Unmasking Bacillus Calmette–Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission—A Case Report
Abstract
1. Introduction
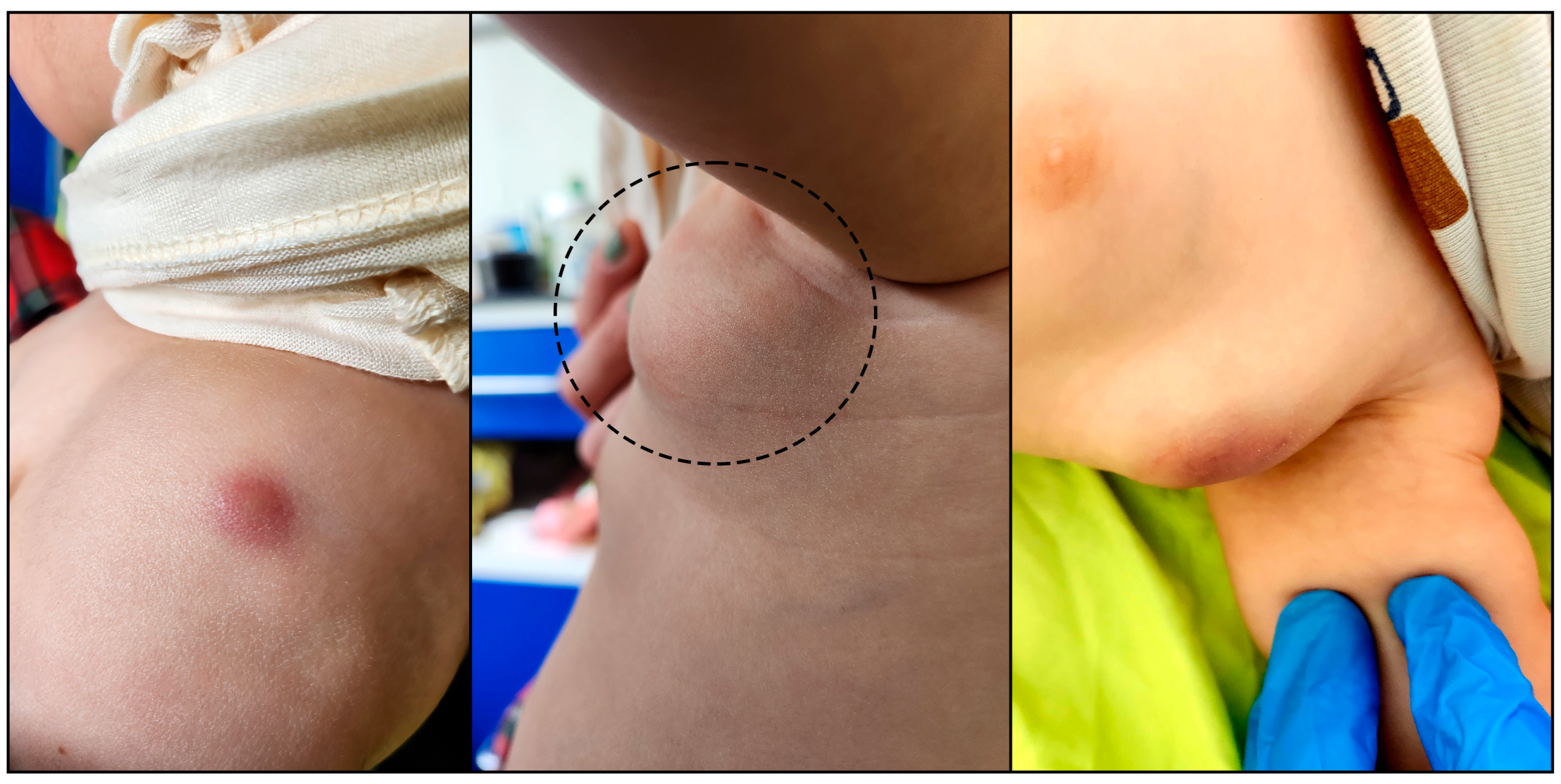
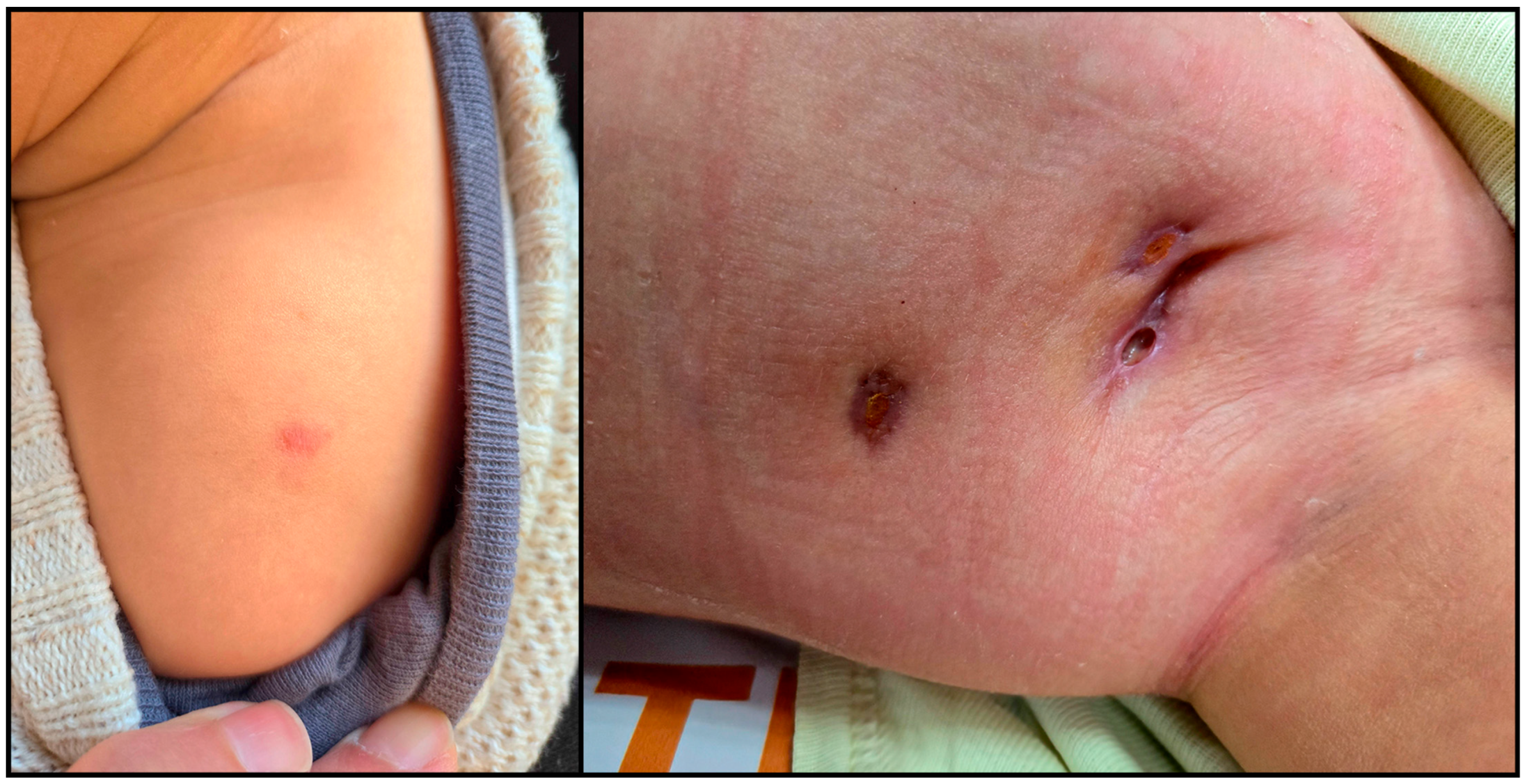
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotton, M.F.; Rabie, H.; Nemes, E.; Mujuru, H.; Bobat, R.; Njau, B.; Violari, A.; Mave, V.; Mitchell, C.; Oleske, J.; et al. A prospective study of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected children from high prevalence countries. PLoS ONE 2019, 14, e0211155. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.C.; de Araújo, L.C.; Medina-Acosta, E. Reduced rate of adverse reactions to the BCG vaccine in children exposed to the vertical transmission of HIV infection and in HIV-infected children from an endemic setting in Brazil. Eur. J. Pediatr. 2009, 168, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, M.; Liu, F.; Xu, X.; Feng, W.; Han, G.; Liu, X.; Zheng, B.; Geng, L.; Fu, T. The role of surgical management of BCG vaccine-induced regional suppurative lymphadenitis in children: A 7 years’ experience from one medical center. BMC Infect. Dis. 2021, 21, 801. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Yusuff, M.; Liebeschuetz, S.; Riddell, A.; Prendergast, A.J. Management and outcome of Bacille Calmette-Guérin vaccine adverse reactions. Vaccine 2015, 33, 5470–5474. [Google Scholar] [CrossRef] [PubMed]
- Cuello-García, C.A.; Pérez-Gaxiola, G.; Gutiérrez, C.J. Treating BCG-induced disease in children. Cochrane Database Syst. Rev. 2013, 2013, CD008300. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.M.K.; Sivanandham, R.; Pandrea, I.; Apetrei, C. BCG Vaccination and Mother-to-Infant Transmission of HIV. J. Infect. Dis. 2020, 222, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, R.V.; Veazey, R.S.; Wang, X. Immunopathogenesis in HIV-associated pediatric tuberculosis. Pediatr. Res. 2022, 91, 21–26. [Google Scholar] [CrossRef] [PubMed]
- WHO. BCG Vaccines: WHO Position Paper—February 2018—Vaccins BCG: Note de Synthèse de l’OMS—Février 2018; Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2018; Volume 93, pp. 73–96. [Google Scholar]
- Violari, A.; Cotton, M.F.; Gibb, D.M.; Babiker, A.G.; Steyn, J.; Madhi, S.A.; Jean-Philippe, P.; McIntyre, J.A.; CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N. Engl. J. Med. 2008, 359, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Rabie, H.; Violari, A.; Duong, T.; Madhi, S.A.; Josipovic, D.; Innes, S.; Dobbels, E.; Lazarus, E.; Panchia, R.; Babiker, A.G.; et al. Early antiretroviral treatment reduces risk of bacille Calmette-Guérin immune reconstitution adenitis. Int. J. Tuberc. Lung Dis. 2011, 15, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Muenchhoff, M.; Prendergast, A.J.; Goulder, P.J. Immunity to HIV in Early Life. Front. Immunol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Rockstroh, J.K. HIV 2023/2024; Medizin Fokus: Hamburg, Germany, 2023; pp. 8–234. [Google Scholar]
- Laughton, B.; Cornell, M.; Boivin, M.; Van Rie, A. Neurodevelopment in perinatally HIV-infected children: A concern for adolescence. J. Int. AIDS Soc. 2013, 16, 18603. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.A.; Crowel, C.S.; Njuguna, I.; Cranmer, L.M.; Wamalwa, D.; Chebet, D.; Otieno, V.; Maleche-Obimbo, E.; Gladstone, M.; John-Stewart, G.; et al. Improved Neurodevelopment After Initiation of Antiretroviral Therapy in Human Immunodeficiency Virus-infected Children. Pediatr. Infect. Dis. J. 2018, 37, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Callens, S.; Pahwa, S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr. Opin. HIV AIDS 2008, 3, 461–467. [Google Scholar] [CrossRef] [PubMed]
- NaserEddi, A.; Dinur-Schejter, Y.; Shadur, B.; Zaidman, I.; Even-Or, E.; Averbuch, D.; Shamriz, O.; Tal, Y.; Shaag, A.; Warnatz, K.; et al. Bacillus Calmette-Guerin (BCG) Vaccine-associated Complications in Immunodeficient Patients Following Stem Cell Transplantation. J. Clin. Immunol. 2021, 41, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Machava, P.; Joaquim, W.; Borrell, J.; Richardson, S.; Cassia, U.; Sidat, M.; Maieca, A.; Massitela, C.; Quelhas, Y.; Mucuila, C.; et al. Severe BCG immune reconstitution inflammatory syndrome lymphadenitis successfully managed with pre-antiretroviral counseling and a non-surgical approach: A case report. AIDS Res. Ther. 2024, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Elsidig, N.; Alshahrani, D.; Alshehri, M.; Alzahrani, M.; Alhajjar, S.; Aljummah, S.; Hussain, I.B.; Alshaalan, M.; Alzamil, F.; Alodyani, A.; et al. Bacillus Calmette-Guérin vaccine related lymphadenitis in children: Management guidelines endorsed by the Saudi Pediatric Infectious Diseases Society (SPIDS). Int. J. Pediatr. Adolesc. Med. 2015, 2, 89–95. [Google Scholar] [CrossRef] [PubMed]
- WHO Operational Handbook on Tuberculosis. Module 1: Prevention—Tuberculosis Preventive Treatment, 2nd ed.; World Health Organization: Geneva, Switzerland, 2024; pp. 25–65. [Google Scholar]
- WHO Operational Handbook on Tuberculosis. Module 5: Management of Tuberculosis in Children and Adolescents; World Health Organization: Geneva, Switzerland, 2022; pp. 5–52. [Google Scholar]
- Baghaei, P.; Tabarsi, P.; Farnia, P.; Radaei, A.H.; Kazempour, M.; Faghani, Y.A.; Mirsaeidi, M.; Novin, A.; Chitsaz, E.; Mansouri, D.; et al. Utility of Gastric Lavage for Diagnosis of Tuberculosis in Patients who are Unable to Expectorate Sputum. J. Glob. Infect. Dis. 2011, 3, 339–343. [Google Scholar] [CrossRef] [PubMed]
- CHIVA. HIV-Tuberculosis Co-Infections in Children. Available online: https://www.chiva.org.uk/wp-content/uploads/2022/02/CHIVA-TB_HIV_Coinfection.pdf (accessed on 7 March 2025).
- Fry, S.H.; Barnabas, S.L.; Cotton, M.F. Tuberculosis and HIV-An Update on the “Cursed Duet” in Children. Front. Pediatr. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, D.; Strashimirov, D.; Grozdeva, R.; Penchev, E.; Georgieva, E.; Yancheva, N. Unmasking Bacillus Calmette–Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission—A Case Report. Trop. Med. Infect. Dis. 2025, 10, 148. https://doi.org/10.3390/tropicalmed10060148
Ivanov D, Strashimirov D, Grozdeva R, Penchev E, Georgieva E, Yancheva N. Unmasking Bacillus Calmette–Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission—A Case Report. Tropical Medicine and Infectious Disease. 2025; 10(6):148. https://doi.org/10.3390/tropicalmed10060148
Chicago/Turabian StyleIvanov, Daniel, Dimitar Strashimirov, Rusina Grozdeva, Evgeni Penchev, Elena Georgieva, and Nina Yancheva. 2025. "Unmasking Bacillus Calmette–Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission—A Case Report" Tropical Medicine and Infectious Disease 10, no. 6: 148. https://doi.org/10.3390/tropicalmed10060148
APA StyleIvanov, D., Strashimirov, D., Grozdeva, R., Penchev, E., Georgieva, E., & Yancheva, N. (2025). Unmasking Bacillus Calmette–Guérin Immune Reconstitution Inflammatory Syndrome in a Perinatal HIV Transmission—A Case Report. Tropical Medicine and Infectious Disease, 10(6), 148. https://doi.org/10.3390/tropicalmed10060148




