Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases
Abstract
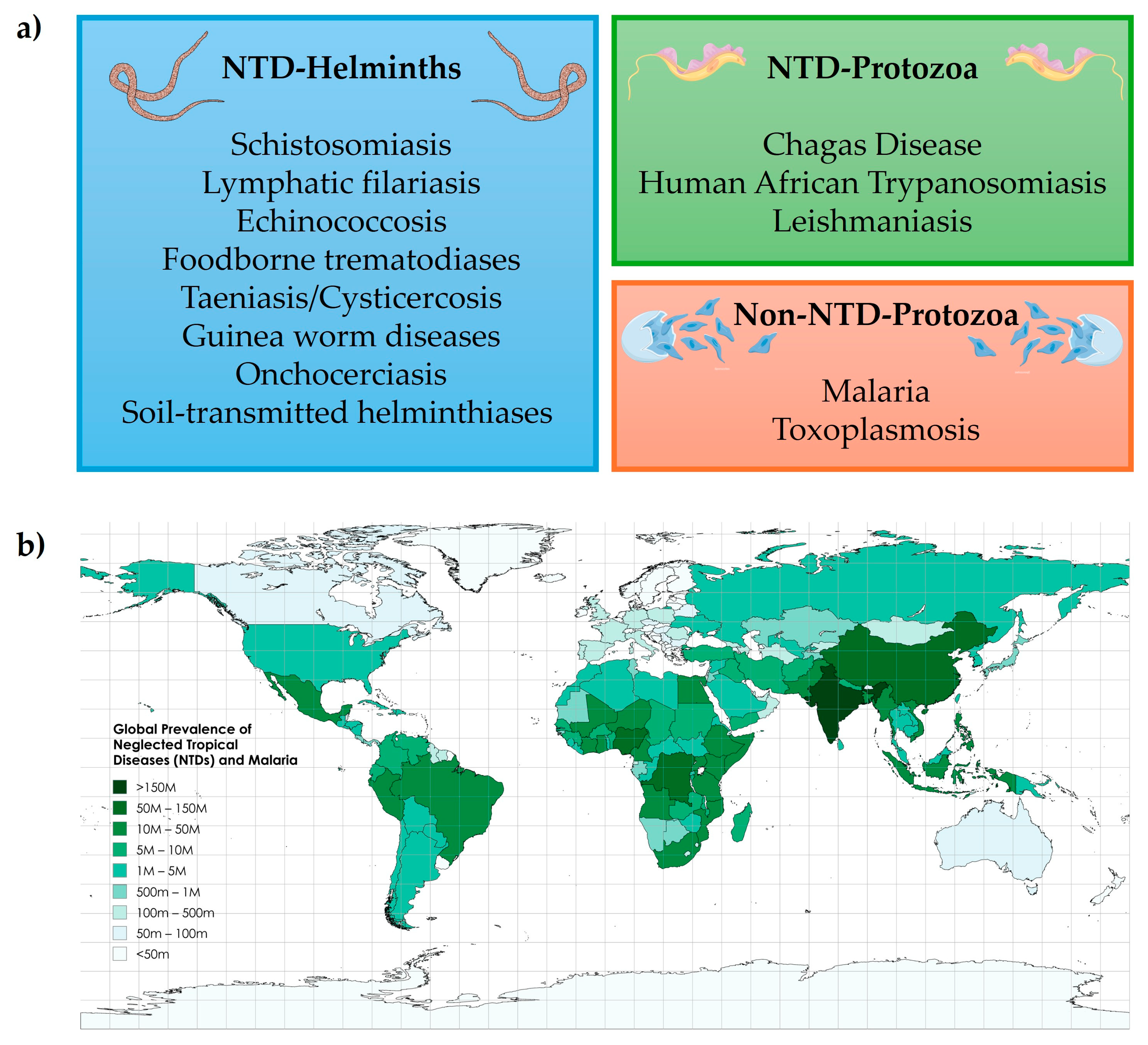
:1. Introduction
2. Structural and Mechanistic Features of Triazoles in Antiparasitic Drug Design
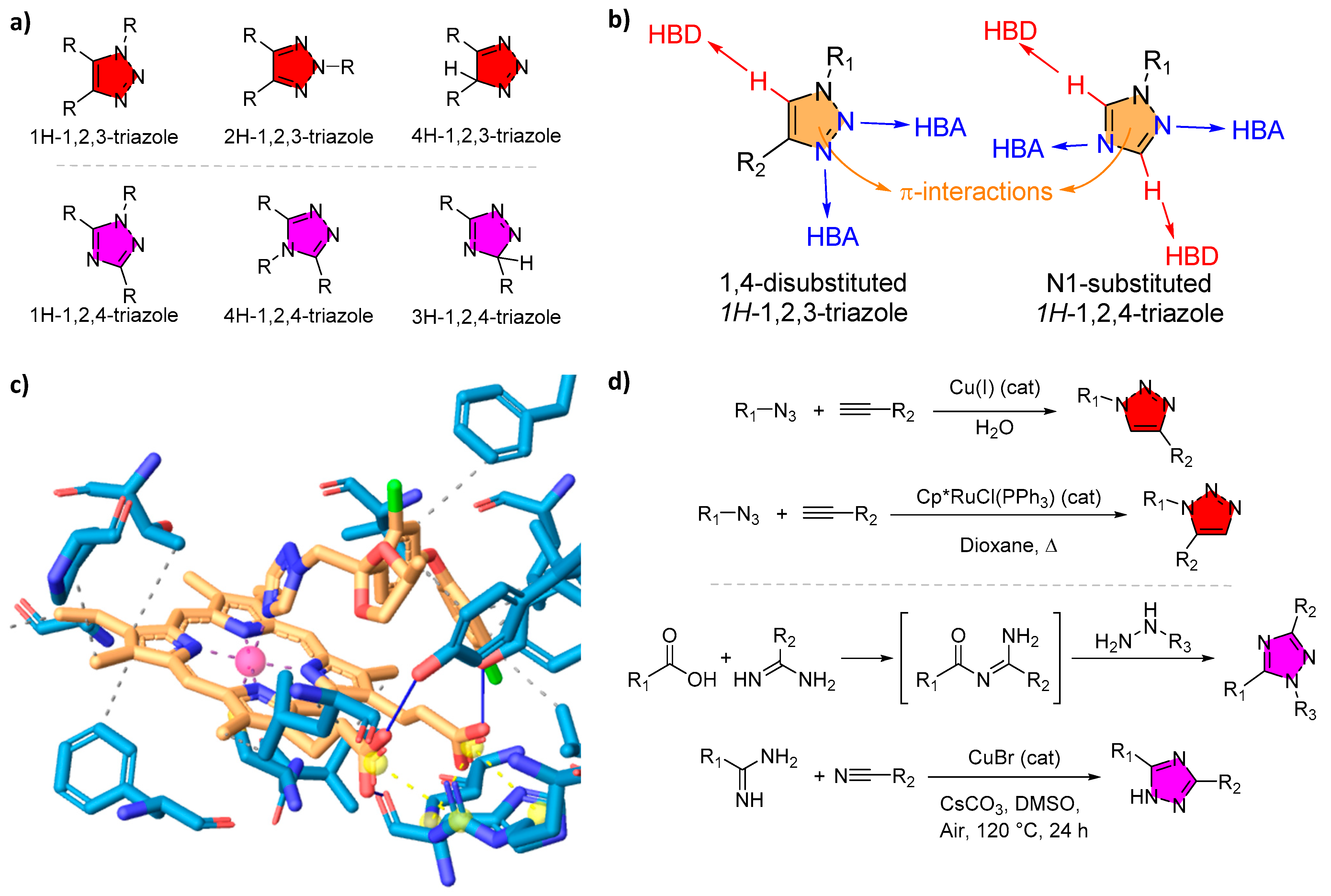
2.1. Triazole Isomers and Pharmacophore Properties
2.2. Coordination with Metals and the Inhibition of CYP Enzymes
2.3. “Click Chemistry” and Derivatization
2.4. Pharmacokinetics and Metabolic Stability
3. Current Progress of Triazole-Based Therapies for Protozoan Parasitic Diseases
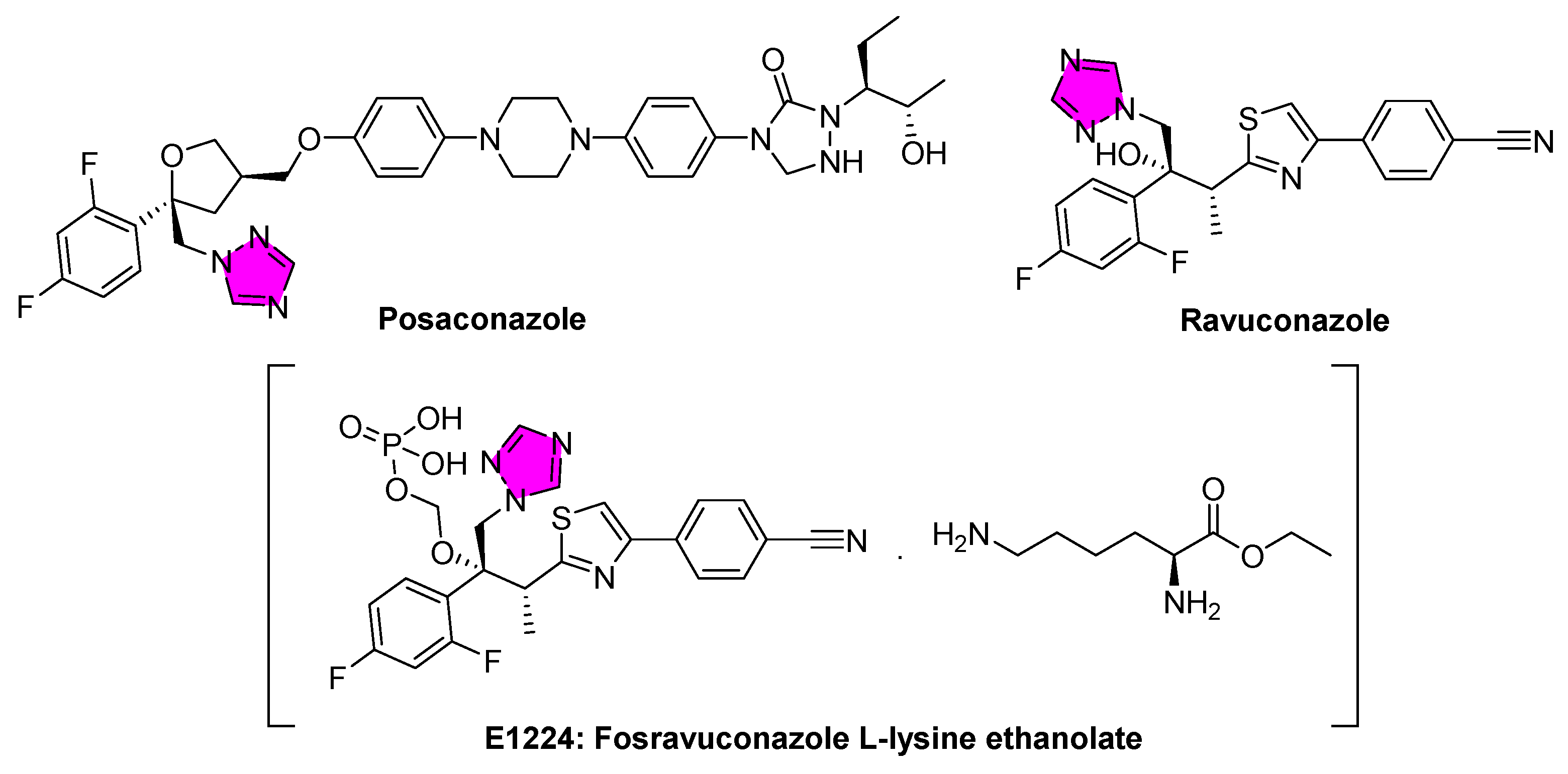
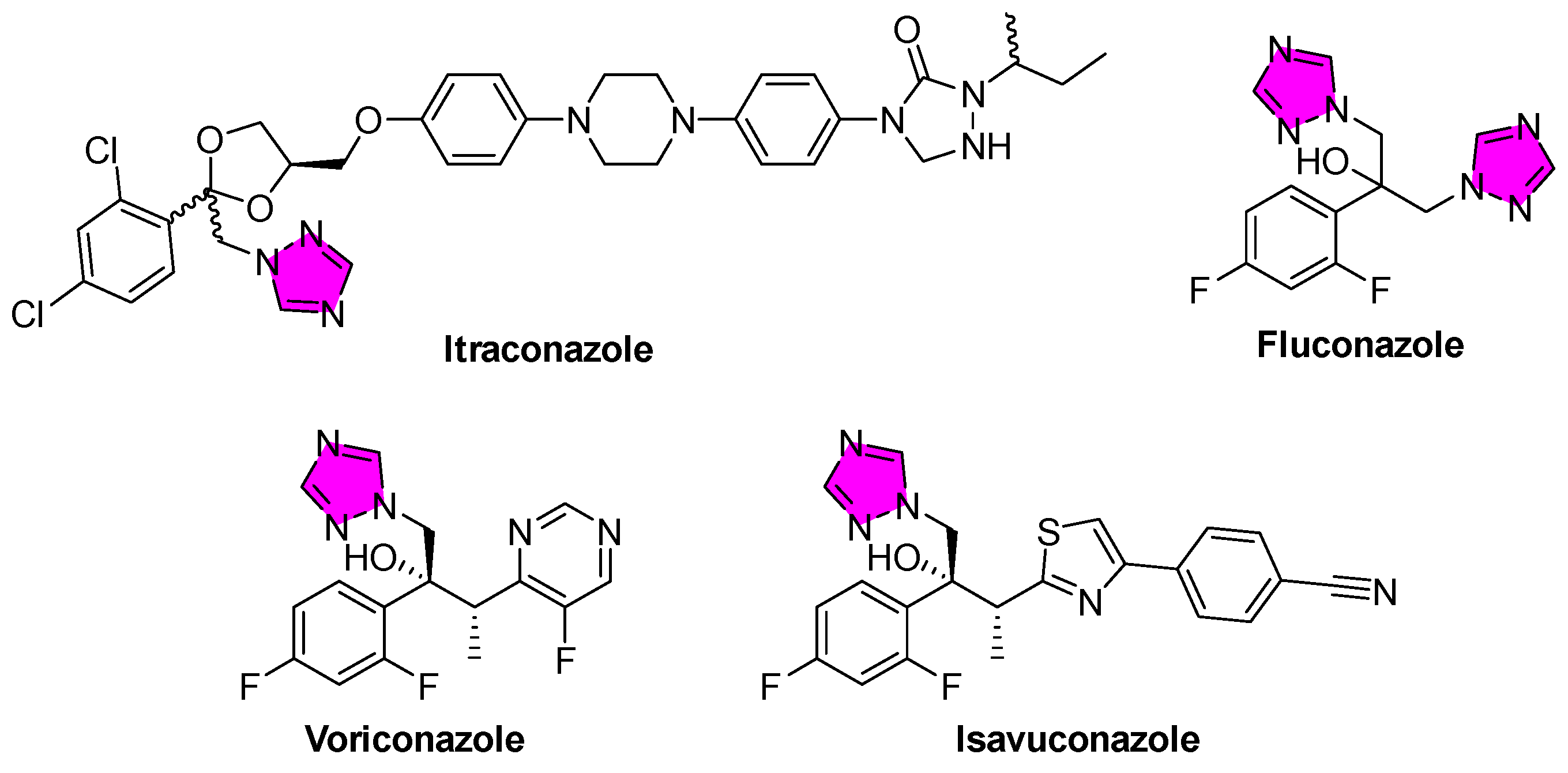
3.1. American Trypanosomiasis (Chagas Disease)
3.2. Leishmaniasis
3.3. Human African Trypanosomiasis (HAT)
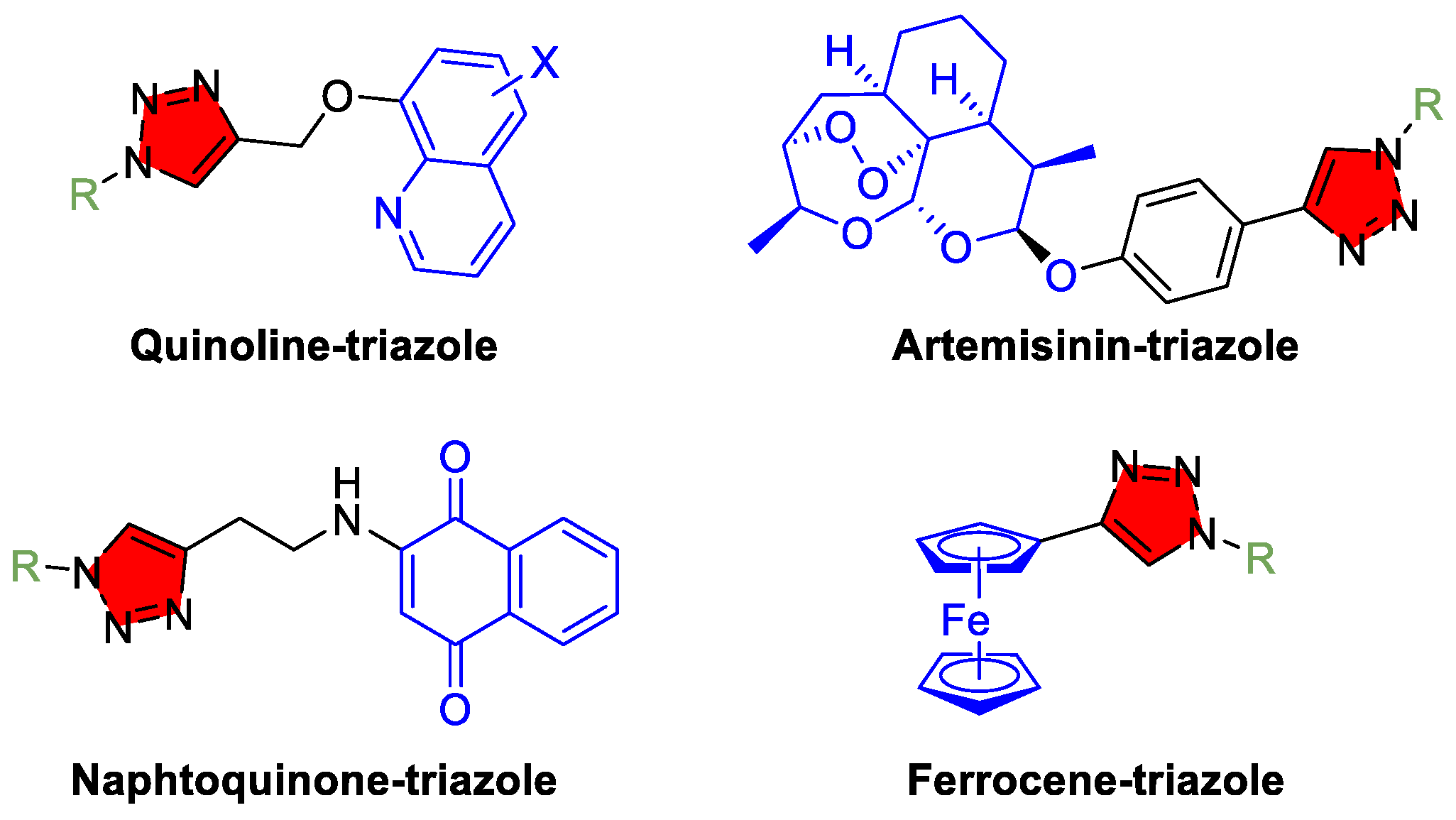
3.4. Malaria
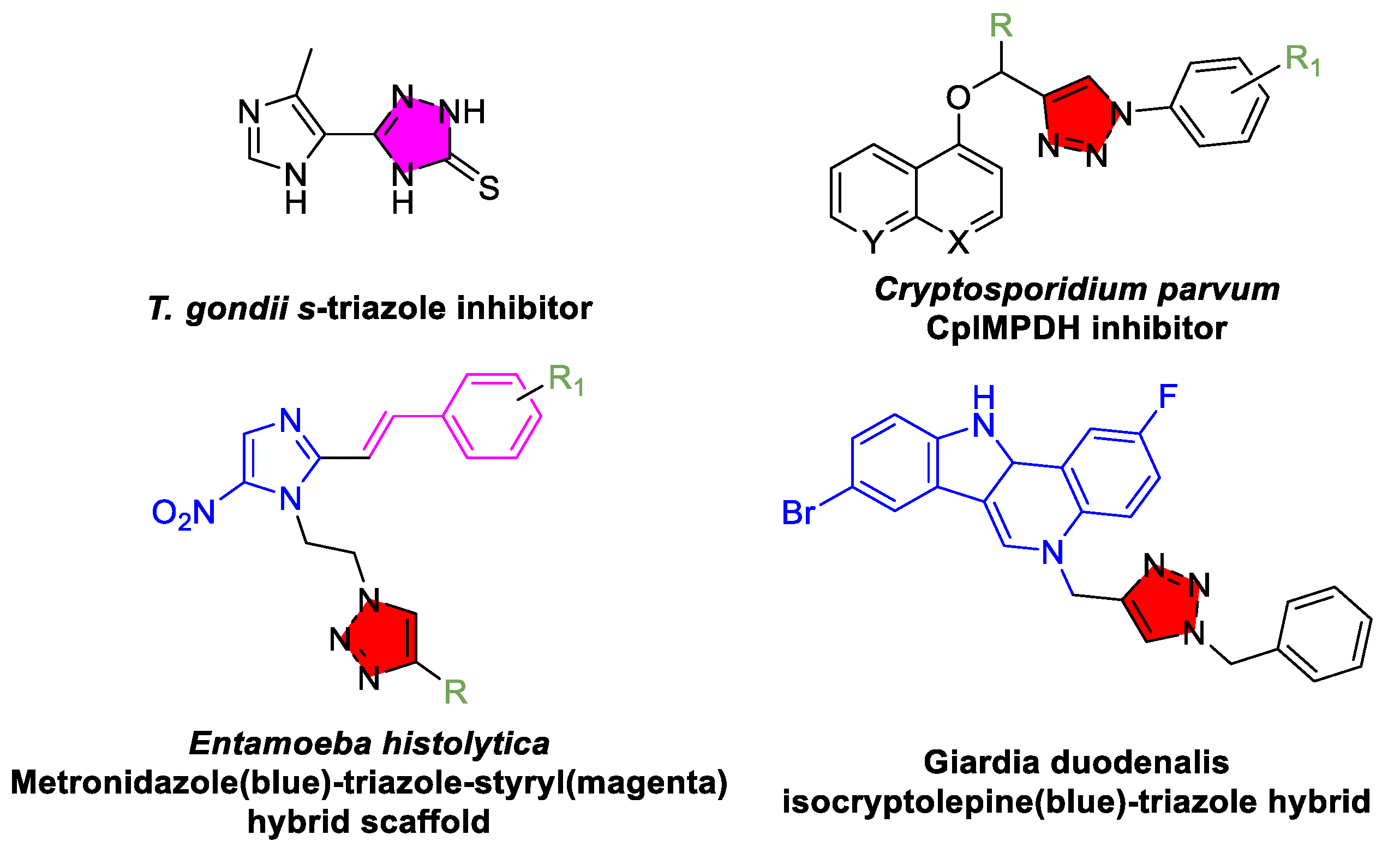
3.5. Toxoplasmosis and Other Protozoan Parasitic Diseases
4. Current Progress of Triazole-Based Therapies in Helminth Infections
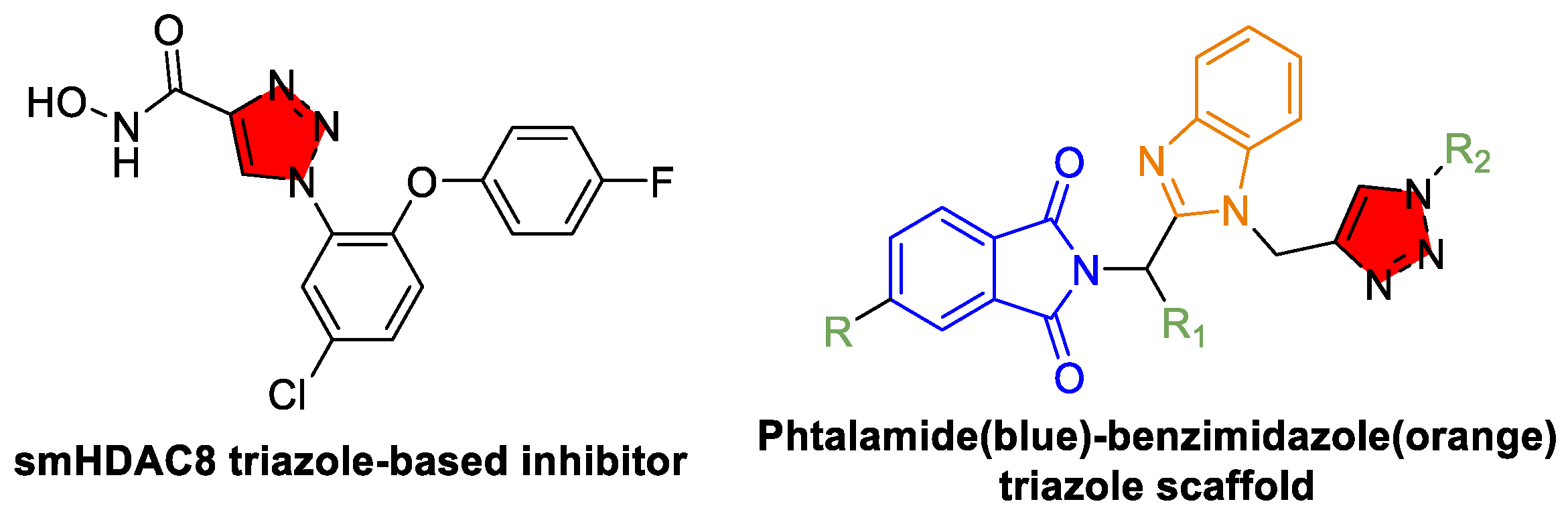
4.1. Schistosomiasis
4.2. Other Helminthic Infections
5. Resistance, Safety, and Other Considerations
5.1. Drug Resistance Mechanisms
5.2. Safety and Toxicity
5.3. Regulatory and Implementation Prospects
5.4. Innovative Delivery Systems
5.5. Environmental and One Health Considerations
6. Recent Trends and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTs | Artemisinin-based combination therapies |
| ADME | Absorption, distribution, metabolism, and excretion |
| CL | Cutaneous Leishmaniasis |
| CYP3A4 | Cytochrome P450 3A4 |
| CYP51 | Cytochrome P450 51 |
| CuAAC | Copper(I)-catalyzed azide–alkyne cycloaddition |
| DFMO | Difluoromethylornithine |
| DHODH | Dihydroorotate dehydrogenase |
| DNDi | Drugs for Neglected Diseases Initiative |
| DSM265 | Experimental triazole inhibitor of dihydroorotate dehydrogenase |
| E1224 | Fosravuconazole prodrug (phosphorylated form of ravuconazole) |
| FAERS | FDA Adverse Event Reporting System |
| FDA | Food and Drug Administration |
| HAT | Human African trypanosomiasis |
| HIV | Human immunodeficiency virus |
| IC50 | Half maximal inhibitory concentration |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LC50 | Lethal concentration 50 |
| MCL | Mucocutaneous Leishmaniasis |
| NTDs | Neglected tropical diseases |
| PDB | Protein Data Bank |
| PCR | Polymerase chain reaction |
| PLIP | Protein–ligand interaction profiler |
| PZQ | Praziquantel |
| SCYX-7158 | Acoziborole (experimental drug for HAT) |
| smHDAC8 | Schistosoma mansoni histone deacetylase 8 |
| VL | Visceral Leishmaniasis |
| VNI | Vinyl-imidazole inhibitor |
References
- WHO. Available online: https://www.who.int/health-topics/neglected-tropical-diseases (accessed on 2 January 2025).
- Fitzpatrick, C.; Nwankwo, U.; Lenk, E.; de Vlas, S.J.; Bundy, D.A. An Investment Case for Ending Neglected Tropical Diseases. In Major Infectious Diseases, 3rd ed.; Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; Chapter 17. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 2 January 2025).
- Andrade, M.V.; Noronha, K.; Diniz, B.P.C.; Guedes, G.; Carvalho, L.R.; Silva, V.A.; Calazans, J.A.; Santos, A.S.; Silva, D.N.; Castro, M.C. The economic burden of malaria: A systematic review. Malar. J. 2022, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, D.; Perez-Saez, J.; Vounatsou, P.; Utzinger, J.; Arcand, J.L. The economic impact of schistosomiasis. Infect. Dis. Poverty 2021, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef]
- Ribeiro, A.L.P.; RAISE Study Collaborators; Machado, Í.; Cousin, E.; Perel, P.; Demacq, C.; Geissbühler, Y.; de Souza, A.; Liprandi, A.S.; Nascimento, B.R.; et al. The Burden of Chagas Disease in the Contemporary World: The RAISE Study. Glob. Heart 2024, 19, 2. [Google Scholar] [CrossRef]
- Curtin, J.M.; Aronson, N.E. Leishmaniasis in the United States: Emerging Issues in a Region of Low Endemicity. Microorganisms 2021, 9, 578. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2022; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 1 May 2025).
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef]
- Brindha, J.; Balamurali, M.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases–Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 622286. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Asua, V.; Bailey, J.A.; Conrad, M.D.; Ishengoma, D.S.; Kamya, M.R.; Rasmussen, C.; Tadesse, F.G.; Uwimana, A.; Fidock, D.A. The Emergence of Artemisinin Partial Resistance in Africa: How Do We Respond? Lancet Infect. Dis. 2024, 24, e591–e600. [Google Scholar] [CrossRef]
- Porta, E.O.J. The Crucial Role of Drug Repositioning in Tackling Chagas Disease, Sleeping Sickness, and Leishmaniasis. INNOSC Theranostics Pharmacol. Sci. 2024, 7, 3721. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis Methods of 1,2,3-/1,2,4-Triazoles: A Review. Front. Chem. 2022, 10, 891484. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole Antifungals: A Review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.P.; Vennam, P.; Woods, C.M.; Krzyaniak, M.D.; Bowman, M.K.; Atkins, W.M. 1,2,3-Triazole-Heme Interactions in Cytochrome P450: Functionally Competent Triazole–Water–Heme Complexes. Biochemistry 2012, 51, 6441–6457. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An Insight on Medicinal Attributes of 1,2,4-Triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Friggeri, L.; Waterman, M.R. CYP51 as Drug Targets for Fungi and Protozoan Parasites: Past, Present and Future. Parasitology 2018, 145, 1820–1836. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, R.; Luo, Z.; Zhang, J.; Gao, Z.; Liu, R.; Liu, N.; Zhang, H.; Li, K.; Wu, X.; et al. Identification of Novel and Potent Triazoles Targeting CYP51 for Antifungal: Design, Synthesis, and Biological Study. Eur. J. Med. Chem. 2024, 280, 116942. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Lamb, D.C.; Guengerich, F.P.; Lepesheva, G.I. Structure-Functional Characterization of Cytochrome P450 Sterol 14α-Demethylase (CYP51B) from Aspergillus fumigatus and Molecular Basis for the Development of Antifungal Drugs. J. Biol. Chem. 2015, 290, 23916–23934. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Kalesh, K.; Steel, P.G. Navigating Drug Repurposing for Chagas Disease: Advances, Challenges, and Opportunities. Front. Pharmacol. 2023, 14, 1233253. [Google Scholar] [CrossRef]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a Strategy for the Discovery of New Anti-Leishmanials: The-State-of-the-Art. Parasitology 2018, 145, 219–236. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Villalta, F.; Waterman, M.R. Targeting Trypanosoma cruzi Sterol 14α-Demethylase (CYP51). Adv. Parasitol. 2011, 75, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4- and 1,5-Disubstituted 1,2,3-Triazoles Good Pharmacophoric Groups? ChemMedChem 2014, 9, 2497–2508. [Google Scholar] [CrossRef]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-Triazole “All-in-One” Ring System in Drug Discovery: A Good Bioisostere, a Good Pharmacophore, a Good Linker, and a Versatile Synthetic Tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef] [PubMed]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-Triazole Ring as a Bioisostere in Medicinal Chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Song, B.; Park, E.Y.; Kim, K.J.; Ki, S.H. Repurposing of Benzimidazole Anthelmintic Drugs as Cancer Therapeutics. Cancers 2022, 14, 4601. [Google Scholar] [CrossRef]
- Martin, F.; Halvarsson, P.; Delhomme, N.; Höglund, J.; Tydén, E. Exploring the β-Tubulin Gene Family in a Benzimidazole-Resistant Parascaris univalens Population. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 84–91. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma., A.; Joshi., K.; Singh., B.; Sharma, S.; Batra, K.; Kaur, K.; Singh, D.; Chadha, R.; et al. 1,2,3-Triazole Derivatives as an Emerging Scaffold for Antifungal Drug Development against Candida albicans: A Comprehensive Review. Chem. Biodivers. 2023, 20, e202300024. [Google Scholar] [CrossRef]
- McCreary, E.K.; Davis, M.R.; Narayanan, N.; Andes, D.R.; Cattaneo, D.; Christian, R.; Lewis, R.E.; Watt, K.M.; Wiederhold, N.P.; Johnson, M.D. Utility of triazole antifungal therapeutic drug monitoring: Insights from the Society of Infectious Diseases Pharmacists: Endorsed by the Mycoses Study Group Education and Research Consortium. Pharmacotherapy 2023, 43, 1043–1050. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Humayun, M.J.; Wadhwa, R. Rufinamide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557595/ (accessed on 28 February 2025).
- Carlucci, R.; Lisa, M.N.; Labadie, G.R. 1,2,3-Triazoles in Biomolecular Crystallography: A Geometrical Data-Mining Approach. J. Med. Chem. 2023, 66, 14377–14390. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, J.; Hu, H.; Yu, S.; Sun, Q.; Dan, Z.; Jiang, Y.; Wu, Q. Design, Synthesis, and Biological Evaluation of Novel Triazole Derivatives as Inhibitors of Cytochrome P450 14α-Demethylase. Eur. J. Med. Chem. 2009, 44, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.; Parker, J.E.; Kelly, D.E.; Kelly, S.L. Azole Affinity of Sterol 14α-Demethylase (CYP51) Enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 2013, 57, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.H.; Warhurst, D.C.; Bhattacharyya, T.; Au, H.Y.A.; Le, H.; Giardini, M.A.; Pascoalino, B.S.; Torrecilhas, A.C.; Romera, L.M.D.; Madeira, R.P.; et al. Novel Structural CYP51 Mutation in Trypanosoma cruzi Associated with Multidrug Resistance to CYP51 Inhibitors and Reduced Infectivity. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 107–120. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A Review: Pharmacological Aspects of Metal Based 1,2,4-Triazole Derived Schiff Bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef]
- Couto Rodrigues, S.; Silva Moratório de Moraes, R.; Tavares de Almeida Pinto, G.; Miranda Martins, M.T.; Antunes do Nascimento, P.; Alves Soares, D.L.; Mestre Botelho, A.B.; Cardoso Cruz, C.; Cunha, A.C. A Review on Chemistry and Methods of Synthesis of 1,2,4-Triazole Derivatives. Chem. Rec. 2025, 25, e202400190. [Google Scholar] [CrossRef]
- Vala, D.P.; Vala, R.M.; Patel, H.M. Versatile Synthetic Platform for 1,2,3-Triazole Chemistry. ACS Omega 2022, 7, 36945–36987. [Google Scholar] [CrossRef]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Lotfi Efrit, M.; Prim, D. Recent Advances in the Chemistry of 1,2,4-Triazoles: Synthesis, Reactivity and Biological Activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Lv, Z.; Zhang, G.; Xu, Z. Recent Updates on 1,2,3-Triazole-Containing Hybrids with In Vivo Therapeutic Potential Against Cancers: A Mini-Review. Eur. J. Med. Chem. 2023, 251, 115254. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahman, S.M.; Bhatti, J.S.; Thareja, S.; Monga, V. Current Development of 1,2,3-Triazole Derived Potential Antimalarial Scaffolds: Structure-Activity Relationship (SAR) and Bioactive Compounds. Eur. J. Med. Chem. 2023, 259, 115699. [Google Scholar] [CrossRef]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Hassan, N.I. Triazole Hybrid Compounds: A New Frontier in Malaria Treatment. Eur. J. Med. Chem. 2023, 259, 115694. [Google Scholar] [CrossRef]
- Xia, J.; Huang, J.; Cai, M. Heterogeneous Copper(I)-Catalyzed Cascade Addition–Oxidative Cyclization of Nitriles with 2-Aminopyridines or Amidines: Efficient and Practical Synthesis of 1,2,4-Triazoles. Synthesis 2019, 51, 2014–2022. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Degirmenci, A.; Sanyal, R.; Sanyal, A. Metal-Free Click-Chemistry: A Powerful Tool for Fabricating Hydrogels for Biomedical Applications. Bioconjug. Chem. 2024, 35, 433–452. [Google Scholar] [CrossRef]
- Laverdiere, M.; Bow, E.J.; Rotstein, C.; Autmizguine, J.; Broady, R.; Garber, G.; Haider, S.; Hussaini, T.; Husain, S.; Ovetchkine, P.; et al. Therapeutic Drug Monitoring for Triazoles: A Needs Assessment Review and Recommendations from a Canadian Perspective. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 327–343. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Bělonožníková, K.; Jakl, M.; Chmelík, J.; Kovač, I.; Ryšlavá, H. Non-Target Biotransformation Enzymes as a Target for Triazole-Zinc Mixtures. Chem. Biol. Interact. 2023, 382, 110625. [Google Scholar] [CrossRef]
- Azanza, J.R.; Mensa, J.; Barberán, J.; Vázquez, L.; Pérez de Oteyza, J.; Kwon, M.; Yáñez, L.; Aguado, J.M.; Cubillo Gracian, A.; Solano, C.; et al. Recommendations on the Use of Azole Antifungals in Hematology-Oncology Patients. Rev. Esp. Quimioter. 2023, 36, 236–258. [Google Scholar] [CrossRef]
- Dooley, K.E.; Flexner, C.; Andrade, A.S. Drug interactions involving combination antiretroviral therapy and other anti-infective agents: Repercussions for resource-limited countries. J. Infect. Dis. 2008, 198, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., 2nd; Wiederhold, N.P.; Hakki, M.; Thompson, G.R. 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob. Agents Chemother. 2022, 66, e0017722. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease Drug Development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.L.; Soares, M.J.; Probst, C.M.; Krieger, M.A. Trypanosoma cruzi Response to Sterol Biosynthesis Inhibitors: Morphophysiological Alterations Leading to Cell Death. PLoS ONE 2013, 8, e55497. [Google Scholar] [CrossRef]
- Molina, J.; Martins-Filho, O.; Brener, Z.; Romanha, A.J.; Loebenberg, D.; Urbina, J.A. Activities of the Triazole Derivative SCH 56592 (Posaconazole) against Drug-Resistant Strains of the Protozoan Parasite Trypanosoma (Schizotrypanum) cruzi in Immunocompetent and Immunosuppressed Murine Hosts. Antimicrob. Agents Chemother. 2000, 44, 150–155. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I. Treatment of Adult Chronic Indeterminate Chagas Disease with Benznidazole and Three E1224 Dosing Regimens: A Proof-of-Concept, Randomised, Placebo-Controlled Trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Calvet, C.M.; Silva, T.A.; Thomas, D.; Suzuki, B.; Hirata, K.; Siqueira-Neto, J.L.; McKerrow, J.H. Long Term Follow-Up of Trypanosoma cruzi Infection and Chagas Disease Manifestations in Mice Treated with Benznidazole or Posaconazole. PLoS Negl. Trop. Dis. 2020, 14, e0008726. [Google Scholar] [CrossRef]
- Diniz Lde, F.; Urbina, J.A.; de Andrade, I.M.; Mazzeti, A.L.; Martins, T.A.; Caldas, I.S.; Talvani, A.; Ribeiro, I.; Bahia, M.T. Benznidazole and Posaconazole in Experimental Chagas Disease: Positive Interaction in Concomitant and Sequential Treatments. PLoS Negl. Trop. Dis. 2013, 7, e2367. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Diniz, L.F.; Gonçalves, K.R.; WonDollinger, R.S.; Assíria, T.; Ribeiro, I.; Bahia, M.T. Synergic Effect of Allopurinol in Combination with Nitroheterocyclic Compounds against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2019, 63, e02264-18. [Google Scholar] [CrossRef]
- Almeida-Silva, J.; Menezes, D.S.; Fernandes, J.M.P.; Almeida, M.C.; Vasco-Dos-Santos, D.R.; Saraiva, R.M.; Viçosa, A.L.; Perez, S.A.C.; Andrade, S.G.; Suarez-Fontes, A.M.; et al. The Repositioned Drugs Disulfiram/Diethyldithiocarbamate Combined to Benznidazole: Searching for Chagas Disease Selective Therapy, Preventing Toxicity and Drug Resistance. Front. Cell Infect. Microbiol. 2022, 12, 926699. [Google Scholar] [CrossRef]
- Saraiva, R.M.; Portela, L.F.; Silveira, G.P.E.; Gomes, N.L.S.; Pinto, D.P.; Silva, A.C.d.A.d.; Sangenis, L.H.; Carneiro, F.M.; Almeida-Silva, J.; Marinho, P.W.; et al. Disulfiram Repurposing in the Combined Chemotherapy of Chagas Disease: A Protocol for Phase I/II Clinical Trial. Med. Case Rep. Study Protoc. 2021, 2, e0110. [Google Scholar] [CrossRef]
- Porta, E.O.; Carvalho, P.B.; Avery, M.A.; Tekwani, B.L.; Labadie, G.R. Click Chemistry Decoration of Amino Sterols as a Promising Strategy to Develop New Leishmanicidal Drugs. Steroids 2014, 79, 28–36. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Jäger, S.N.; Nocito, I.; Lepesheva, G.I.; Serra, E.C.; Tekwani, B.L.; Labadie, G.R. Antitrypanosomal and Antileishmanial Activity of Prenyl-1,2,3-Triazoles. Medchemcomm 2017, 8, 1015–1021. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Ballari, M.S.; Carlucci, R.; Wilkinson, S.; Ma, G.; Tekwani, B.L.; Labadie, G.R. Systematic Study of 1,2,3-Triazolyl Sterols for the Development of New Drugs against Parasitic Neglected Tropical Diseases. Eur. J. Med. Chem. 2023, 254, 115378. [Google Scholar] [CrossRef]
- Chen, C.K.; Leung, S.S.; Guilbert, C.; Jacobson, M.P.; McKerrow, J.H.; Podust, L.M. Structural Characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei Bound to the Antifungal Drugs Posaconazole and Fluconazole. PLoS Negl. Trop. Dis. 2010, 4, e651. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Muñoz, J.; Posada, E.; López-Chejade, P.; Gállego, M.; Ayala, E.; del Cacho, E.; Soy, D.; Gascon, J. Tolerance of Benznidazole in Treatment of Chagas’ Disease in Adults. Antimicrob. Agents Chemother. 2010, 54, 4896–4899. [Google Scholar] [CrossRef]
- Buckner, F.S. Sterol 14-Demethylase Inhibitors for Trypanosoma cruzi Infections. Adv. Exp. Med. Biol. 2008, 625, 61–80. [Google Scholar] [CrossRef]
- Diniz Lde, F.; Caldas, I.S.; Guedes, P.M.; Crepalde, G.; de Lana, M.; Carneiro, C.M.; Talvani, A.; Urbina, J.A.; Bahia, M.T. Effects of Ravuconazole Treatment on Parasite Load and Immune Response in Dogs Experimentally Infected with Trypanosoma cruzi. Antimicrob. Agents Chemother. 2010, 54, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Specific Chemotherapy of Chagas Disease: Relevance, Current Limitations and New Approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H. Potential of Ravuconazole and Its Prodrugs as the New Oral Therapeutics for Onychomycosis. Med. Mycol. J. 2016, 57, E93–E110. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.F.; Mazzeti, A.L.; Caldas, I.S.; Ribeiro, I.; Bahia, M.T. Outcome of E1224-Benznidazole Combination Treatment for Infection with a Multidrug-Resistant Trypanosoma cruzi Strain in Mice. Antimicrob. Agents Chemother. 2018, 62, e00401-18. [Google Scholar] [CrossRef]
- Ribeiro, I.; Blum, B.; Fernandes, J.; Santina, G.; Asada, M.; Everson, M.; Schuck, E.; Feleder, E.; Evene, E.; Gualano, V. Drug-Drug Interaction Study of Benznidazole and E1224 in Healthy Male Volunteers. Antimicrob. Agents Chemother. 2021, 65, e02150-19. [Google Scholar] [CrossRef] [PubMed]
- Hata, K. Development of E1224 by Leveraging a Strategic Partnership for the Medicines Creation Against Neglected Tropical Diseases. Parasitol. Int. 2021, 81, 102278. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New Regimens of Benznidazole Monotherapy and in Combination with Fosravuconazole for Treatment of Chagas Disease (BENDITA): A Phase 2, Double-Blind, Randomised Trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized Trial of Posaconazole and Benznidazole for Chronic Chagas’ Disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An Update on Pharmacotherapy for Leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Pandharkar, T.; Zhu, X.; Mathur, R.; Jiang, J.; Schmittgen, T.D.; Shaha, C.; Werbovetz, K.A. Studies on the Antileishmanial Mechanism of Action of the Arylimidamide DB766: Azole Interactions and Role of CYP5122A1. Antimicrob. Agents Chemother. 2014, 58, 4682–4689. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Wali, J.P.; Aggarwal, P.; Gupta, U.; Saluja, S.; Singh, S. Ketoconazole in Treatment of Visceral Leishmaniasis. Lancet 1990, 336, 810–811. [Google Scholar] [CrossRef]
- Calvopina, M.; Guevara, A.G.; Armijos, R.X.; Hashiguchi, Y.; Davidson, R.N.; Cooper, P.J. Itraconazole in the Treatment of New World Mucocutaneous Leishmaniasis. Int. J. Dermatol. 2004, 43, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jin, Y.; Yang, S.; Joachim, A.M.; Ning, Y.; Mori-Quiroz, L.M.; Fromm, J.; Perera, C.; Zhang, K.; Werbovetz, K.A.; et al. Sterol Profiling of Leishmania Parasites Using a New HPLC-Tandem Mass Spectrometry-Based Method and Antifungal Azoles as Chemical Probes Reveals a Key Intermediate Sterol That Supports a Branched Ergosterol Biosynthetic Pathway. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 27–42. [Google Scholar] [CrossRef]
- Benaim, G.; Sanders, J.M.; Garcia-Marchán, Y.; Colina, C.; Lira, R.; Caldera, A.R.; Payares, G.; Sanoja, C.; Burgos, J.M.; Leon-Rossell, A.; et al. Amiodarone Has Intrinsic Anti-Trypanosoma cruzi Activity and Acts Synergistically with Posaconazole. J. Med. Chem. 2006, 49, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdely, H.M.; Graybill, J.R.; Loebenberg, D.; Melby, P.C. Efficacy of the Triazole SCH 56592 against Leishmania amazonensis and Leishmania donovani in Experimental Murine Cutaneous and Visceral Leishmaniases. Antimicrob. Agents Chemother. 1999, 43, 2910–2914. [Google Scholar] [CrossRef]
- Fernández, O.L.; Rosales-Chilama, M.; Quintero, N.; Travi, B.L.; Wetzel, D.M.; Gómez, M.A.; Saravia, N.G. Potency and Preclinical Evidence of Synergy of Oral Azole Drugs and Miltefosine in an Ex Vivo Model of Leishmania (Viannia) panamensis Infection. Antimicrob. Agents Chemother. 2022, 66, e0142521. [Google Scholar] [CrossRef]
- Gupta, Y.; Goicoechea, S.; Romero, J.G.; Mathur, R.; Caulfield, T.R.; Becker, D.P.; Durvasula, R.; Kempaiah, P. Repurposing Lansoprazole and Posaconazole to Treat Leishmaniasis: Integration of In Vitro Testing, Pharmacological Corroboration, and Mechanisms of Action. J. Food Drug Anal. 2022, 30, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Paniz Mondolfi, A.E.; Stavropoulos, C.; Gelanew, T.; Loucas, E.; Perez Alvarez, A.M.; Benaim, G.; Polsky, B.; Schoenian, G.; Sordillo, E.M. Successful Treatment of Old World Cutaneous Leishmaniasis Caused by Leishmania infantum with Posaconazole. Antimicrob. Agents Chemother. 2011, 55, 1774–1776. [Google Scholar] [CrossRef]
- Mengeot, L.; Yombi, J.C.; Baeck, M. Cutaneous Leishmaniasis Due to Leishmania aethiopica: A Therapeutic Challenge. JAAD Case Rep. 2021, 20, 72–75. [Google Scholar] [CrossRef]
- Elfatoiki, F.Z.; Boumezzourh, A.; Maksouri, H.; Elkhalfaoui, N.; Dessay, M.; Riyad, M.; Chiheb, S. Leishmaniose Cutanée du Vermillon avec Atteinte Muqueuse de la Lèvre Supérieure [Cutaneous Leishmaniasis of the Vermilion Border of the Upper Lip Extending to the Oral Mucosa]. Ann. Dermatol. Venereol. 2020, 147, 116–118. [Google Scholar] [CrossRef]
- Benzaquen, M.; Chambelland, A.; Fongue, J.; Melenotte, C.; Christen, J.R.; Ranque, S.; Buono, J.P.; Parola, P.; Berbis, P. Cutaneous Sporotrichoid Leishmaniasis Treated with Oral Fluconazole. Dermatol. Ther. 2019, 32, e12976. [Google Scholar] [CrossRef]
- Prates, F.V.; Dourado, M.E.; Silva, S.C.; Schriefer, A.; Guimarães, L.H.; Brito, M.D.; Almeida, J.; Carvalho, E.M.; Machado, P.R. Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 64, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, V.A.; Francesconi, F.; Ramasawmy, R.; Romero, G.A.S.; Alecrim, M.D.G.C. Failure of Fluconazole in Treating Cutaneous Leishmaniasis Caused by Leishmania guyanensis in the Brazilian Amazon: An Open, Nonrandomized Phase 2 Trial. PLoS Negl. Trop. Dis. 2018, 12, e0006225. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Oryan, A.; Bemani, E. Efficacy of Amiodarone and Voriconazole Combination Therapy in Cutaneous Leishmaniasis in the Mice Experimentally Infected with Leishmania major. J. Infect. Chemother. 2021, 27, 984–990. [Google Scholar] [CrossRef]
- Galvão, E.L.; Rabello, A.; Cota, G.F. Efficacy of Azole Therapy for Tegumentary Leishmaniasis: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0186117. [Google Scholar] [CrossRef]
- de Macedo-Silva, S.T.; Urbina, J.A.; de Souza, W.; Rodrigues, J.C. In Vitro Activity of the Antifungal Azoles Itraconazole and Posaconazole against Leishmania amazonensis. PLoS ONE 2013, 8, e83247. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; Hachem, R.Y.; Chemaly, R.F.; Kontoyiannis, D.P.; Raad, I.I. Posaconazole: A Broad-Spectrum Triazole Antifungal. Lancet Infect. Dis. 2005, 5, 775–785. [Google Scholar] [CrossRef]
- Mashayekhi Goyonlo, V.; Derakhshan, Z.; Darchini-Maragheh, E. Treatment of Cutaneous Leishmaniasis with Allopurinol Plus Itraconazole in Iran. Am. J. Trop. Med. Hyg. 2023, 108, 1164–1166. [Google Scholar] [CrossRef]
- Bhusal, C.K.; Beniwal, P.; Singh, S.; Kaur, D.; Kaur, U.; Kaur, S.; Sehgal, R. Possibility of Re-Purposing Antifungal Drugs Posaconazole & Isavuconazole against Promastigote Form of Leishmania major. Indian J. Med. Res. 2024, 160, 466–478. [Google Scholar] [CrossRef]
- Valverde Mordt, O.; Tarral, A.; Strub-Wourgaft, N. Development and Introduction of Fexinidazole into the Global Human African Trypanosomiasis Program. Am. J. Trop. Med. Hyg. 2022, 106, 61–66. [Google Scholar] [CrossRef]
- Nes, C.R.; Singha, U.K.; Liu, J.; Ganapathy, K.; Villalta, F.; Waterman, M.R.; Lepesheva, G.I.; Chaudhuri, M.; Nes, W.D. Novel sterol metabolic network of Trypanosoma brucei procyclic and bloodstream forms. Biochem. J. 2012, 443, 267–277. [Google Scholar] [CrossRef]
- Wiedemar, N.; Graf, F.E.; Zwyer, M.; Ndomba, E.; Kunz Renggli, C.; Cal, M.; Schmidt, R.S.; Wenzler, T.; Mäser, P. Beyond immune escape: A variant surface glycoprotein causes suramin resistance in Trypanosoma brucei. Mol. Microbiol. 2018, 107, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, V.; Black, S.J. Selective inhibition of the uptake by bloodstream form Trypanosoma brucei brucei of serum lipoprotein-associated phospholipid and cholesteryl ester. Mol. Biochem. Parasitol. 1990, 41, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cleghorn, L.A.T.; Wall, R.J.; Albrecht, S.; MacGowan, S.A.; Norval, S.; De Rycker, M.; Woodland, A.; Spinks, D.; Thompson, S.; Patterson, S.; et al. Development of a 2,4-Diaminothiazole Series for the Treatment of Human African Trypanosomiasis Highlights the Importance of Static-Cidal Screening of Analogues. J. Med. Chem. 2023, 66, 8896–8916. [Google Scholar] [CrossRef] [PubMed]
- Seetsi, A.; N’Da, D.D.; Nyembe, N.; Suganuma, K.; Ramatla, T.; Thekisoe, O. In Vitro Antitrypanosomal Activity of Synthesized Nitrofurantoin-Triazole Hybrids against Trypanosoma Species Causing Animal African Trypanosomosis. Exp. Parasitol. 2024, 259, 108711. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The Pipeline for Drugs for Control and Elimination of Neglected Tropical Diseases: 1. Anti-Infective Drugs for Regulatory Registration. Parasit. Vectors 2023, 16, 82. [Google Scholar] [CrossRef]
- Betu Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Valverde Mordt, O.; Ngolo Tete, D.; Prêtre, A.; Delhomme, S.; Ilunga Wa Kyhi, M.; Camara, M.; Catusse, J.; et al. Efficacy and Safety of Acoziborole in Patients with Human African Trypanosomiasis Caused by Trypanosoma brucei gambiense: A Multicentre, Open-Label, Single-Arm, Phase 2/3 Trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Wang, W.L.; Liang, L.L.; Liu, W.; Gong, K.K.; Sun, K.L. Triazole Derivatives and Their Antiplasmodial and Antimalarial Activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Iso-O, N.; Komatsuya, K.; Tokumasu, F.; Isoo, N.; Ishigaki, T.; Yasui, H.; Yotsuyanagi, H.; Hara, M.; Kita, K. Malaria Parasites Hijack Host Receptors from Exosomes to Capture Lipoproteins. Front. Cell Dev. Biol. 2021, 9, 749153. [Google Scholar] [CrossRef]
- Tran, P.N.; Brown, S.H.; Rug, M.; Ridgway, M.C.; Mitchell, T.W.; Maier, A.G. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 2016, 15, 73. [Google Scholar] [CrossRef]
- Herrmann, L.; Leidenberger, M.; Quadros, H.C.; Grau, B.W.; Hampel, F.; Friedrich, O.; Moreira, D.R.M.; Kappes, B.; Tsogoeva, S.B. Access to Artemisinin-Triazole Antimalarials via Organo-Click Reaction: High In Vitro/In Vivo Activity against Multi-Drug-Resistant Malaria Parasites. JACS Au 2024, 4, 951–957. [Google Scholar] [CrossRef]
- Costa Souza, R.M.; Montenegro Pimentel, L.M.L.; Ferreira, L.K.M.; Pereira, V.R.A.; Santos, A.C.D.S.; Dantas, W.M.; Silva, C.J.O.; De Medeiros Brito, R.M.; Andrade, J.L.; De Andrade-Neto, V.F. Biological Activity of 1,2,3-Triazole-2-amino-1,4-naphthoquinone Derivatives and Their Evaluation as Therapeutic Strategy for Malaria Control. Eur. J. Med. Chem. 2023, 255, 115400. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Kaushik, C.P. Quinoline-1,2,3-Triazole Hybrids: Design, Synthesis, Antimalarial and Antimicrobial Evaluation. J. Mol. Struct. 2024, 1316, 138882. [Google Scholar] [CrossRef]
- Milićević, D.; Kimmel, R.; Gazvoda, M.; Urankar, D.; Kafka, S.; Košmrlj, J. Synthesis of Bis(1,2,3-Triazole) Functionalized Quinoline-2,4-Diones. Molecules 2018, 23, 2310. [Google Scholar] [CrossRef] [PubMed]
- Krstulović, L.; Mišković Špoljarić, K.; Rastija, V.; Filipović, N.; Bajić, M.; Glavaš-Obrovac, L. Novel 1,2,3-Triazole-Containing Quinoline-Benzimidazole Hybrids: Synthesis, Antiproliferative Activity, In Silico ADME Predictions, and Docking. Molecules 2023, 28, 6950. [Google Scholar] [CrossRef]
- Haque, A.; Hsieh, M.F.; Hassan, S.I.; Faizi, S.H.; Saha, A.; Dege, N.; Rather, J.A.; Khan, M.S. Synthesis, Characterization, and Pharmacological Studies of Ferrocene-1H-1,2,3-Triazole Hybrids. J. Mol. Struct. 2017, 1146, 536–545. [Google Scholar] [CrossRef]
- Biot, C.; Dessolin, J.; Ricard, I.; Dive, D. Easily Synthesized Antimalarial Ferrocene Triazacyclononane Quinoline Conjugates. J. Organomet. Chem. 2004, 689, 4678–4682. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial Drug Discovery: Progress and Approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Nosten, F.; White, N.J. Artemisinin-Based Combination Treatment of Falciparum Malaria. Am. J. Trop. Med. Hyg. 2007, 77, 181–192. [Google Scholar] [CrossRef]
- Roy, K.K. Targeting the Active Sites of Malarial Proteases for Antimalarial Drug Discovery: Approaches, Progress and Challenges. Int. J. Antimicrob. Agents 2017, 50, 287–302. [Google Scholar] [CrossRef]
- Merritt, C.; Silva, L.E.; Tanner, A.L.; Stuart, K.; Pollastri, M.P. Kinases as Druggable Targets in Trypanosomatid Protozoan Parasites. Chem. Rev. 2014, 114, 11280–11304. [Google Scholar] [CrossRef]
- Phillips, M.A.; Lotharius, J.; Marsh, K.; White, J.; Dayan, A.; White, K.L.; Njoroge, J.W.; El Mazouni, F.; Lao, Y.; Kokkonda, S.; et al. A Long-Duration Dihydroorotate Dehydrogenase Inhibitor (DSM265) for Prevention and Treatment of Malaria. Sci. Transl. Med. 2015, 7, 296ra111. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Rathod, P.K. Plasmodium Dihydroorotate Dehydrogenase: A Promising Target for Novel Anti-Malarial Chemotherapy. Infect. Disord. Drug Targets 2010, 10, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Rückle, T.; Roth, A.; Mürbeth, R.E.; Chalon, S.; Kerr, N.; Samec, S.S.; Gobeau, N.; Calle, C.L.; Ibáñez, J.; et al. DSM265 for Plasmodium falciparum Chemoprophylaxis: A Randomised, Double-Blinded, Phase 1 Trial with Controlled Human Malaria Infection. Lancet Infect. Dis. 2017, 17, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.C.; Duke, E.R.; Shipman, K.J.; Jensen, R.L.; Fong, Y.; Ferguson, S.; Janes, H.E.; Gillespie, K.; Seilie, A.M.; Hanron, A.E.; et al. A Randomized Trial Evaluating the Prophylactic Activity of DSM265 Against Preerythrocytic Plasmodium falciparum Infection During Controlled Human Malaria Infection by Mosquito Bites and Direct Venous Inoculation. J. Infect. Dis. 2018, 217, 693–702. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Lotharius, J.; Rückle, T.; Chalon, S.; Phillips, M.A.; Elliott, S.; Sekuloski, S.; Griffin, P.; Ng, C.L.; Fidock, D.A.; et al. Safety, Tolerability, Pharmacokinetics, and Activity of the Novel Long-Acting Antimalarial DSM265: A Two-Part First-in-Human Phase 1a/1b Randomised Study. Lancet Infect. Dis. 2017, 17, 626–635. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Casapia, M.; Chuquiyauri, R.; Hinojosa, J.C.; Kerr, N.; Rosario, M.; Toovey, S.; Arch, R.H.; Phillips, M.A.; Rozenberg, F.D.; et al. Antimalarial Activity of Single-Dose DSM265, a Novel Plasmodium Dihydroorotate Dehydrogenase Inhibitor, in Patients with Uncomplicated Plasmodium falciparum or Plasmodium vivax Malaria Infection: A Proof-of-Concept, Open-Label, Phase 2a Study. Lancet Infect. Dis. 2018, 18, 874–883. [Google Scholar] [CrossRef]
- Rosenthal, P.J. Artefenomel: A Promising New Antimalarial Drug. Lancet Infect. Dis. 2016, 16, 6–8. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Rückle, T.; Elliott, S.L.; Ballard, E.; Collins, K.A.; Marquart, L.; Griffin, P.; Chalon, S.; Möhrle, J.J. A Single-Dose Combination Study with the Experimental Antimalarials Artefenomel and DSM265 to Determine Safety and Antimalarial Activity against Blood-Stage Plasmodium falciparum in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 64, e01371-19. [Google Scholar] [CrossRef]
- Dini, S.; Zaloumis, S.G.; Price, D.J.; Gobeau, N.; Kümmel, A.; Cherkaoui, M.; Moehrle, J.J.; McCarthy, J.S.; Simpson, J.A. Seeking an Optimal Dosing Regimen for OZ439/DSM265 Combination Therapy for Treating Uncomplicated Falciparum Malaria. J. Antimicrob. Chemother. 2021, 76, 2325–2334. [Google Scholar] [CrossRef]
- Węglińska, L.; Bekier, A.; Trotsko, N.; Kaproń, B.; Plech, T.; Dzitko, K.; Paneth, A. Inhibition of Toxoplasma gondii by 1,2,4-Triazole-Based Compounds: Marked Improvement in Selectivity Relative to the Standard Therapy Pyrimethamine and Sulfadiazine. J. Enzyme Inhib. Med. Chem. 2022, 37, 2621–2634. [Google Scholar] [CrossRef]
- Arafa, F.M.; Osman, D.H.; Tolba, M.M.; Rezki, N.; Aouad, M.R.; Hagar, M.; Osman, M.; Said, H. Sulfadiazine analogs: Anti-Toxoplasma in vitro study of sulfonamide triazoles. Parasitol. Res. 2023, 122, 2353–2365. [Google Scholar] [CrossRef]
- Massimine, K.M.; Doan, L.T.; Atreya, C.A.; Stedman, T.T.; Anderson, K.S.; Joiner, K.A.; Coppens, I. Toxoplasma gondii is capable of exogenous folate transport. A likely expansion of the BT1 family of transmembrane proteins. Mol. Biochem. Parasitol. 2005, 144, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Gollapalli, D.R.; Kirubakaran, S.; Zhang, M.; Johnson, C.R.; Benjamin, N.N.; Hedstrom, L.; Cuny, G.D. Triazole Inhibitors of Cryptosporidium parvum Inosine 5′-Monophosphate Dehydrogenase. J. Med. Chem. 2009, 52, 4623–4630. [Google Scholar] [CrossRef]
- Negi, B.; Poonan, P.; Ansari, M.F.; Kumar, D.; Aggarwal, S.; Singh, R.; Azam, A.; Rawat, D.S. Synthesis, Antiamoebic Activity and Docking Studies of Metronidazole-Triazole-Styryl Hybrids. Eur. J. Med. Chem. 2018, 150, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Popruk, S.; Tummatorn, J.; Sreesai, S.; Ampawong, S.; Thiangtrongjit, T.; Tipthara, P.; Tarning, J.; Thongsornkleeb, C.; Ruchirawat, S.; Reamtong, O. Inhibition of Giardia duodenalis by Isocryptolepine–Triazole Adducts and Derivatives. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100561. [Google Scholar] [CrossRef]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth Infections: The Great Neglected Tropical Diseases. J. Clin. Invest. 2008, 118, 1311–1321. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- CDC—Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/schistosomiasis/about/index.html (accessed on 14 February 2025).
- Wang, W.; Wang, L.; Liang, Y.S. Susceptibility or Resistance of Praziquantel in Human Schistosomiasis: A Review. Parasitol. Res. 2012, 111, 1871–1877. [Google Scholar] [CrossRef]
- Thomas, C.M.; Timson, D.J. The Mechanism of Action of Praziquantel: Can New Drugs Exploit Similar Mechanisms? Curr. Med. Chem. 2020, 27, 676–696. [Google Scholar] [CrossRef]
- Abla, N.; Keiser, J.; Vargas, M.; Reimers, N.; Haas, H.; Spangenberg, T. Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model. PLoS Negl. Trop. Dis. 2017, 11, e0005942. [Google Scholar] [CrossRef]
- Elzoheiry, M.A.; Elmehankar, M.S.; Aboukamar, W.A.; El-Gamal, R.; Sheta, H.; Zenezan, D.; Nabih, N.; Elhenawy, A.A. Fluconazole as Schistosoma mansoni Cytochrome P450 Inhibitor: In Vivo Murine Experimental Study. Exp. Parasitol. 2022, 239, 108291. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.A.; Salem, M.B.; William, S.; Hammam, O.A.; El-Lakkany, N.M. Itraconazole, a Cytochrome P450 Inhibitor, Enhanced the Efficacy of Praziquantel against Schistosoma mansoni Infection and Alleviated Liver Injury in Mice. Exp. Parasitol. 2022, 239, 108293. [Google Scholar] [CrossRef]
- Kalinin, D.V.; Jana, S.K.; Pfafenrot, M.; Chakrabarti, A.; Melesina, J.; Shaik, T.B.; Lancelot, J.; Pierce, R.J.; Sippl, W.; Romier, C.; et al. Structure-Based Design, Synthesis, and Biological Evaluation of Triazole-Based smHDAC8 Inhibitors. ChemMedChem 2020, 15, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; El-Sakkary, N.; Skinner, D.E.; Sharma, P.P.; Ottilie, S.; Antonova-Koch, Y.; Kumar, P.; Winzeler, E.; Poonam; Caffrey, C.R.; et al. Synthesis and Bioactivity of Phthalimide Analogs as Potential Drugs to Treat Schistosomiasis, a Neglected Disease of Poverty. Pharmaceuticals 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Chin, C.M. The Use of Heterocyclic-Based Azo Compounds Bearing Pyrrolidine, Imidazole, Triazole, and Thiazole Moieties for the Treatment of Neglected Tropical Disease Caused by Schistosoma mansoni. Eur. J. Med. Chem. Rep. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Lin, Y.; Jung, H.; Bulman, C.A.; Ng, J.; Vinck, R.; O’Beirne, C.; Zhong, S.; Moser, M.S.; Tricoche, N.; Peguero, R.; et al. Discovery of New Broad-Spectrum Anti-Infectives for Eukaryotic Pathogens Using Bioorganometallic Chemistry. J. Med. Chem. 2023, 66, 15867–15882. [Google Scholar] [CrossRef]
- Paprocka, R.; Kołodziej, P.; Wiese-Szadkowska, M.; Helmin-Basa, A.; Bogucka-Kocka, A. Evaluation of Anthelmintic and Anti-Inflammatory Activity of 1,2,4-Triazole Derivatives. Molecules 2022, 27, 4488. [Google Scholar] [CrossRef]
- Sudhir, M.S.; Venkata Nadh, R. Evaluation of In Vitro Anthelmintic Activities of Novel 1,2,3-Benzotriazole Derivatives Synthesized in Ultrasonic and Solvent Free Conditions. J. Pharm. Res. 2013, 7, 47–52. [Google Scholar] [CrossRef]
- Logan, A.; Wolfe, A.; Williamson, J.C. Antifungal Resistance and the Role of New Therapeutic Agents. Curr. Infect. Dis. Rep. 2022, 24, 105–116. [Google Scholar] [CrossRef]
- Nywening, A.V.; Rybak, J.M.; Rogers, P.D.; Fortwendel, J.R. Mechanisms of Triazole Resistance in Aspergillus fumigatus. Environ. Microbiol. 2020, 22, 4934–4952. [Google Scholar] [CrossRef]
- Salazar-Villamizar, M.E.; Escobar, P. In Vitro Selection of Ketoconazole-Pentamidine-Resistant Leishmania (Viannia) braziliensis Strains. Exp. Parasitol. 2022, 233, 108206. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.M.; Hall, B.S.; Taylor, M.C.; Gómez-Palacio, A.; Wilkinson, S.R.; Triana-Chávez, O.; Kelly, J.M. Benznidazole-Resistance in Trypanosoma cruzi Is a Readily Acquired Trait That Can Arise Independently in a Single Population. J. Infect. Dis. 2012, 206, 220–228. [Google Scholar] [CrossRef]
- Campos, M.C.; Leon, L.L.; Taylor, M.C.; Kelly, J.M. Benznidazole-Resistance in Trypanosoma cruzi: Evidence That Distinct Mechanisms Can Act in Concert. Mol. Biochem. Parasitol. 2014, 193, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current Trends in the Pharmacological Management of Chagas Disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.S.; Lo, D.C.; Zheng, W. Drug Combination Therapy for Emerging Viral Diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Zhan, J.L.; Zhao, L.M.; Liu, X.D. Safety of Triazole Antifungals: A Pharmacovigilance Study from 2004 to 2021 Based on FAERS. Ther. Adv. Drug Saf. 2022, 13, 20420986221143266. [Google Scholar] [CrossRef]
- Neofytos, D.; Avdic, E.; Magiorakos, A.P. Clinical Safety and Tolerability Issues in Use of Triazole Derivatives in Management of Fungal Infections. Drug Healthc. Patient Saf. 2010, 2, 27–38. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xiang, Z.J.; Yang, J.H.; Wang, W.J.; Xu, Z.C.; Xiang, R.L. Adverse Effects Associated with Currently Commonly Used Antifungal Agents: A Network Meta-Analysis and Systematic Review. Front. Pharmacol. 2021, 12, 697330. [Google Scholar] [CrossRef]
- Deuschle, M.; Lecei, O.; Stalla, G.K.; Landgraf, R.; Hamann, B.; Lederbogen, F.; Uhr, M.; Luppa, P.; Maras, A.; Colla, M.; et al. Steroid Synthesis Inhibition with Ketoconazole and Its Effect upon the Regulation of the Hypothalamus-Pituitary-Adrenal System in Healthy Humans. Neuropsychopharmacology 2003, 28, 379–383. [Google Scholar] [CrossRef]
- Alvar, J.; den Boer, M.; Dagne, D.A. Towards the Elimination of Visceral Leishmaniasis as a Public Health Problem in East Africa: Reflections on an Enhanced Control Strategy and a Call for Action. Lancet Glob. Health 2021, 9, e1763–e1769. [Google Scholar] [CrossRef]
- Maertens, J.A. History of the Development of Azole Derivatives. Clin. Microbiol. Infect. 2004, 10 (Suppl. S1), 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Aliporewala, V.M.; Patel, D.A. Common Antifungal Drugs in Pregnancy: Risks and Precautions. J. Obstet. Gynaecol. India 2021, 71, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Fahal, A.H.; Ahmed, E.S.; Bakhiet, S.M.; Bakhiet, O.E.; Fahal, L.A.; Mohamed, A.A.; Mohamedelamin, E.S.W.; Bahar, M.E.N.; Attalla, H.Y.; Siddig, E.E.; et al. Two Dose Levels of Once-Weekly Fosravuconazole versus Daily Itraconazole in Combination with Surgery in Patients with Eumycetoma in Sudan: A Randomised, Double-Blind, Phase 2, Proof-of-Concept Superiority Trial. Lancet Infect. Dis. 2024, 24, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tsubouchi, I.; Okubo, A. Efficacy and Safety of Fosravuconazole L-Lysine Ethanolate, a Novel Oral Triazole Antifungal Agent, for the Treatment of Onychomycosis: A Multicenter, Double-Blind, Randomized Phase III Study. J. Dermatol. 2018, 45, 1151–1159. [Google Scholar] [CrossRef]
- González, S.; Wall, R.J.; Thomas, J.; Braillard, S.; Brunori, G.; Camino Díaz, I.; Cantizani, J.; Carvalho, S.; Castañeda Casado, P.; Chatelain, E.; et al. Short-Course Combination Treatment for Experimental Chronic Chagas Disease. Sci. Transl. Med. 2023, 15, eadg8105. [Google Scholar] [CrossRef]
- Grace, A.G.; Mittal, A.; Jain, S.; Tripathy, J.P.; Satyanarayana, S.; Tharyan, P.; Kirubakaran, R. Shortened Treatment Regimens versus the Standard Regimen for Drug-Sensitive Pulmonary Tuberculosis. Cochrane Database Syst. Rev. 2019, 12, CD012918. [Google Scholar] [CrossRef]
- Quijia Quezada, C.; Azevedo, C.S.; Charneau, S.; Santana, J.M.; Chorilli, M.; Carneiro, M.B.; Bastos, I.M.D. Advances in Nanocarriers as Drug Delivery Systems in Chagas Disease. Int. J. Nanomed. 2019, 14, 6407–6424. [Google Scholar] [CrossRef]
- Garza-Tovar, T.F.; Sacriste-Hernández, M.I.; Juárez-Durán, E.R.; Arenas, R. An Overview of the Treatment of Cutaneous Leishmaniasis. Fac. Rev. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC); European Chemicals Agency (ECHA); European Environment Agency (EEA); European Medicines Agency (EMA); European Commission’s Joint Research Centre (JRC). Impact of the use of azole fungicides, other than as human medicines, on the development of azole-resistant Aspergillus spp. EFSA J. 2025, 23, e9200. [Google Scholar] [CrossRef]
- Picot, S.; Beugnet, F.; Leboucher, G.; Bienvenu, A.L. Drug Resistant Parasites and Fungi from a One-Health Perspective: A Global Concern That Needs Transdisciplinary Stewardship Programs. One Health 2022, 14, 100368. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Bofill Verdaguer, I.; Perez, C.; Banchio, C.; Ferreira de Azevedo, M.; Katzin, A.M.; Labadie, G.R. Repositioning Salirasib as a New Antimalarial Agent. Medchemcomm 2019, 10, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Dean, T.T.; Jelú-Reyes, J.; Allen, A.C.; Moore, T.W. Peptide-Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics. J. Med. Chem. 2024, 67, 1641–1661. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, J.P.; Diniz, L.A.; Santos, V.C.; Vilchez Larrea, S.C.; Alonso, G.D.; Ferreira, R.S.; Dehaen, W.; Quevedo, M.A. Structure-Aided Computational Design of Triazole-Based Targeted Covalent Inhibitors of Cruzipain. Molecules 2024, 29, 4224. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Available online: https://go.drugbank.com/ (accessed on 1 March 2025).
- Google Scholar. Available online: https://scholar.google.com/ (accessed on 1 March 2025).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 March 2025).
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 1 March 2025).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isern, J.A.; Carlucci, R.; Labadie, G.R.; Porta, E.O.J. Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases. Trop. Med. Infect. Dis. 2025, 10, 142. https://doi.org/10.3390/tropicalmed10050142
Isern JA, Carlucci R, Labadie GR, Porta EOJ. Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases. Tropical Medicine and Infectious Disease. 2025; 10(5):142. https://doi.org/10.3390/tropicalmed10050142
Chicago/Turabian StyleIsern, Jaime A., Renzo Carlucci, Guillermo R. Labadie, and Exequiel O. J. Porta. 2025. "Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases" Tropical Medicine and Infectious Disease 10, no. 5: 142. https://doi.org/10.3390/tropicalmed10050142
APA StyleIsern, J. A., Carlucci, R., Labadie, G. R., & Porta, E. O. J. (2025). Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases. Tropical Medicine and Infectious Disease, 10(5), 142. https://doi.org/10.3390/tropicalmed10050142





