Aguilar, P.; Gardner, J.; Medefindt, J.; Horrocks, A.; Sterling, S.; Aguero-Rosenfeld, M.; et al. The evaluation of electrolyzed water, sodium dichloroisocyanurate, and peracetic acid with hydrogen peroxide for the disinfection of patient room surfaces. Am. J. Infect. Control 2023, 51, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Voorn, M.G.; Kelley, A.M.; Chaggar, G.K.; Li, X.; Teska, P.J.; Oliver, H.F. Contact time and disinfectant formulation significantly impact the efficacies of disinfectant towelettes against Candida auris on hard, non-porous surfaces. Sci. Rep. 2023, 13, 5849. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.M.; Ciftci, Y.; Peddinti, B.S.T.; Ghiladi, R.A.; Vediyappan, G.; Spontak, R.J.; Govind, R. Preventing the spread of life-threatening gastrointestinal microbes on the surface of a continuously self-disinfecting block polymer. J. Colloid Interface Sci. 2023, 652, 718–726. [Google Scholar] [CrossRef]
- Cox, C.A.; Manavathu, E.K.; Wakade, S.; Myntti, M.; Vazquez, J.A. Efficacy of biofilm disrupters against Candida auris and other Candida species in monomicrobial and polymicrobial biofilms. Mycoses 2024, 67, e13684. [Google Scholar] [CrossRef] [PubMed]
- Erganis, S.; Ozturk, A.; Uzuntas, S.T.; Kirca, F.; Dogan, A.; Dinc, B.; Kalkanci, A. Variable sensitivity of clinical Candida auris strains to biocides: Implications for infection control in healthcare settings. BMC Microbiol. 2024, 24, 447. [Google Scholar] [CrossRef]
- Gierke, A.M.; Hessling, M. Photoinactivation by UVA radiation and visible light of Candida auris compared to other fungi. Photochem. Photobiol. Sci. 2024, 23, 681–692. [Google Scholar] [CrossRef]
- Gupta, N.; Haughton, S.; Kemper, S.; Koehler, M.; Antoon, R.; Edwards, C.G.; Bardin, A. The antimicrobial effectiveness of chlorhexidine and chlorhexidine-silver sulfadiazine-impregnated central venous catheters against the emerging fungal pathogen Candida auris. Am. J. Infect. Control 2024, 52, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Kaple, C.E.; Memic, S.; Cadnum, J.L.; Donskey, C.J. Evaluation of an automated far ultraviolet-C light technology for decontamination of surfaces and aerosolized viruses in bathrooms. Antimicrob. Resist. Infect. Control 2024, 13, 114. [Google Scholar] [CrossRef]
- Koutras, C.; Wade, R.L. Ultraviolet-C mediated inactivation of Candida auris, a rapid emerging health threat. Am. J. Infect. Control 2024, 52, 133–135. [Google Scholar] [CrossRef]
- Lee, E.H.; Choi, M.H.; Lee, K.H.; Kim, D.; Jeong, S.H.; Song, Y.G.; Han, S.H. Intrahospital transmission and infection control of Candida auris originating from a severely infected COVID-19 patient transferred abroad. J. Hosp. Infect. 2024, 143, 140–149. [Google Scholar] [CrossRef]
- Lemons, A.R.; McClelland, T.L.; Martin, S.B., Jr.; Lindsley, W.G.; Green, B.J. Inactivation of the multi-drug resistant pathogen Candida auris using ultraviolet germicidal irradiation (UVGI). J. Hosp. Infect. 2020, 105, 495–501. [Google Scholar] [CrossRef]
- Loftus, R.W.; Brindeiro, C.T.; Dexter, F.; Parra, M.C.; Hwang, S.M.; Wanta, B.; Szeluga, D.J.; Hadder, B.A.; Seering, M.S.; Charnin, J.E. Importance of Ultraviolet-C (UV-C) emitter configuration for the attenuation of Staphylococcus aureus and Candida auris pathogens. Cureus 2024, 16, e71612. [Google Scholar] [CrossRef] [PubMed]
- Memic, S.; Osborne, A.O.; Cadnum, J.L.; Donskey, C.J. Efficacy of a far-ultraviolet-C light technology for continuous decontamination of air and surfaces. Infect. Control Hosp. Epidemiol. 2024, 45, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Retore, Y.I.; Lucini, F.; Pimentel, L.R.; de Oliveira, H.C.; Simionatto, S.; Rossato, L. Screening of the global health priority BoxⓇ reveals potential new disinfectants against the emerging multidrug-resistant pathogen Candida auris. Microb. Pathog. 2024, 194, 106828. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Bolomey, A.C.; Cadnum, J.L.; Donskey, C.J. Inactivation and/or physical removal of Candida auris from floors by detergent cleaner, disinfectants, microfiber, and ultraviolet C light (UV-C). Infect. Control Hosp. Epidemiol. 2024, 45, 390–392. [Google Scholar] [CrossRef]
- Krishnan, S.; Venkatachalam, P.; Shanmugam, S.R.; Paramasivam, N. Fractional inhibitory concentration of bio-actives from agricultural waste disassembles biofilms and quenches virulence of nosocomial pathogens. J. Med. Microbiol. 2025, 74, 001980. [Google Scholar] [CrossRef]
- Memic, S.; Torres-Teran, M.M.; Cadnum, J.L.; Donskey, C.J. Evaluation of a far ultraviolet-C device for decontamination of portable equipment in clinical areas. Am. J. Infect. Control 2025, 53, 403–406. [Google Scholar] [CrossRef]
- Sathyapalan, D.T.; Antony, R.; Nampoothiri, V.; Kumar, A.; Shashindran, N.; James, J.; Thomas, J.; Prasanna, P.; Sudhir, A.S.; Philip, J.M.; et al. Evaluating the measures taken to contain a Candida auris outbreak in a tertiary care hospital in South India: An outbreak investigational study. BMC Infect. Dis. 2021, 21, 425. [Google Scholar] [CrossRef]
- Gierke, A.M.; Hessling, M. Sensitivity analysis of C. auris, S. cerevisiae, and C. cladosporioides by irradiation with Far-UVC, UVC, and UVB. Pathog. Immun. 2024, 9, 135–151. [Google Scholar] [CrossRef]
- Stoffel, J.J.; Kohler Riedi, P.L.; Hadj Romdhane, B. A multimodel regime for evaluating effectiveness of antimicrobial wound care products in microbial biofilms. Wound Repair Regen. 2020, 28, 438–447. [Google Scholar] [CrossRef]
- Huang, X.; Hurabielle, C.; Drummond, R.A.; Bouladoux, N.; Desai, J.V.; Sim, C.K.; Belkaid, Y.; Lionakis, M.S.; Segre, J.A. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 2021, 29, 210–221.e6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.J.; Eix, E.F.; Lam, B.C.; Wartman, K.M.; Meudt, J.J.; Shanmuganayagam, D.; Nett, J.E. Augmenting the activity of chlorhexidine for decolonization of Candida auris from porcine skin. J. Fungi 2021, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, R.; Feng, R.; Li, Q.; Su, M.; Hou, C.; Zhuang, K.; Dai, Y.; Lei, N.; Jiang, Y.; et al. Cage-modified hypocrellin against multidrug-resistant Candida spp. with unprecedented activity in light-triggered combinational photodynamic therapy. Drug Resist. Updat. 2022, 65, 100887. [Google Scholar] [CrossRef]
- Elshaer, M.; Herrada, J.; Gamal, A.; McCormick, T.S.; Ghannoum, M. Efficacy of chlorhexidine in advanced performance technology formulation in decolonizing the skin using Candida auris skin colonization mouse model. Am. J. Infect. Control 2023, 51, 836–837. [Google Scholar] [CrossRef]
- Elbahr, U.; Khairy, A.; Dayyab, F.; Delos Reyes, C.S.; Pastrana, J.; Vineeth, C.; Hejres, S.; Sudha, S.P.; Keskin, O.; Rana, S.S.; et al. Can daily bathing with 4% chlorhexidine + daily chlorhexidine wipe for 1 week be effective in decolonizing Candida auris colonization? Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 243–247. [Google Scholar] [CrossRef]
- Gugsch, F.; Tan, C.K.; Oh, D.Y.; Paßvogel, L.; Steinhauer, K. Efficacy of octenidine- and chlorhexidine-based wash-mitts against Candida albicans and Candida auris—A comparative study. J. Hosp. Infect. 2024, 143, 91–96. [Google Scholar] [CrossRef]
- Rosa, R.; Abbo, L.M.; Jimenez, A.; Carter, C.; Ruiz, M.; Gerald, W.; Jimenez Hamann, M. Effectiveness of a sodium hypochlorite isotonic solution in decolonization of patients with Candida auris: Learnings from a county health care system. Am. J. Infect. Control 2024, 52, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Gerges, B.Z.; Rosenblatt, J.; Truong, Y.L.; Jiang, Y.; Raad, I.I. The antifungal activity of a polygalacturonic and caprylic acid ointment in an in vitro, three-dimensional wound biofilm model. J. Fungi 2025, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Gavilanes-Martínez, M.A.; Coral-Garzón, A.; Cáceres, D.H.; García, A.M. Antifungal activity of boric acid, triclosan and zinc oxide against different clinically relevant Candida species. Mycoses 2021, 64, 1045–1052. [Google Scholar] [CrossRef]
- Wu, W.G.; Luk, K.S.; Hung, M.F.; Tsang, W.Y.; Lee, K.P.; Lam, B.H.; Cheng, K.L.; Cheung, W.S.; Tang, H.L.; To, W.K. Antifungal efficacy of natural antiseptic products against Candida auris. Med. Mycol. 2024, 62, myae060. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and implications for infection control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).