Navigating the Parasitic Landscape: Insights into Infection Patterns and Public Health Strategies in West Africa
Abstract
1. Introduction
1.1. Prevalence
1.2. Factors Influencing Parasitic Disease Transmission
1.3. Morbidity and Mortality
1.4. Diagnostics and Treatments
1.5. One-Health Approach
1.6. Public Health Challenges
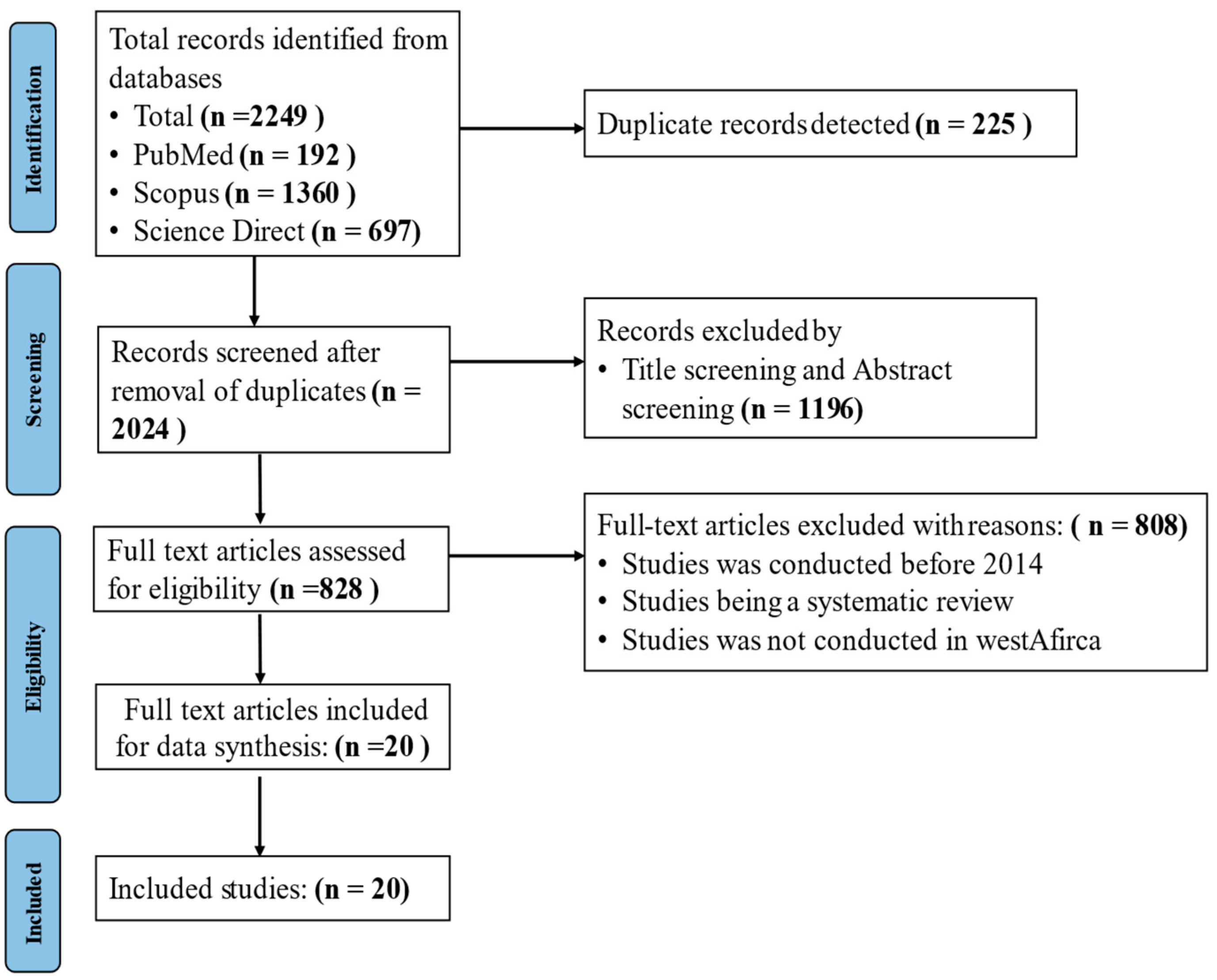
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
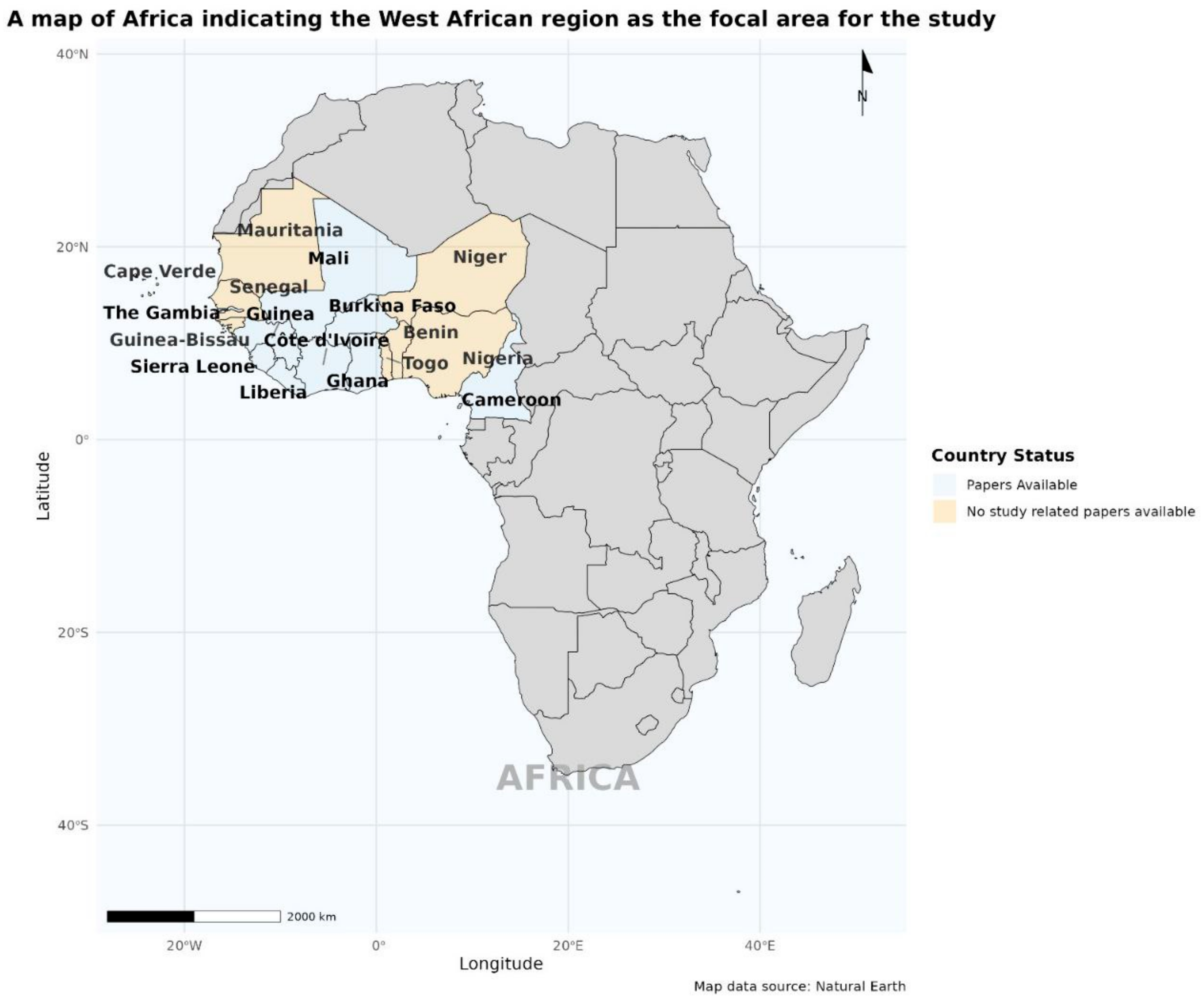
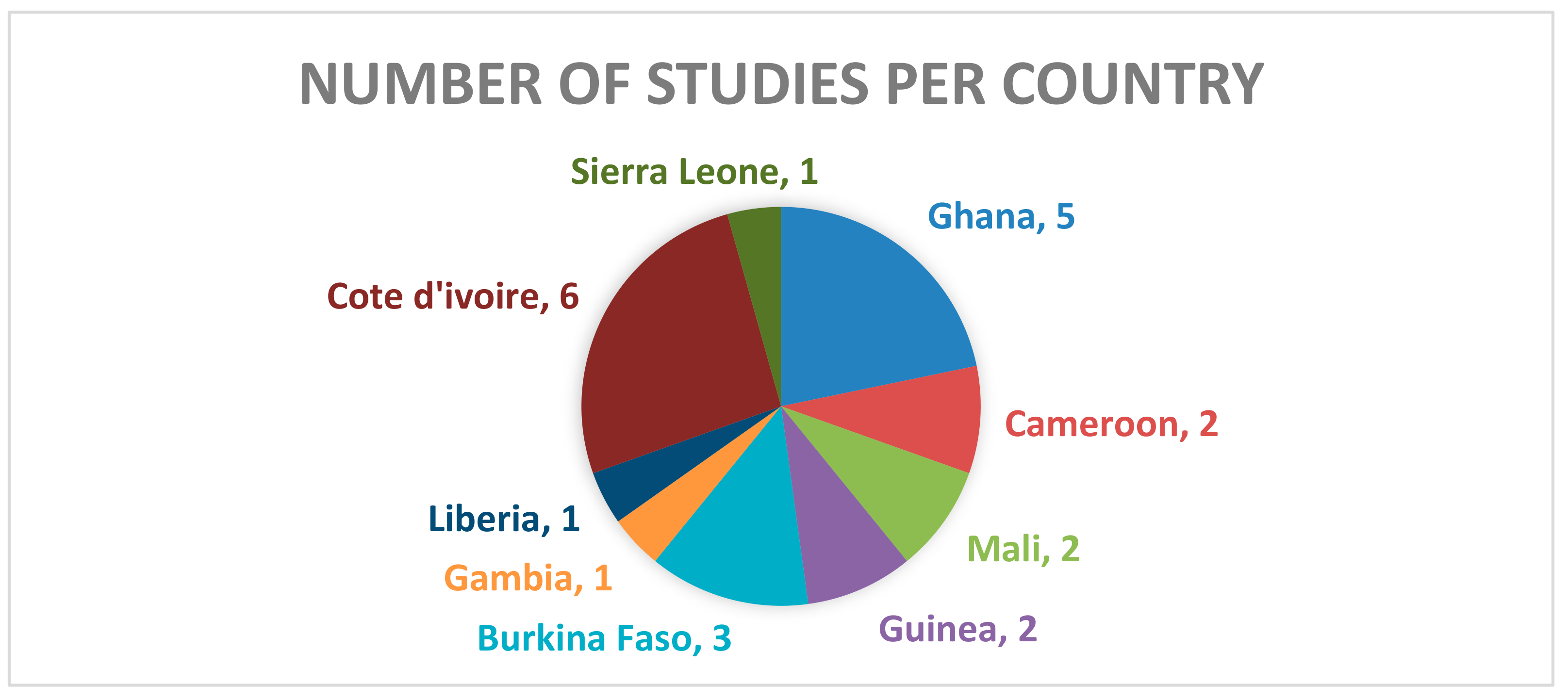
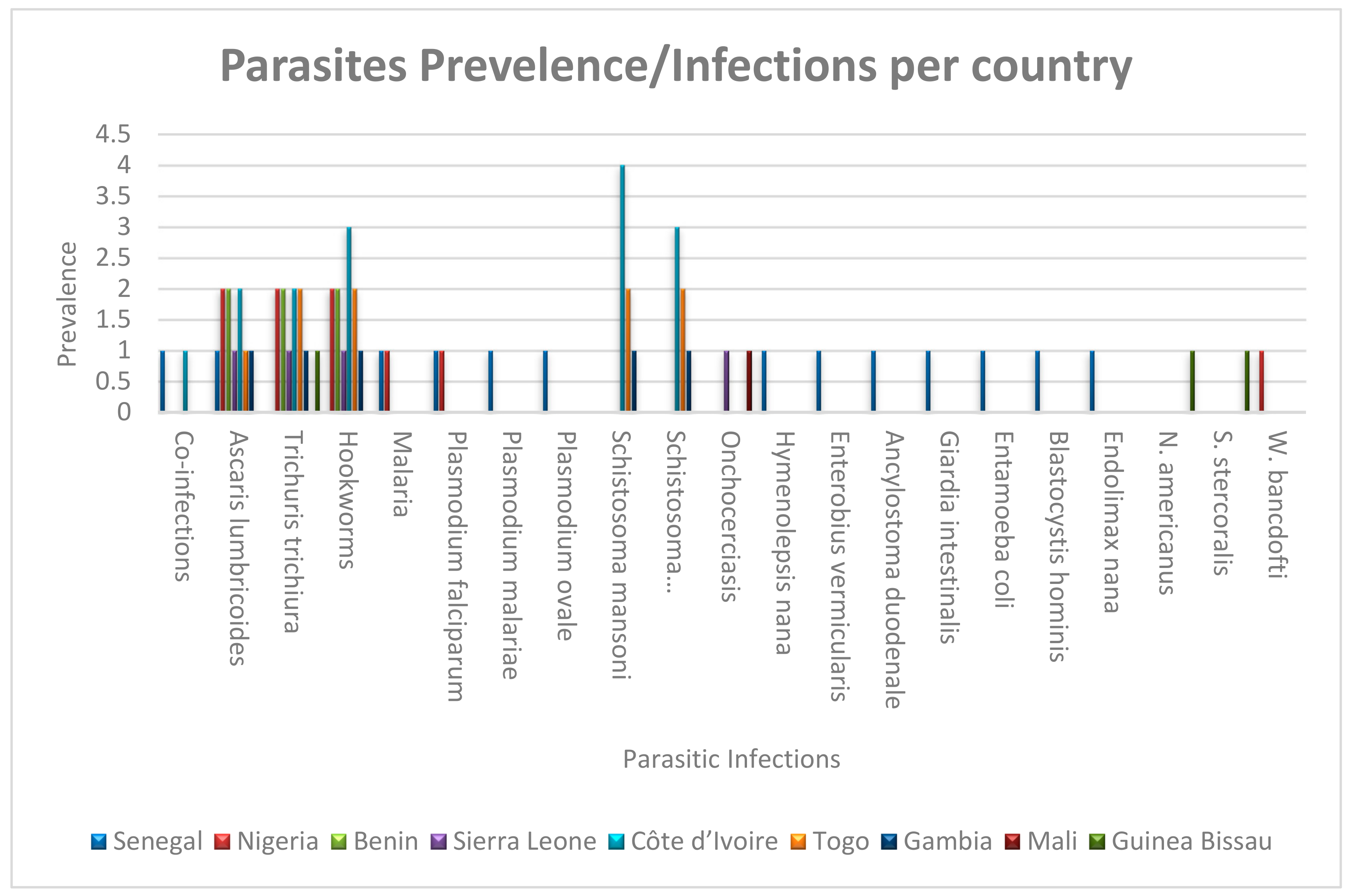
3. Results
4. Discussion
4.1. The Burden of Parasitic Diseases
4.2. Drivers of Transmission: Ecology, Poverty, and Culture
4.3. Progress and Pitfalls in Current Interventions
4.4. Emerging Challenges in a Changing World
4.5. Toward Sustainable and Equitable Solutions
4.6. Recommendations
- Longitudinal Studies: The need to court longitudinal studies as a means of tracking infection trends over time will provide deeper insights into the effectiveness of intervention strategies.
- Impact Assessment: There is the need for research to be focused on assessing the impact of integrated control programs on both individual health outcomes and broader public health metrics.
- Socioeconomic Factors: Investigating the influence of socioeconomic factors on infection prevalence can inform targeted interventions that address underlying vulnerabilities within communities.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 2021, 19, 623–638. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Malaria Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- MeMedeiros, Z.M.; Vieira, A.V.B.; Xavier, A.T.; Bezerra, G.S.N.; Lopes, M.d.F.C.; Bonfim, C.V.; Aguiar-Santos, A.M. Lymphatic filariasis: A systematic review on morbidity and its repercussions in countries in the Americas. Int. J. Environ. Res. Public Health 2022, 19, 316. [Google Scholar] [CrossRef]
- Adu-Gyasi, D.; Asante, K.P.; Frempong, M.T.; Gyasi, D.K.; Iddrisu, L.F.; Ankrah, L.; Dosoo, D.; Adeniji, E.; Agyei, O.; Gyaase, S.; et al. Epidemiology of soil transmitted Helminth infections in the middle-belt of Ghana, Africa. Parasite Epidemiol. Control. 2018, 3, e00071. [Google Scholar] [CrossRef] [PubMed]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba Histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef]
- Andrade, M.V.; Noronha, K.; Diniz, B.P.C.; Guedes, G.; Carvalho, L.R.; Silva, V.A.; Calazans, J.A.; Santos, A.S.; Silva, D.N.; Castro, M.C. The economic burden of malaria: A systematic review. Malar. J. 2022, 21, 283. [Google Scholar] [CrossRef]
- Ezeh, C.O.; Onyekwelu, K.C.; Akinwale, O.P.; Shan, L.; Wei, H. Urinary schistosomiasis in Nigeria: A 50 year review of prevalence, distribution and disease burden. Parasite 2019, 26, 19. [Google Scholar] [CrossRef]
- Owusu, E.D.A.; Buabeng, V.; Dadzie, S.; Brown, C.A.; Grobusch, M.P.; Mens, P. Characteristics of asymptomatic Plasmodium spp. parasitaemia in Kwahu-Mpraeso, a malaria endemic mountainous district in Ghana, West Africa. Malar. J. 2016, 15, 38. [Google Scholar] [CrossRef]
- Rahim, S.; Karim, M.M. Lymphatic Filariasis: A systematic review on its morbidity and road to elimination. Afr. J. Biol. Sci. 2022, 4, 116–126. [Google Scholar] [CrossRef]
- Liao, C.W.; Fu, C.J.; Kao, C.Y.; Lee, Y.L.; Chen, P.C.; Chuang, T.W.; Naito, T.; Chou, C.M.; Huang, Y.C.; Bonfim, I.; et al. Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of São Tomé and Príncipe, West Africa. Afr. Health Sci. 2016, 16, 690–697. [Google Scholar] [CrossRef][Green Version]
- Darko, B.A.; Owusu-Asenso, C.M.; Addo-Osafo, K.; Appiah-Lawson, E.; Afrane, Y.A.; Tette, E.M.A. Malaria, gastrointestinal parasite infection and nutritional status among febrile children in Accra, Ghana. Res. Sq. 2023. [Google Scholar] [CrossRef]
- World Bank. The Global Malaria Action Plan; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Sartorius, B.; Cano, J.; Tusting, L.S.; Pullan, R.L.; Marczak, L.B.; Miller-Petrie, M.K.; Hay, S.I.; Sartorius, B.; Cano, J.; Simpson, H.; et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000–2018: A geospatial analysis. Lancet Glob. Health 2020, 9, e52–e60. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Pattnaik, S.; Kshatri, J.S.; Kanungo, S.; Mandal, N.; Palo, S.K.; Pati, S. Prevalence and correlates of soil-transmitted helminths in schoolchildren aged 5 to 18 years in low- and middle-income countries: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1283054. [Google Scholar] [CrossRef] [PubMed]
- Ahiadorme, M.; Morhe, E. Soil transmitted helminth infections in Ghana: A ten year review. Pan Afr. Med. J. 2020, 35, 131. [Google Scholar] [CrossRef]
- Gidey, K.; Gidey, M.T.; Hailu, B.Y.; Gebreamlak, Z.B.; Niriayo, Y.L. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS ONE 2023, 18, e0282141. [Google Scholar] [CrossRef]
- Rinaldo, D.; Perez-Saez, J.; Vounatsou, P.; Utzinger, J.; Arcand, J.L. The economic impact of schistosomiasis. Infect. Dis. Poverty 2021, 10, 134. [Google Scholar] [CrossRef]
- Cable, J.; Barber, I.; Boag, B.; Ellison, A.R.; Morgan, E.R.; Murray, K.; Pascoe, E.L.; Sait, S.M.; Wilson, A.J.; Booth, M. Global change, parasite transmission and disease control: Lessons from ecology. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate Change and Cascading Risks from Infectious Disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef]
- Ge, Y.; Liang, D.; Cao, J.; Gosling, R.; Mushi, V.; Huang, J. How socioeconomic status affected the access to health facilities and malaria diagnosis in children under five years: Findings from 19 sub-Saharan African countries. Infect. Dis. Poverty 2023, 12, 29. [Google Scholar] [CrossRef]
- Kpoda, N.W.; Darimani, H.S.; Oueda, A.; Ouédraogo, I.; Kabré, G.B. Risk of Waterborne Parasitic Infection among Vegetables Producers in the City of Ouagadougou, Burkina Faso: An Attempt to Quantify Them Using the Quantitative Microbiological Risk Assessment Method. Agric. Sci. 2022, 13, 10–24. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. Available online: http://infection.thelancet.com (accessed on 17 December 2024). [CrossRef]
- Adegoke, A.A.; Amoah, I.D.; Stenström, T.A.; Verbyla, M.E.; Mihelcic, J.R. Epidemiological evidence and health risks associated with agricultural reuse of partially treated and untreated wastewater: A review. Front. Public Health 2018, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J.; Johansen, Ø.H.; Kifleyohannes, T.; Efunshile, A.M.; Terefe, G. Cryptosporidium Infections in Africa—How Important Is Zoonotic Transmission? A Review of the Evidence. Front. Vet. Sci. 2020, 7, 575881. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Lajaunie, C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated With Changes in Forest Cover and Oil Palm Expansion at Global Scale. Front. Vet. Sci. 2021, 8, 661063. [Google Scholar] [CrossRef] [PubMed]
- Bylicka-Szczepanowska, E.; Korzeniewski, K. Asymptomatic Malaria Infections in the Time of COVID-19 Pandemic: Experience from the Central African Republic. Int. J. Environ. Res. Public Health 2022, 19, 3544. [Google Scholar] [CrossRef]
- Shi, D.; Wei, L.; Liang, H.; Yan, D.; Zhang, J.; Wang, Z. Trends of the Global, Regional and National Incidence, Mortality, and Disability-Adjusted Life Years of Malaria, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. Risk Manag. Healthc. Policy 2023, 16, 1187–1201. [Google Scholar] [CrossRef]
- Zeng, M.; Niu, L. Exploring spatiotemporal trends and impacts of health resources and services on under-5 mortality in West African countries, 2010–2019: A spatial data analysis. Front. Public Health 2023, 11, 1193319. [Google Scholar] [CrossRef]
- UNICEF. Levels and Trends of Child Mortality in West and Central Africa; UNICEF: New York, NY, USA, 2023. [Google Scholar]
- Derbew Molla, Y.; Tesfahun Alemu, H. New Developments in Diagnosis of Intestinal Parasites. 2024. Available online: www.intechopen.com (accessed on 29 January 2025).
- Zhang, X.; Zhao, Y.; Zeng, Y.; Zhang, C. Evolution of the Probe-Based Loop-Mediated Isothermal Amplification (LAMP) Assays in Pathogen Detection. Diagnostics 2023, 13, 1530. [Google Scholar] [CrossRef]
- Ruenchit, P. State-of-the-art techniques for diagnosis of medical parasites and arthropods. Diagnostics 2021, 11, 1545. [Google Scholar] [CrossRef]
- Mahdavi, R.; Shams-Eldin, H.; Witt, S.; Latz, A.; Heinz, D.; Fresco-Taboada, A.; Aira, C.; Hübner, M.P.; Sukyte, D.; Visekruna, A.; et al. Development of a Novel Enzyme-Linked Immunosorbent Assay and Lateral Flow Test System for Improved Serodiagnosis of Visceral Leishmaniasis in Different Areas of Endemicity. Microbiol. Spectr. 2023, 11, e04338-22. [Google Scholar] [CrossRef]
- Abuaku, B.; Boateng, P.; Peprah, N.Y.; Asamoah, A.; Duah-Quashie, N.O.; Matrevi, S.A.; Amoako, E.O.; Quashie, N.; Owusu-Antwi, F.; Malm, K.L.; et al. Therapeutic efficacy of dihydroartemisinin-piperaquine combination for the treatment of uncomplicated malaria in Ghana. Front. Cell. Infect. Microbiol. 2023, 12, 1058660. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Da Costa, J.M.C. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar] [CrossRef] [PubMed]
- Husin, N.; Pasaribu, A.P.; Ali, M.; Suteno, E.; Suteno, E.; Wijaya, W.; Pasaribu, S. Comparison of Albendazole and Mebendazole on Soil-Transmitted Helminth Infections among School-Aged Children. Open Access Maced. J. Med. Sci. 2022, 10, 1264–1270. [Google Scholar] [CrossRef]
- Gonzales, M.L.M.; Dans, L.F.; Sio-Aguilar, J. Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst. Rev. 2019, 1, CD006085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundar, S.; Chakravarty, J. Liposomal amphotericin B and leishmaniasis: Dose and response. J. Glob. Infect. Dis. 2010, 2, 159–166. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Treatment of Human African Trypanosomiasis. 2024. Available online: https://iris.who.int/handle/10665/378083 (accessed on 4 March 2025).
- Banek, K.; Diliberto, D.D.; Webb, E.L.; Smith, S.J.; Chandramohan, D.; Staedke, S.G. Exploring barriers and facilitators of adherence to artemisinin-based combination therapies for the treatment of uncomplicated malaria in children in freetown, sierra leone. Healthcare 2021, 9, 1233. [Google Scholar] [CrossRef]
- Ogongo, P.; Nyakundi, R.K.; Chege, G.K.; Ochola, L. The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game. Front. Immunol. 2022, 13, 846108. [Google Scholar] [CrossRef]
- Takala-Harrison, S.; Laufer, M.K. Antimalarial drug resistance in Africa: Key lessons for the future. Ann. N. Y. Acad. Sci. 2015, 1342, 62–67. [Google Scholar] [CrossRef]
- Erkyihun, G.A.; Alemayehu, M.B. One Health Approach for the Control of Zoonotic Diseases. Zoonoses 2022, 2, 963. [Google Scholar] [CrossRef]
- Carpenter, A.; Waltenburg, M.A.; Hall, A.; Kile, J.; Killerby, M.; Knust, B.; Negron, M.; Nichols, M.; Wallace, R.M.; Behravesh, C.B.; et al. Vaccine Preventable Zoonotic Diseases: Challenges and Opportunities for Public Health Progress. Vaccines 2022, 10, 993. [Google Scholar] [CrossRef]
- Li, J.; Docile, H.J.; Fisher, D.; Pronyuk, K.; Zhao, L. Current Status of Malaria Control and Elimination in Africa: Epidemiology, Diagnosis, Treatment, Progress and Challenges. J. Epidemiol. Glob. Health 2024, 14, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Oyegoke, O.O.; Maharaj, L.; Akoniyon, O.P.; Kwoji, I.; Roux, A.T.; Adewumi, T.S.; Maharaj, R.; Oyebola, B.T.; Adeleke, M.A.; Okpeku, M. Malaria diagnostic methods with the elimination goal in view. Parasitol. Res. 2022, 121, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Sritart, H.; Tuntiwong, K.; Miyazaki, H.; Taertulakarn, S. Disparities in healthcare services and spatial assessments of mobile health clinics in the border regions of Thailand. Int. J. Environ. Res. Public Health 2021, 18, 10782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayeh-Kumi, P.F.; Tetteh-Quarcoo, P.B.; Aryee, I.N.A.; Baddoo, P.N.A.; Ocansey, J.T.; Otoboah, M.K. Navigating the Parasitic Landscape: Insights into Infection Patterns and Public Health Strategies in West Africa. Trop. Med. Infect. Dis. 2025, 10, 125. https://doi.org/10.3390/tropicalmed10050125
Ayeh-Kumi PF, Tetteh-Quarcoo PB, Aryee INA, Baddoo PNA, Ocansey JT, Otoboah MK. Navigating the Parasitic Landscape: Insights into Infection Patterns and Public Health Strategies in West Africa. Tropical Medicine and Infectious Disease. 2025; 10(5):125. https://doi.org/10.3390/tropicalmed10050125
Chicago/Turabian StyleAyeh-Kumi, Patrick F., Patience B. Tetteh-Quarcoo, Isabella N. A. Aryee, Peter Nii Apai Baddoo, James Teye Ocansey, and Michael Kofi Otoboah. 2025. "Navigating the Parasitic Landscape: Insights into Infection Patterns and Public Health Strategies in West Africa" Tropical Medicine and Infectious Disease 10, no. 5: 125. https://doi.org/10.3390/tropicalmed10050125
APA StyleAyeh-Kumi, P. F., Tetteh-Quarcoo, P. B., Aryee, I. N. A., Baddoo, P. N. A., Ocansey, J. T., & Otoboah, M. K. (2025). Navigating the Parasitic Landscape: Insights into Infection Patterns and Public Health Strategies in West Africa. Tropical Medicine and Infectious Disease, 10(5), 125. https://doi.org/10.3390/tropicalmed10050125






