Changes in Malaria Patterns in Comoros from 2010 to 2021: A Comparative Study with Sub-Saharan Africa
Abstract
1. Introduction
2. Method and Materials
2.1. Data Sources
2.2. Disease Definition
2.3. Disability-Adjusted Life Year (DALY)
2.4. Risk Factors
2.5. Relative Change and Estimated Annual Percentage Change
2.6. Decomposition Analysis
2.7. Statistical Analysis and Visualization
3. Results
3.1. Trend Analysis
3.2. Sex and Age Patterns
3.3. Decomposition Analysis
3.4. Risk Factors
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guidelines for Malaria. 2024. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria (accessed on 30 November 2024).
- White, N.J. Anaemia and Malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 11 December 2024).
- Andrade, M.V.; Noronha, K.; Diniz, B.P.C.; Guedes, G.; Carvalho, L.R.; Silva, V.A.; Calazans, J.A.; Santos, A.S.; Silva, D.N.; Castro, M.C. The Economic Burden of Malaria: A Systematic Review. Malar. J. 2022, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020 (accessed on 30 November 2021).
- Chakir, I.; Said, A.I.; Affane, B.; Jambou, R. Control of Malaria in the Comoro Islands over the Past Century. Malar. J. 2017, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, J.-P. Modelling Determinants of Child Mortality and Poverty in the Comoros. Health Place 2004, 10, 13–42. [Google Scholar] [CrossRef]
- Global Technical Strategy for Malaria 2016–2030; 2021 update; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031357 (accessed on 19 July 2021).
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER Statement. Lancet 2016, 388, e19–e23. [Google Scholar] [CrossRef]
- Girum, T.; Shumbej, T.; Shewangizaw, M. Burden of malaria in Ethiopia, 2000–2016: Findings from the Global Health Estimates 2016. Trop. Dis. Travel. Med. Vaccines 2019, 5, 11. [Google Scholar] [CrossRef]
- GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, W.; Kang, L.; Liu, J.; Liu, M. Trends of the global, regional and national incidence of malaria in 204 countries from 1990 to 2019 and implications for malaria prevention. J. Travel. Med. 2021, 28, taab046. [Google Scholar] [CrossRef]
- Cen, J.; Wang, Q.; Cheng, L.; Gao, Q.; Wang, H.; Sun, F. Global, regional, and national burden and trends of migraine among women of childbearing age from 1990 to 2021: Insights from the Global Burden of Disease Study 2021. J. Headache Pain 2024, 25, 96. [Google Scholar] [CrossRef]
- Lei, S.; Huang, G.; Li, X.; Xi, P.; Yao, Z.; Lin, X. Global Burden, Trends, and Inequalities of Gallbladder and Biliary Tract Cancer, 1990-2021: A Decomposition and Age-Period-Cohort Analysis. Liver Int. 2025, 45, e16199. [Google Scholar] [CrossRef] [PubMed]
- Apeagyei, A.E.; Cogswell, I.; Patel, N.K.; O’rourke, K.; Tsakalos, G.; Dieleman, J.L. Examining malaria treatment and prevention spending efficiency in malaria-endemic countries, 2000–2020. Malar. J. 2024, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Okumu, F.; Moonen, B. The Fight against Malaria: Diminishing Gains and Growing Challenges. Sci. Transl. Med. 2022, 14, eabn3256. [Google Scholar] [CrossRef]
- Okoyo, C.; Githinji, E.; Muia, R.W.; Masaku, J.; Mwai, J.; Nyandieka, L.; Munga, S.; Njenga, S.M.; Kanyi, H.M. Assessment of malaria infection among pregnant women and children below five years of age attending rural health facilities of Kenya: A cross-sectional survey in two counties of Kenya. PLoS ONE 2021, 6, e0257276. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, J.N.; Dolan, C.; Malik, A.A.; Tavel, A. China’s hidden role in malaria control and elimination in Africa. BMJ Glob. Health 2023, 8, e013349. [Google Scholar] [CrossRef]
- Slater, H.C.; Griffin, J.T.; Ghani, A.C.; Okell, L.C. Assessing the potential impact of artemisinin and partner drug resistance in sub-Saharan Africa. Malar. J. 2016, 15, 10. [Google Scholar] [CrossRef]
- Ryan, S.J.; Lippi, C.A.; Zermoglio, F. Shifting transmission risk for malaria in Africa with climate change: A framework for planning and intervention. Malar. J. 2020, 19, 170. [Google Scholar] [CrossRef]
- Oxborough, R.M.; Figueroa Chilito, K.C.; Tokponnon, F.; Messenger, A.L. Malaria vector control in sub-Saharan Africa: Complex trade-offs to combat the growing threat of insecticide resistance. Lancet Planet. Health 2024, 8, e804–e812. [Google Scholar] [CrossRef]
- Agaba, B.B.; Yeka, A.; Nsobya, S.; Arinaitwe, E.; Nankabirwa, J.; Opigo, J.; Mbaka, P.; Lim, C.S.; Kalyango, J.N.; Karamagi, C.; et al. Systematic review of the status of pfhrp2 and pfhrp3 gene deletion, approaches and methods used for its estimation and reporting in Plasmodium falciparum populations in Africa: Review of published studies 2010-2019. Malar. J. 2019, 18, 355. [Google Scholar] [CrossRef]
- Oladipo, H.J.; Tajudeen, Y.A.; Oladunjoye, I.O.; Yusuff, S.I.; Yusuf, R.O.; Oluwaseyi, E.M.; AbdulBasit, M.O.; Adebisi, Y.A.; El-Sherbini, M.S. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann. Med. Surg. 2022, 81, 104366. [Google Scholar] [CrossRef]
- Heuschen, A.-K.; Lu, G.; Razum, O.; Abdul-Mumin, A.; Sankoh, O.; von Seidlein, L.; D’alessandro, U.; Müller, O. Public health-relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: A scoping review. Malar. J. 2021, 20, 339. [Google Scholar] [CrossRef]
- Newby, G.; Hwang, J.; Koita, K.; Chen, I.; Greenwood, B.; von Seidlein, L.; Shanks, G.D.; Slutsker, L.; Kachur, S.P.; Wegbreit, J.; et al. Review of mass drug administration for malaria and its operational challenges. Am. J. Trop. Med. Hyg. 2015, 93, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wang, Q.; Zheng, S.; Zhou, C.; Gao, Y.; Guo, J.; Mliva, A.M.; Oithik, F.; Bacar, A.; Attoumane, R.; et al. Mass Drug Administration of Artemisinin-piperaquine on High Malaria Epidemic Area. Trop. Med. Health 2014, 42, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Huang, B.; Wang, Q.; Wu, W.; Zheng, S.; Zhang, H.; Li, D.; Feng, D.; Li, G.; Xue, L.; et al. Large-scale Artemisinin–Piperaquine Mass Drug Administration With or Without Primaquine Dramatically Reduces Malaria in a Highly Endemic Region of Africa. Clin. Infect. Dis. 2018, 67, 1670–1676. [Google Scholar] [CrossRef]
- Deng, C.; Wu, W.; Yuan, Y.; Li, G.; Zhang, H.; Zheng, S.; Li, M.; Tan, R.; Wang, Y.; Nadia, J.; et al. Malaria Control by Mass Drug Administration With Artemisinin Plus Piperaquine on Grande Comore Island, Union of Comoros. Open Forum Infect. Dis. 2023, 10, ofad076. [Google Scholar] [CrossRef]
- Shi, J.; Feng, Y.; Wang, M.; Maimaitiming, M.; Jin, Y.; Ren, M.; Zheng, Z. China’s anti-malaria development assistance for health to sub-Saharan Africa and its influencing factors: A panel analysis. J. Glob. Health 2024, 14, 04250. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2019 (accessed on 4 December 2019).
- Nadia, J.; Wang, Y.; Li, G.; Sun, L.; Mmadi, S.A.; Abdallah, K.S.; Abdallah, A.M.; Shu, L.; Bacar, A.; Deng, C.; et al. Knowledge, attitudes, and practices toward malaria and antimalarial mass drug administration among heads of households in villages on grande Comore island, the Comoros. J. Parasitol. 2023, 109, 187–199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2016 (accessed on 19 November 2016).
- Ibraheem Nasir, S.M.; Amarasekara, S.; Wickremasinghe, R.; Fernando, D.; Udagama, P. Prevention of re-establishment of malaria: Historical perspective and future prospects. Malar. J. 2020, 19, 452. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2018 (accessed on 19 November 2018).
- World Health Organization. Mass Drug Administration for Falciparum Malaria: A Practical Field Manual. 2017. Available online: https://iris.who.int/bitstream/handle/10665/259367/9789241513104-eng.pdf?sequence=1 (accessed on 20 September 2017).
- Langhorne, J.; Ndungu, F.M.; Sponaas, A.-M.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef]
- Laurens, M.B. RTS, S/AS01 Vaccine (Mosquirix™): An Overview. Hum. Vaccin. Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef]

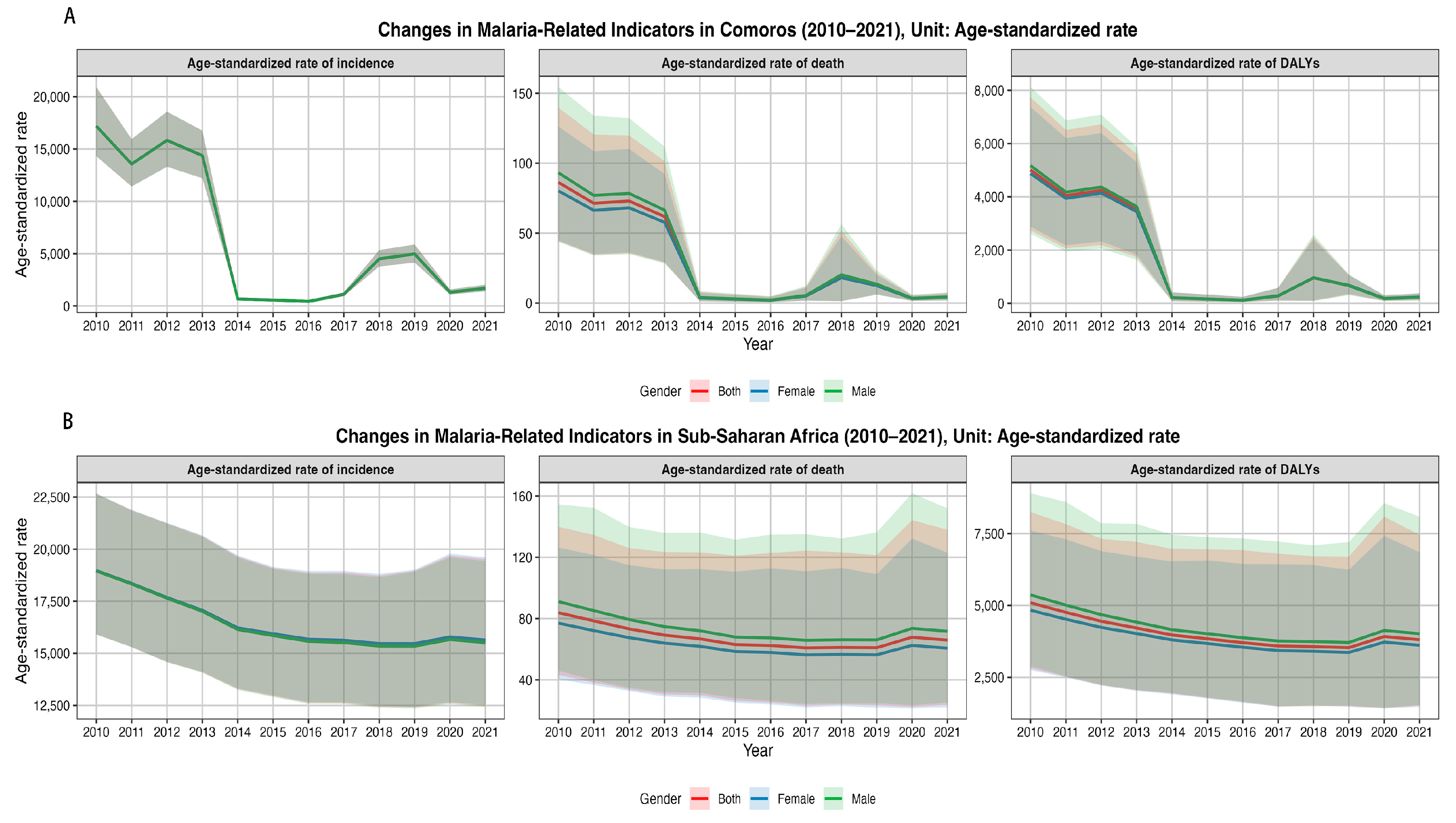
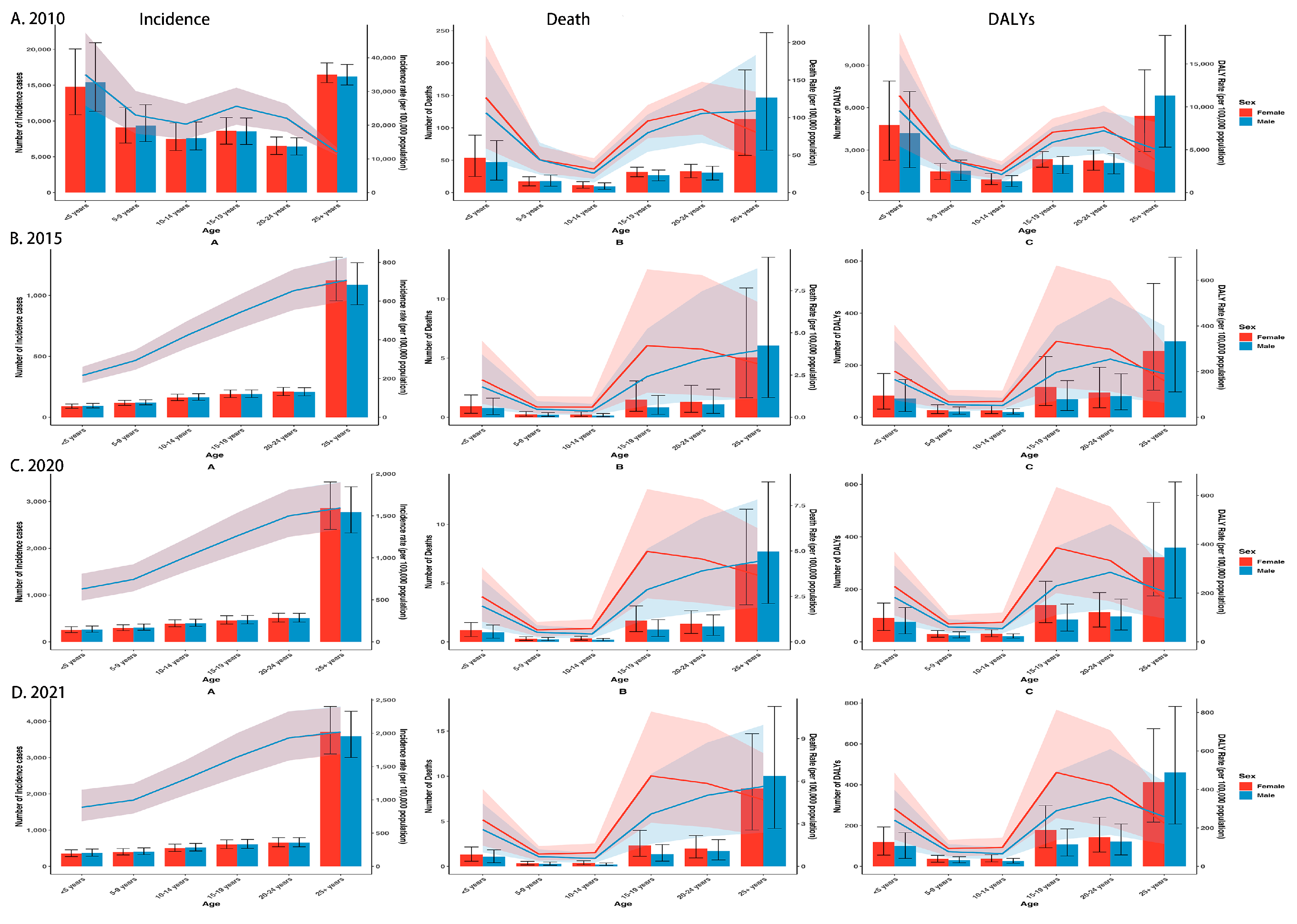
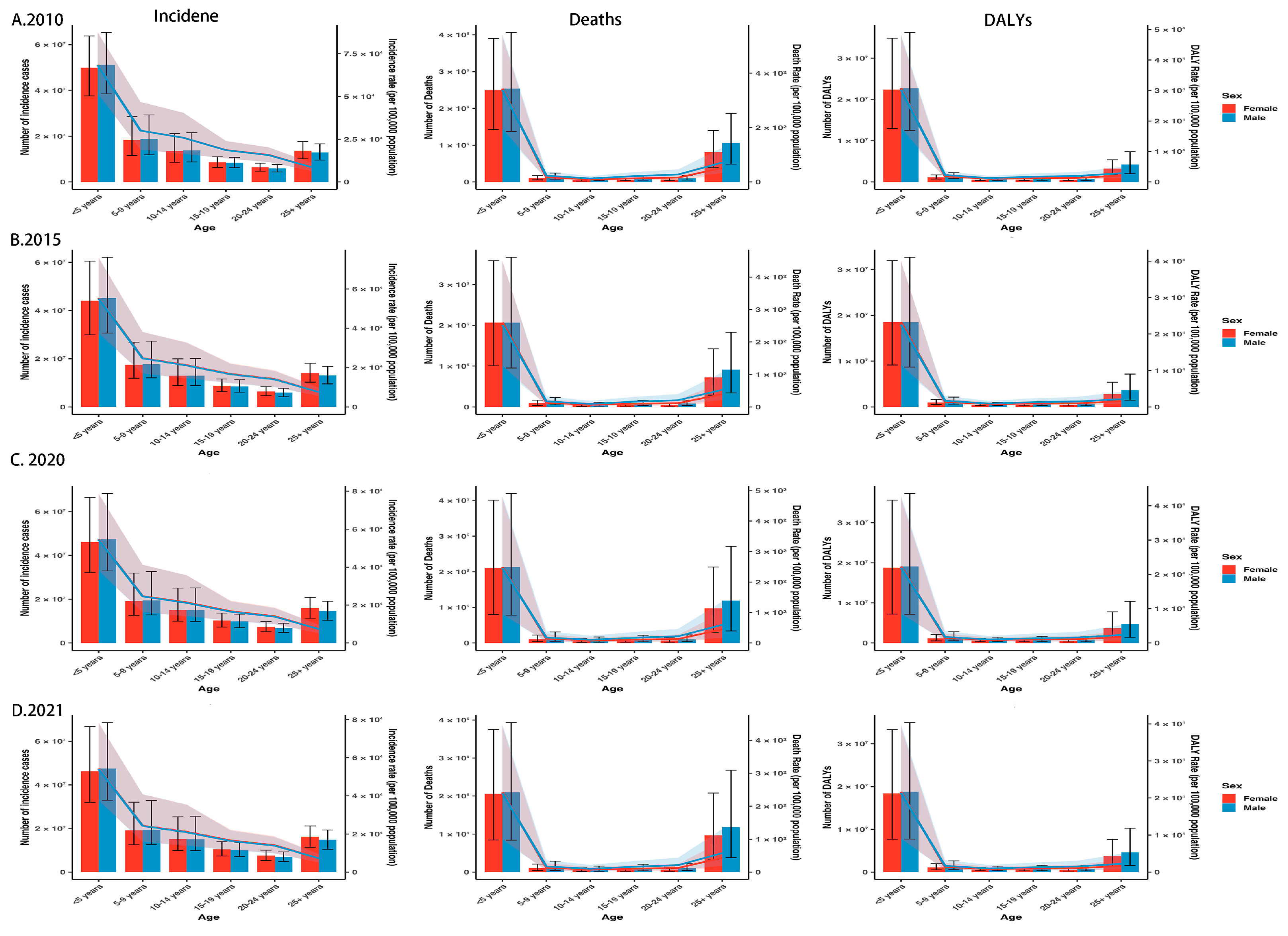
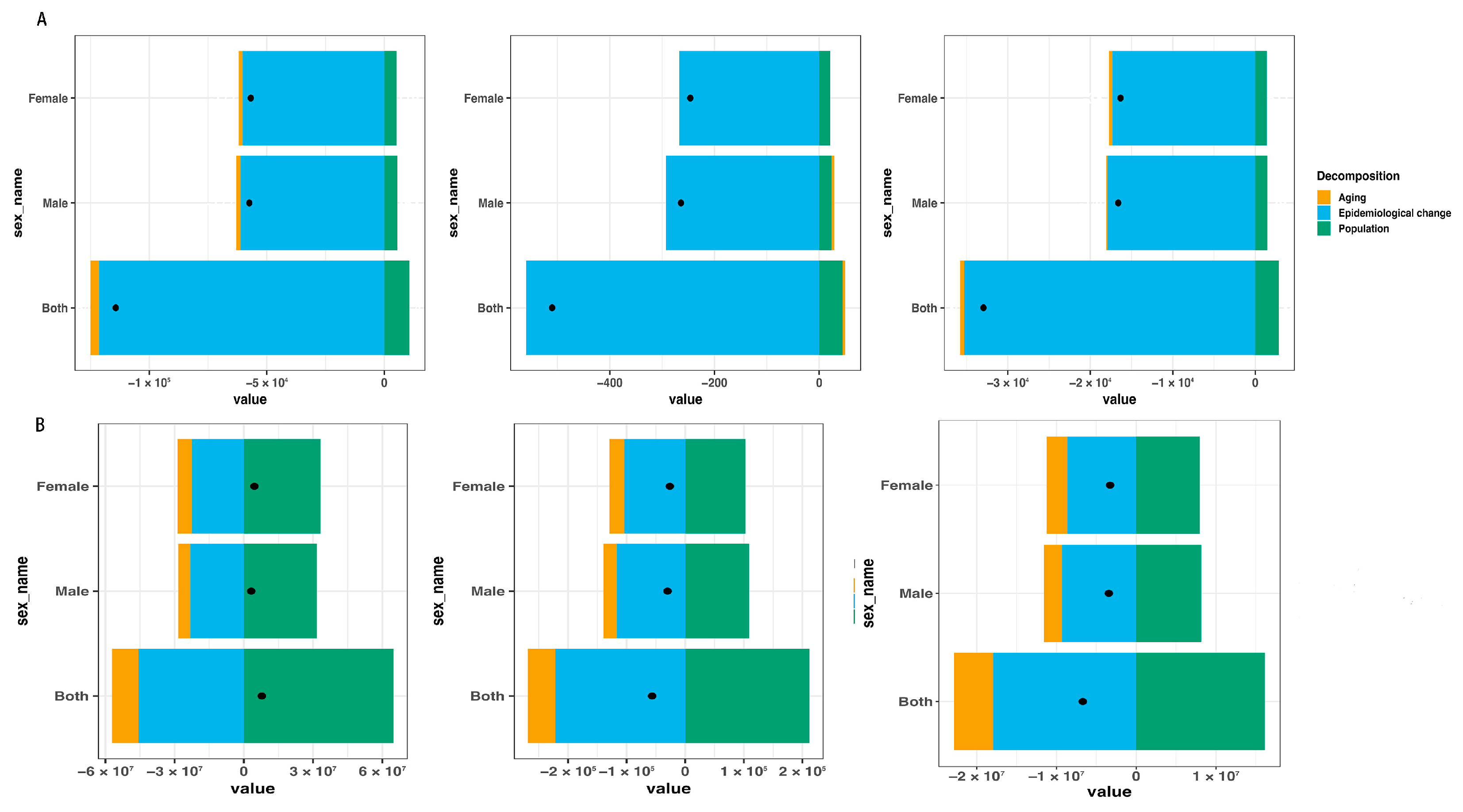

| Measure | Location | Sex | 2010 | 2021 | 2010–2021 | 2010 | 2021 | 2010–2021 |
|---|---|---|---|---|---|---|---|---|
| Number (95%UI) | Number (95%UI) | RC (%) | ASR (per 105, 95%UI) | ASR (per 105, 95%UI) | EAPC (%, 95%CI) | |||
| Incidence | Comoros | Both | 126,651 (102,670 to 157,081) | 12,384 (10,210 to 14,942) | −90.22 | 17,200.31 (14,327.51 to 20,935.67) | 1687.94 (1395.05 to 2031.47) | −18.70 (−33.77 to −0.20) |
| Male | 63,625 (51,485 to 79,017) | 6163 (5080 to 7439) | −90.31 | 17,200.31 (14,327.51 to 20,935.67) | 1687.94 (1395.05 to 2031.47) | −18.70 (−33.77 to −0.20) | ||
| Female | 63,026 (51,184 to 78,064) | 6221 (5131 to 7503) | −90.13 | 17,200.31 (14,327.51 to 20,935.67) | 1687.94 (1395.05 to 2031.47) | −18.70 (−33.77 to −0.20) | ||
| Sub-Saharan Africa | Both | 221,381,206 (185,049,437 to 269,945,919) | 229,191,055 (181,685,394 to 293,064,373) | 3.53 | 18,964.03 (15,909.77 to 22,663.51) | 15,578.4 (12,473.58 to 19,528.01) | −1.78 (−2.35 to −1.21) | |
| Male | 110,937,613 (92,711,851 to 135,452,396) | 114,158,022 (90,433,854 to 146,696,541) | 2.90 | 18,953.67 (15,903.46 to 22,649.87) | 15,512.62 (12,426.43 to 19,437.26) | −1.82 (−2.4 to −1.24) | ||
| Female | 110,443,593 (92,337,586 to 134,516,767) | 115,033,033 (91,251,540 to 146,367,832) | 4.16 | 18975.46 (15917.4 to 22678.50) | 15,643.46 (12,520.36 to 19,617.03) | −1.75 (−2.31 to −1.17) | ||
| Death | Comoros | Both | 540 (288 to 843) | 30 (13 to 50) | −94.44 | 86.34 (44.15 to 139.70) | 4.26 (1.91 to 7.24) | −23.89 (−36.58 to −8.66) |
| Male | 279 (137 to 442) | 15 (6 to 25) | −94.62 | 93.21 (43.88 to 154.54) | 4.41 (1.87 to 7.67) | −24.18 (−36.89 to −8.91) | ||
| Female | 261 (150 to 399) | 15 (7 to 25) | −94.25 | 80.24 (44.74 to 126.22) | 4.15 (1.95 to 6.94) | −23.56 (−36.22 to −8.38) | ||
| Sub-Saharan Africa | Both | 759,438 (406,996 to 1,232,590) | 702,937 (262,494 to 1,397,708) | −7.44 | 83.85 (43.26 to 139.85) | 65.9 (23.66 to 137.94) | −2.13 (−3.29 to −0.95) | |
| Male | 400,693 (208,524 to 668,953) | 370,507 (135,482 to 752,024) | −7.53 | 91.06 (45.95 to 154.46) | 71.7 (25.28 to 151.95) | −2.11 (−3.33 to −0.89) | ||
| Female | 358,745 (198,155 to 573,656) | 332,430 (126,249 to 649,233) | −7.34 | 77.01 (40.35 to 126.36) | 60.63 (22.11 to 122.95) | −2.11 (−3.22 to −0.98) | ||
| DALYs | Comoros | Both | 34,724 (19,541 to 52,520) | 1778 (886 to 2878) | −94.88 | 5011.98 (2743 to 7741.25) | 234.27 (116.63 to 380.55) | −24.49 (−36.88 to −9.66) |
| Male | 17,458 (9118 to 26,594) | 848 (397 to 1398) | −95.14 | 5175.58 (2613.65 to 8124.69) | 228.46 (106.15 to 379.61) | −24.89 (−37.35 to −9.95) | ||
| Female | 17,266 (10,389 to 25,920) | 930 (484 to 1488) | −94.61 | 4872.26 (2875.94 to 7377.94) | 241.11 (125.55 to 386.05) | −24.08 (−36.41 to −9.36) | ||
| Sub-Saharan Africa | Both | 58,907,957 (33,290,778 to 93,197,378) | 52,215,321 (21,277,913 to 99,016,298) | −11.36 | 5096.46 (2814.33 to 8247.95) | 3810.98 (1517.53 to 7452.61) | −2.67 (−3.77 to −1.55) | |
| Male | 30,526,557 (16,596,104 to 49,497,914) | 27,101,669 (10,802,601 to 52,299,987) | −11.22 | 5369.34 (2892.3 to 8899.31) | 4020.39 (1542.91 to 8084.54) | −2.66 (−3.81 to −1.49) | ||
| Female | 28,381,400 (16,503,490 to 44,431,680) | 25,113,652 (10,510,511 to 46,335,752) | −11.51 | 4836.1 (2753.76 to 7604.98) | 3617.94 (1484.20 to 6852.74) | −2.66 (−3.72 to −1.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Yu, L.; Liang, J.; Xie, W.; Li, G.; Deng, C.; Song, J.; Zou, G.; Chen, Y. Changes in Malaria Patterns in Comoros from 2010 to 2021: A Comparative Study with Sub-Saharan Africa. Trop. Med. Infect. Dis. 2025, 10, 138. https://doi.org/10.3390/tropicalmed10050138
Zhou S, Yu L, Liang J, Xie W, Li G, Deng C, Song J, Zou G, Chen Y. Changes in Malaria Patterns in Comoros from 2010 to 2021: A Comparative Study with Sub-Saharan Africa. Tropical Medicine and Infectious Disease. 2025; 10(5):138. https://doi.org/10.3390/tropicalmed10050138
Chicago/Turabian StyleZhou, Sheng, Linxin Yu, Jianming Liang, Wei Xie, Guoming Li, Changsheng Deng, Jianping Song, Guanyang Zou, and Yinhuan Chen. 2025. "Changes in Malaria Patterns in Comoros from 2010 to 2021: A Comparative Study with Sub-Saharan Africa" Tropical Medicine and Infectious Disease 10, no. 5: 138. https://doi.org/10.3390/tropicalmed10050138
APA StyleZhou, S., Yu, L., Liang, J., Xie, W., Li, G., Deng, C., Song, J., Zou, G., & Chen, Y. (2025). Changes in Malaria Patterns in Comoros from 2010 to 2021: A Comparative Study with Sub-Saharan Africa. Tropical Medicine and Infectious Disease, 10(5), 138. https://doi.org/10.3390/tropicalmed10050138





