Neonatal Mucormycosis: A Rare but Highly Lethal Fungal Infection in Term and Preterm Newborns—A 20-Year Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- 1.
- Mucormycosis as an etiologic agent.
- 2.
- Documentation of infection: Mucormycosis was confirmed either histologically by culture or by mycological study (pre- or post-mortem).
- 3.
- Onset age: Less than 1 month.
- 4.
- Gestational weeks: Specifying whether the newborn was preterm or term.
- 5.
- Birth weight.
- 6.
- Sex.
- 7.
- Topography of the mucormycosis lesions.
- 8.
- Therapeutic intervention: Use of antifungal therapy.
- 9.
- Surgical intervention.
- 10.
- Outcome: Mortality was assessed as ‘‘all-cause mortality’’ during the course of mucormycosis.
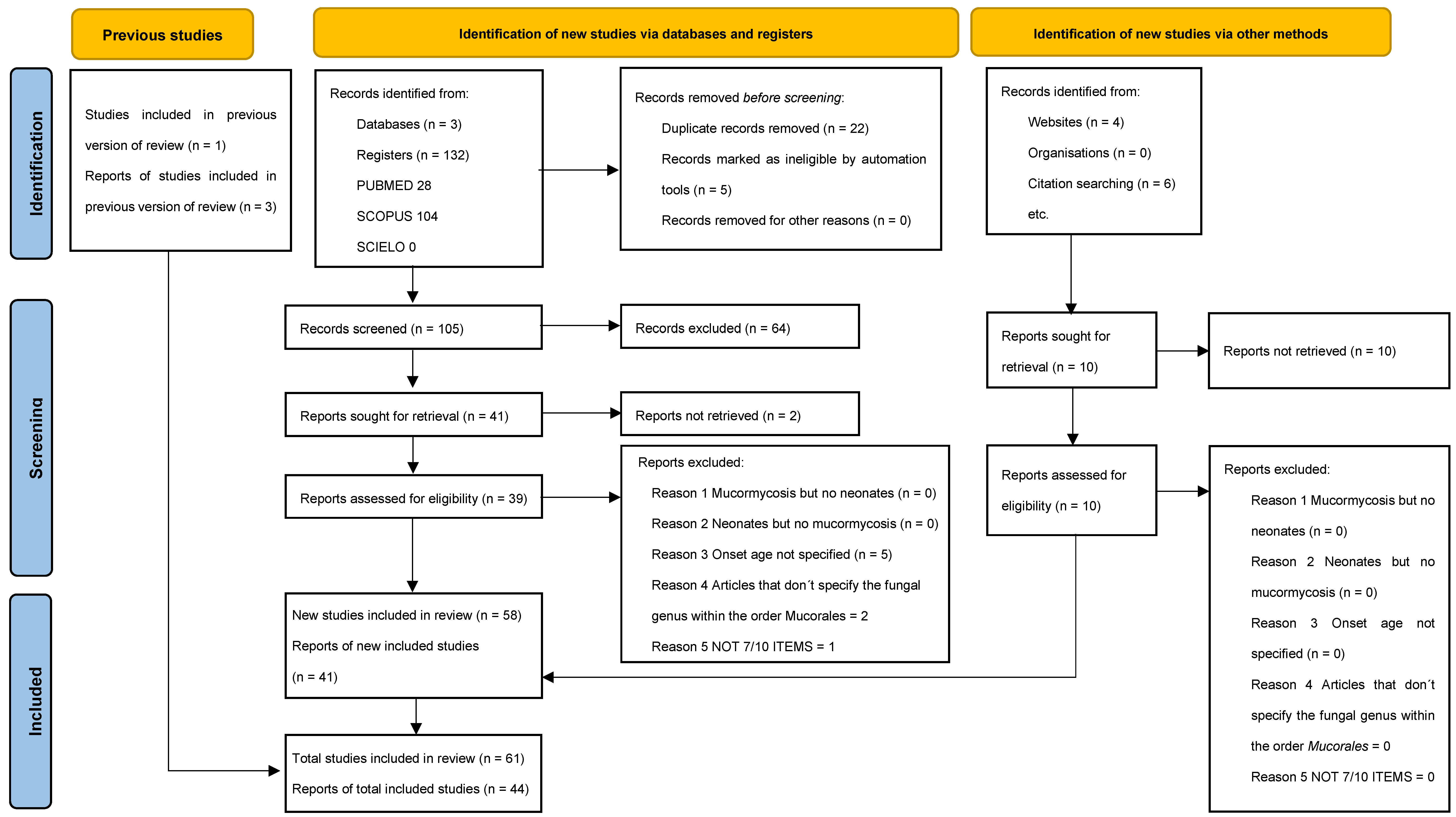
2.1. Quality Assessment
2.2. Statistical Evaluation
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrikkos, G.; Tsioutis, C. Recent advances in the pathogenesis of mucormycoses. Clin. Ther. 2018, 40, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Alqarihi, A.; Kontoyiannis, D.P.; Ibrahim, A.S. Mucormycosis in 2023: An update on pathogenesis and management. Front. Cell. Infect. Microbiol. 2023, 13, 1254919. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Kontoyiannis, D.P. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013, 26, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Özbek, L.; Topçu, U.; Manay, M.; Esen, B.H.; Bektas, S.N.; Aydın, S.; Özdemir, B.; Khostelidi, S.N.; Klimko, N.; Cornely, O.; et al. COVID-19–associated mucormycosis: A systematic review and meta-analysis of 958 cases. Clin. Microbiol. Infect. 2023, 29, 722–731. [Google Scholar] [CrossRef]
- Roilides, E.; Zaoutis, T.E.; Katragkou, A.; Benjamin, D.K., Jr.; Walsh, T.J. Zygomycosis in neonates: An uncommon but life-threatening infection. Am. J. Perinatol. 2009, 26, 565–573. [Google Scholar] [CrossRef]
- Sangild, P.T.; Strunk, T.; Currie, A.J.; Nguyen, D.N. Editorial: Immunity in compromised newborns. Front. Immunol. 2021, 12, 732332. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Simeoni, U.; La Scola, B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol. 2016, 11, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. N. Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Otto, W.R.; Pahud, B.A.; Yin, D.E. Pediatric mucormycosis: A 10-year systematic review of reported cases and review of the literature. J. Pediatr. Infect. Dis. Soc. 2019, 8, 342–350. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kargl, S.; Silye, R.; Pumberger, W. Intestinal mucormycosis in neonates—A surgical disease. Surg. Curr. Res. 2013, S6, 001. [Google Scholar] [CrossRef]
- Siu, K.L.; Lee, W.H. A rare cause of intestinal perforation in an extreme low birth weight infant—Gastrointestinal mucormycosis: A case report. J. Perinatol. 2004, 24, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Hoyosa, Á.; Mejía, M.A.; Herrera, V.; Soto, A.; Rico, C. Necrotizing cellulitis due to Rhizopus arrhizus in an extremely premature infant. IDCases 2020, 20, e00754. [Google Scholar] [CrossRef]
- Veleminsky, M., Sr.; Noll, P.; Hanzl, M.; Veleminsky, M., Jr. Necrotizing enterocolitis in children with low birth-weight induced with mucormycose strains. Neuro Endocrinol. Lett. 2008, 29, 1021–1025. [Google Scholar] [PubMed]
- Kumar, V.; Aggarwal, A.; Taneja, R.; Saha, S.S.; Khazanchi, R.K.; Kler, N.; Saluja, S. Primary cutaneous mucormycosis in a premature neonate and its management by tumescent skin grafting. Br. J. Plast. Surg. 2005, 58, 852–854. [Google Scholar] [CrossRef]
- Shah, A.; Lagvankar, S.; Shah, A. Cutaneous mucormycosis in children. Indian Pediatr. 2006, 43, 167–170. [Google Scholar] [PubMed]
- Dhingra, K.K.; Mandal, S.; Khurana, N. Unsuspected intestinal mucormycosis in a neonate presenting as necrotizing enterocolitis (NEC). Eur. J. Pediatr. Surg. 2008, 18, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Vij, M.; Chirla, D.K.; Kumar, N.; Samal, S.C. Unsuspected invasive neonatal gastrointestinal mucormycosis: A clinico-pathological study of six cases from a tertiary care hospital. J. Indian Assoc. Pediatr. Surg. 2012, 17, 153–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrawal, P.; Saikia, U.; Ramanaathan, S.; Samujh, R. Neonatal small intestinal zygomycosis misdiagnosed as intussusception in a two-day-old child with a review of the literature. Fetal Pediatr. Pathol. 2013, 32, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Veerabhadra, R.; Krishnakumar, G.; Bharathi, B.; Nishad, P.; Sree Rekha, J. Gastrointestinal mucormycosis: A rare but lethal mimicker of necrotising enterocolitis. J. Nepal Paediatr. Soc. 2016, 36, 198–200. [Google Scholar] [CrossRef]
- Athwani, V.K.; Sharma, P.K.; Mahal, T.; Kaur, B. Gastrointestinal mucormycosis mimicking as necrotizing enterocolitis: Case series of 5 neonates. J. Neonatol. 2018, 32, 74–77. [Google Scholar] [CrossRef]
- Mishra, S.; Shelly, D.; Gupta, D.; Bharadwaj, R. Invasive cutaneous mucormycosis in a preterm neonate presenting as a vesicobullous lesion. Indian J. Pathol. Microbiol. 2018, 61, 103–105. [Google Scholar] [CrossRef] [PubMed]
- William, A.; Kaur, R.; Rawat, D.; Kandir, N.S.S.; Sharma, A. Necrotizing fasciitis in neonate by Lichtheimia ramosa: A case study. Access Microbiol. 2022, 4, 000327. [Google Scholar] [CrossRef] [PubMed]
- Kajal, P.; Bhutani, N.; Saini, K.; Sindhu, A. Mucormycosis of the colon in a premature neonate. J. Neonatal Surg. 2022, 11, 1085. [Google Scholar] [CrossRef]
- Inoue, S.; Odaka, A.; Hashimoto, D.; Hoshi, R.; Kurishima, C.; Kunikata, T.; Sobajima, H.; Tamura, M.; Tamaru, J. Rare case of disseminated neonatal zygomycosis mimicking necrotizing enterocolitis with necrotizing fasciitis. J. Pediatr. Surg. 2011, 46, E29–E32. [Google Scholar] [CrossRef] [PubMed]
- Ishiwada, N.; Kitajima, H.; Morioka, I.; Takeuchi, N.; Endo, M.; Watanabe, A.; Kamei, K. Nationwide survey of neonatal invasive fungal infection in Japan. Med. Mycol. 2018, 56, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Tomotaki, S.; Takeyama, E.; Tanaka, M.; Ohyama, M.; Tanaka, Y. Mucor mycelial thrombosis of the portal vein in an extremely low-birthweight infant. Pediatr. Int. 2018, 60, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Kucinskiene, V.; Sutkute, A.; Valiukeviciene, S. Cutaneous fungal infection in a neonatal intensive care unit patient: A case report and literature review. Pediatr. Dermatol. 2014, 31, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Morales-Aguirre, J.J.; Agüero-Echeverría, W.M.; Ornelas-Carsolio, M.E.; Reséndiz-Sánchez, J.; Gómez-Barreto, D.; Cashat-Cruz, M. Successful treatment of a primary cutaneous zygomycosis caused by Absidia corymbifera in a premature newborn. Pediatr. Infect. Dis. J. 2004, 23, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Al Tawil, K.; Eldemerdash, A.; Balkhy, H.; Al Hathlol, K.; Shaalan, M. Neonatal necrotizing fasciitis and fungal infections in preterm infants. J. Neonatal Perinatal Med. 2010, 3, 147–151. [Google Scholar] [CrossRef]
- Oztürk, M.A.; Akin, M.A.; Deniz, K.; Turan, C.; Efendioğlu, B.; Yikilmaz, A.; Sarici, D. Neonatal gastrointestinal mucormycosis in an asphyxiated premature newborn. Turk. J. Pediatr. 2011, 53, 705–708. [Google Scholar] [PubMed]
- White, H.; Emery, F. Case of cutaneous Rhizopus infection in an extremely preterm infant. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 599. [Google Scholar] [CrossRef] [PubMed]
- Chmelova, K.; Tinnion, R.; Zalewski, S. Cutaneous mucormycosis in an extremely premature infant. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 661. [Google Scholar] [CrossRef] [PubMed]
- Nichol, P.F.; Corliss, R.F.; Rajpal, S.; Helin, M.; Lund, D.P. Perforation of the appendix from intestinal mucormycosis in a neonate. J. Pediatr. Surg. 2004, 39, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Diven, S.C.; Angel, C.A.; Hawkins, H.K.; Rowen, J.L.; Shattuck, K.E. Intestinal zygomycosis due to Absidia corymbifera mimicking necrotizing enterocolitis in a preterm neonate. J. Perinatol. 2004, 24, 794–796. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Walker, T.A.; Lockhart, S.R.; Ng, D.; Chiller, T.; Melchreit, R.; Brandt, M.E.; Smith, R.M.; Centers for Disease Control and Prevention (CDC). Notes from the field: Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 155–156. [Google Scholar] [PubMed] [PubMed Central]
- Lowe, C.D.; Sainato, R.J.; Stagliano, D.R.; Morgan, M.M.; Green, B.P. Primary cutaneous mucormycosis in an extremely preterm infant successfully treated with liposomal amphotericin B. Pediatr. Dermatol. 2017, 34, e116–e119. [Google Scholar] [CrossRef] [PubMed]
- Matlock, D.N., Jr.; Miquel-Verges, F.; Courtney, S.E. Invasive fungal dermatitis in preterm newborns. Clin. Pediatr. 2018, 57, 1354–1355. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Abdessalam, S.; Davies, H.D.; Aldrich, A.M.; Bedrnicek, J.; Gollehon, N. Invasive cutaneous mucormycosis in an extremely preterm infant. J. Pediatr. Surg. Case Rep. 2018, 35, 52–56. [Google Scholar] [CrossRef]
- Benjamin, T.; Wattier, R.L.; Dominic, W. Allograft of primary cutaneous mucormycosis in a preterm neonate: A case report. Wounds 2019, 31, E46–E48. [Google Scholar] [PubMed]
- Fatemizadeh, R.; Rodman, E.; Demmler-Harrison, G.J.; Dinu, D. Rhizopus infection in a preterm infant: A novel use of posaconazole. Pediatr. Infect. Dis. J. 2020, 39, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Vittitow, S.L.; Rusu, C.A.; Abubakar, M.O.; Burnsed, J.; Gru, A.A.; Zlotoff, B.J. Primary cutaneous mucormycosis in a premature neonate treated conservatively with amphotericin B. Pediatr. Dermatol. 2022, 39, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Calabria, O.; Acosta, N.; Gallegos, L.; Vargas Montiel, H. Mucormicosis cutánea en un recién nacido pretérmino: A propósito de un caso. Kasmera 2005, 33, 166–172. [Google Scholar]
- Jain, D.; Kohli, K. Neonatal gastrointestinal mucormycosis clinically mimicking necrotizing enterocolitis. Eur. J. Pediatr. Surg. 2009, 19, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.; Alladi, A.; Correa, M.; D’Cruz, A.J. Neonatal colonic mucormycosis—A tropical perspective. J. Trop. Pediatr. 2005, 51, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sarin, Y.K. Intestinal mucormycosis in a neonate: A case report and review. J. Indian Assoc. Pediatr. Surg. 2010, 15, 98–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, R.; Parelkar, S.V.; Oak, S.; Sanghvi, B.; Prakash, A. Neonatal lingual and gastrointestinal mucormycosis in a case of low anorectal malformation—A rare presentation. J. Pediatr. Surg. 2011, 46, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Sathe, P.A.; Ghodke, R.K.; Kandalkar, B.M. A survivor of neonatal intestinal mucormycosis. J. Clin. Diagn. Res. 2015, 9, ED24–ED25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirakhur, H.; Sekhon, V.; Luthra, M. Neonatal cutaneous mucormycosis. J. Clin. Neonatol. 2016, 5, 262–264. [Google Scholar] [CrossRef]
- Devi, R.U.; Balachandran, A.; Kamalarathnam, C.N.; Pappathi, S. Neonatal mucormycosis with gastrointestinal and cutaneous involvement. Indian Pediatr. 2018, 55, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Suresh, D.V.; Rangesh, S.; Mehrunnissa, J.B.; Aqthar, J.B. Neonatal mucormycosis. Indian J. Pediatr. 2021, 88, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Athwani, V.K.; Malviya, L.M.; Lazarus, M.; Sachdeva, K.; Madoriya, K.; Yadav, N. Neonatal rhino-orbito-cerebral mucormycosis. Perinatology 2023, 24, S92–S96. [Google Scholar]
- Sawardekar, K.P. Gangrenous necrotizing cutaneous mucormycosis in an immunocompetent neonate: A case report from Oman. J. Trop. Pediatr. 2018, 64, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Arshad, S.; Idrees, R.; Abdul-Ghafar, J.; Din, N.U. Fatal invasive gastrointestinal fungal infection in three non-immunocompromised patients. J. Coll. Physicians Surg. Pak. 2019, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Castaño, S.; Velásquez-Trujillo, L.A.; Ángel-Correa, M.; Bravo, J.H.; Matta-Cortés, L. Mucormicosis: Un dulce enemigo, serie de casos. Biomédica 2024, 44, 135–143. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Yu, Y.; Ji, H.; Dong, X.; Li, J.; Li, H.; He, H.; Li, Z.; Yang, Z.; et al. Invasive fungal infection is associated with antibiotic exposure in preterm infants: A multi-centre prospective case–control study. J. Hosp. Infect. 2023, 134, 43–49. [Google Scholar] [CrossRef]
- Otten, C.M.; Taylor, S.; Stoll, B.; Goldberg, R.N.; Hansen, N.I.; Sánchez, P.J.; Ambalavanan, N.; Benjamin, D.K., Jr. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009, 123, 58–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jenks, J.D.; Prattes, J.; Wurster, S.; Sprute, R.; Seidel, D.; Oliverio, M.; Egger, M.; Del Rio, C.; Sati, H.; Cornely, O.A.; et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023, 66, 102325. [Google Scholar] [CrossRef]
- Mehler, K.; Cornely, O.; Seifert, H.; Zweigner, J.; Janssen, S.; Oberthuer, A. Molds and more: Rare fungal infections in preterm infants <24 weeks of gestation. Pediatr. Infect. Dis. J. 2022, 41, 352–357. [Google Scholar] [CrossRef]
- Gunathilaka, M.G.; Senevirathna, R.M.; Illappereruma, S.C.; Keragala, K.A.R.; Hathagoda, K.L.; Bandara, H.M. Mucormycosis-causing fungi in humans: A meta-analysis establishing the phylogenetic relationships using internal transcribed spacer (ITS) sequences. J. Med. Microbiol. 2023, 72, 001651. [Google Scholar] [CrossRef]
- Raveenthiran, V. Gastrointestinal mucormycosis mimicking necrotizing enterocolitis of newborn. J. Neonatal Surg. 2013, 2, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the Susceptibility of the Premature Infant to Necrotizing Enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Q.; Tao, X.; Zhang, Z.; Shi, L.; Cheng, L.; He, P.; Fan, X.; Xu, R.; Luo, Y.; et al. Primary cutaneous mucormycosis caused by Mucor irregularis in a Chinese man. Mycopathologia 2024, 189, 39. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Lanternier, F.; Zahar, J.R.; Dannaoui, E.; Bougnoux, M.E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54 Suppl. 1, S44–S54. [Google Scholar] [CrossRef]
- Clark, N.M.; Grim, S.A.; Lynch, J.P., III. Posaconazole: Use in the prophylaxis and treatment of fungal infections. Semin. Respir. Crit. Care Med. 2015, 36, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Harpf, V.; Rambach, G.; Parth, N.; Neurauter, M.; Fleischer, V.; Lackner, M.; Lass-Flörl, C.; Würzner, R.; Speth, C. Complement, but not platelets, plays a pivotal role in the outcome of mucormycosis in vivo. J. Fungi 2023, 9, 162. [Google Scholar] [CrossRef]
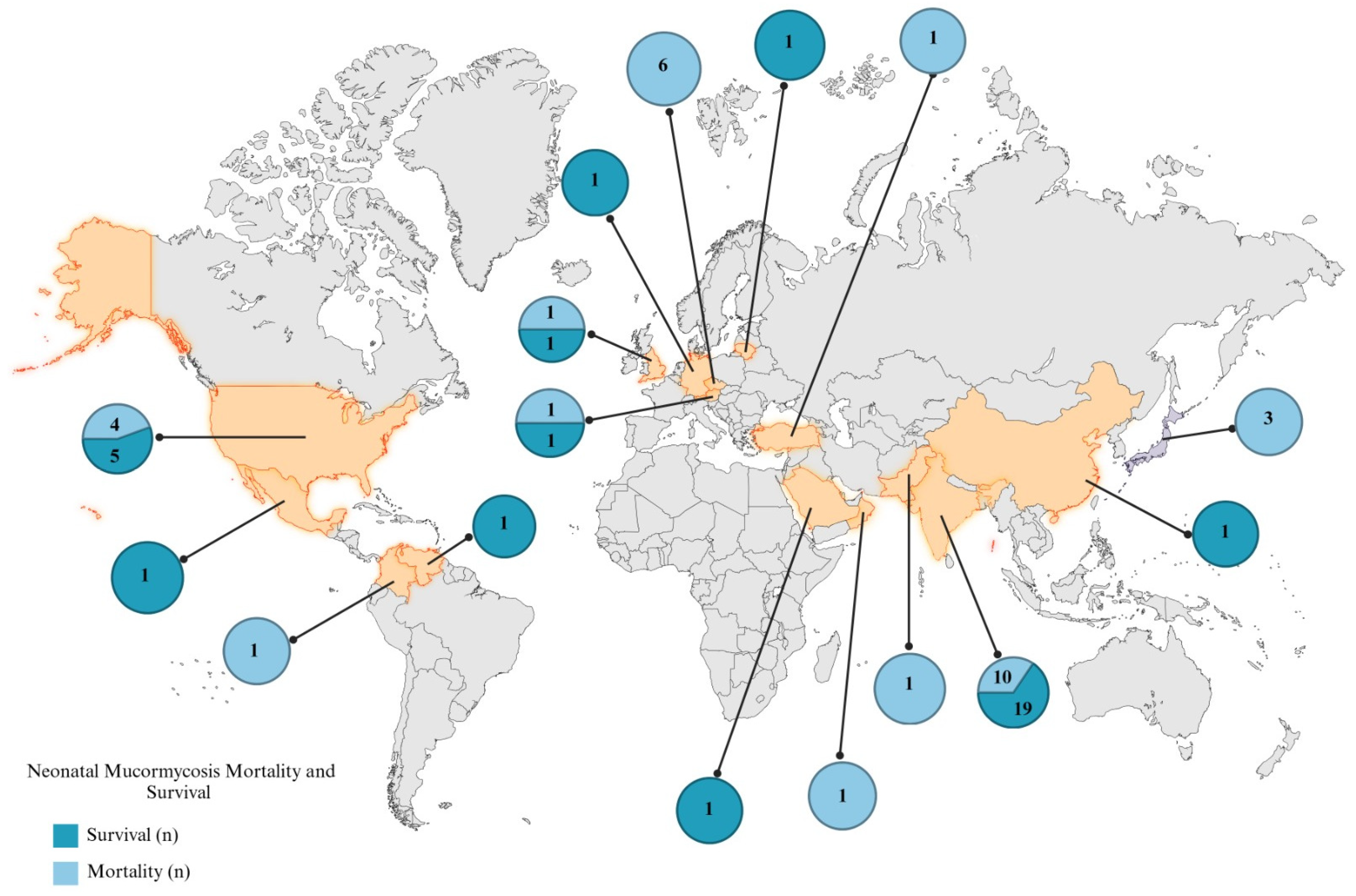
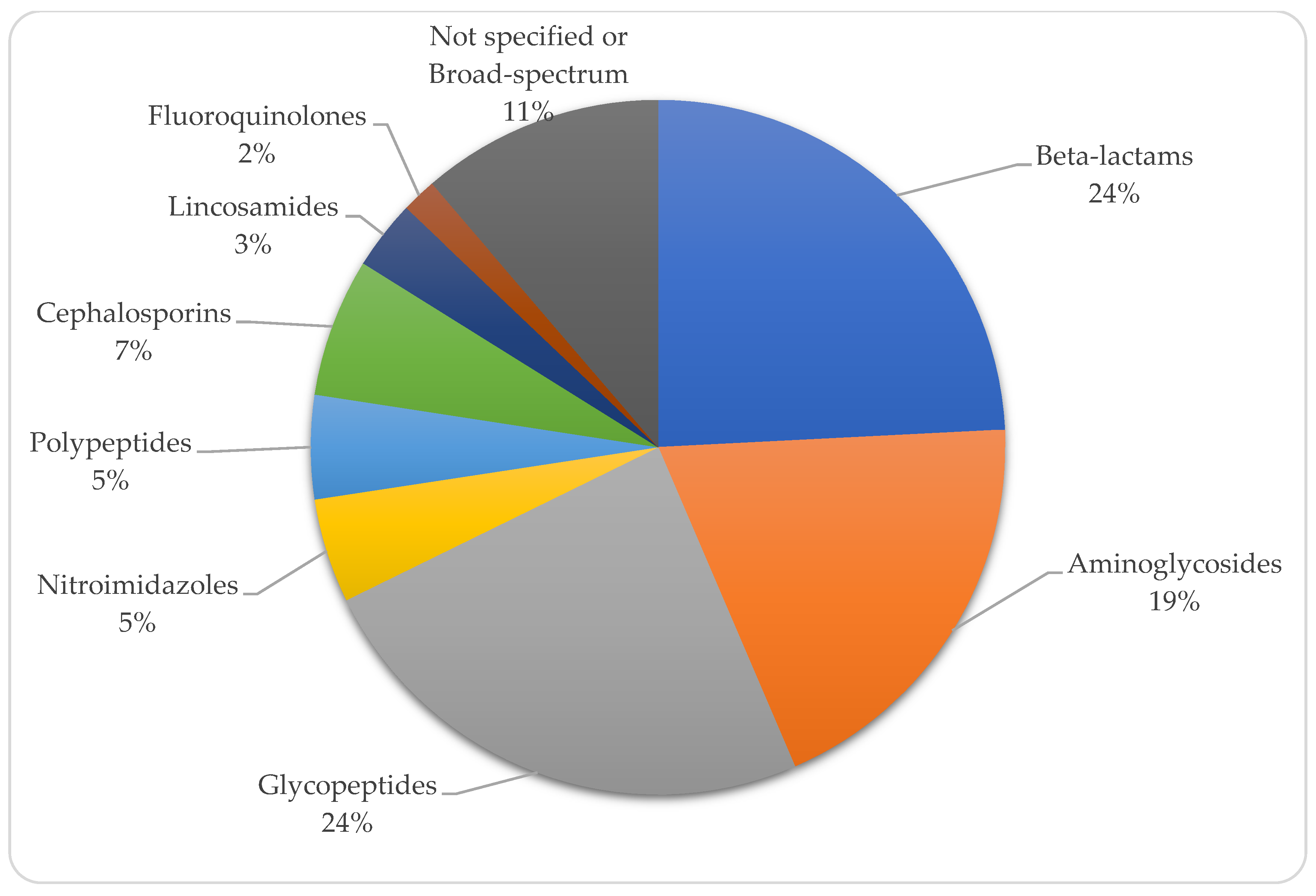
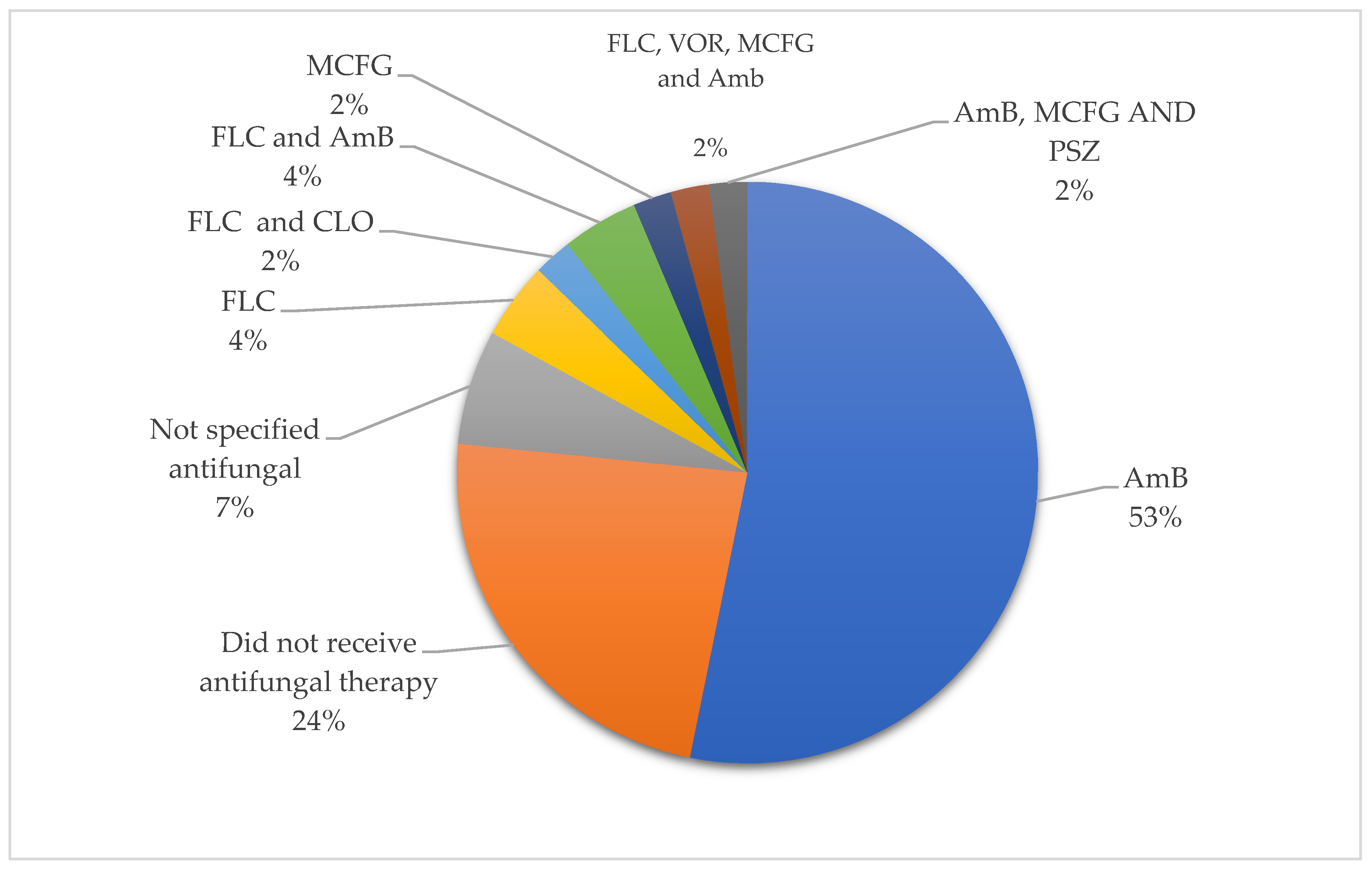
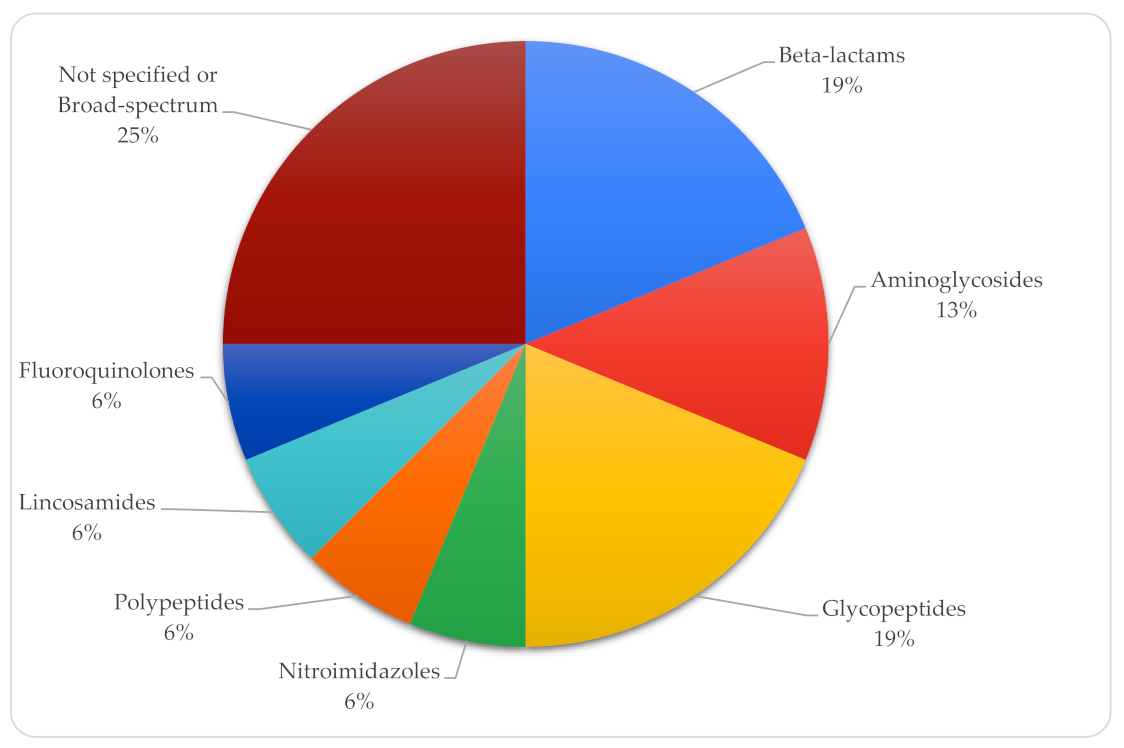
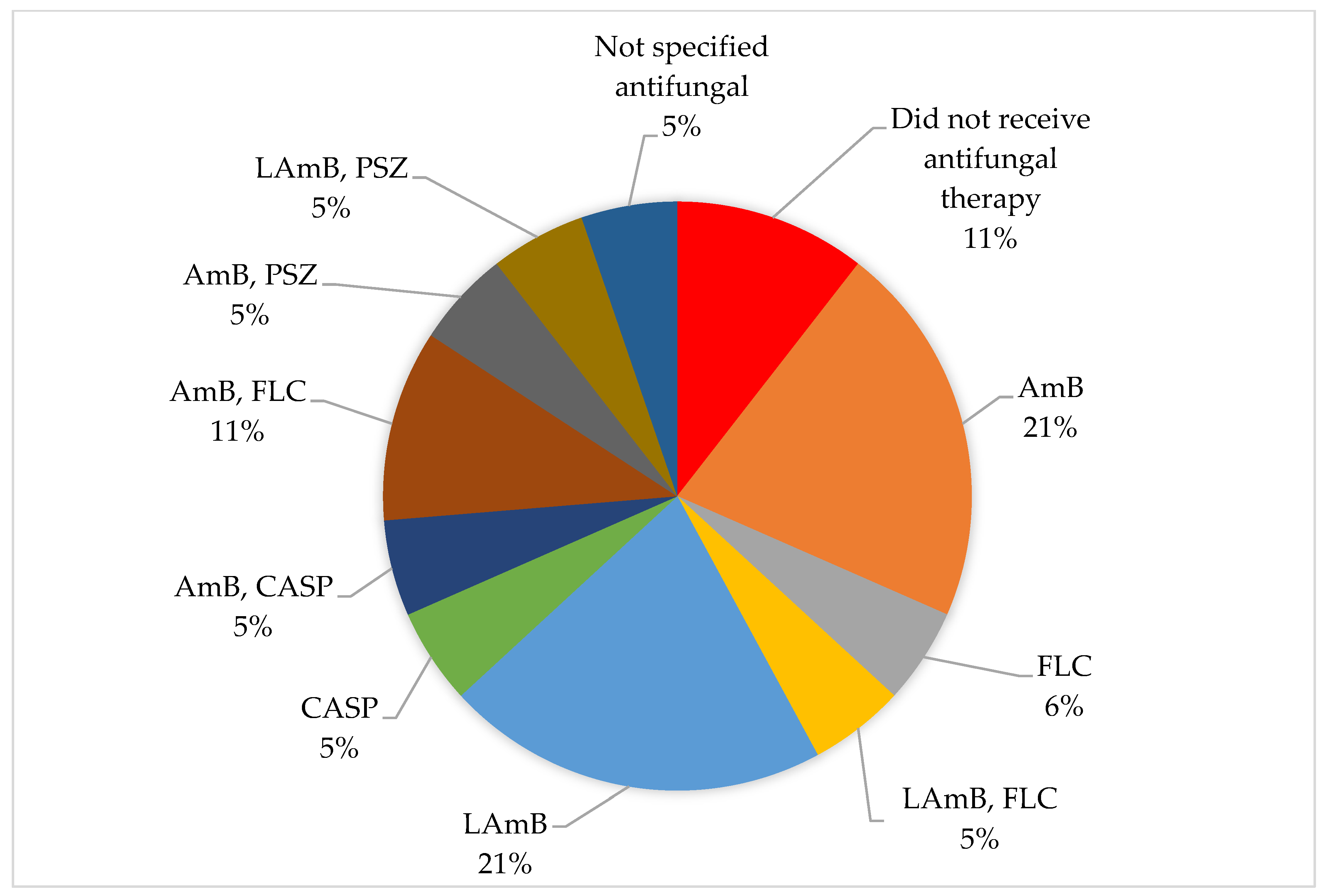
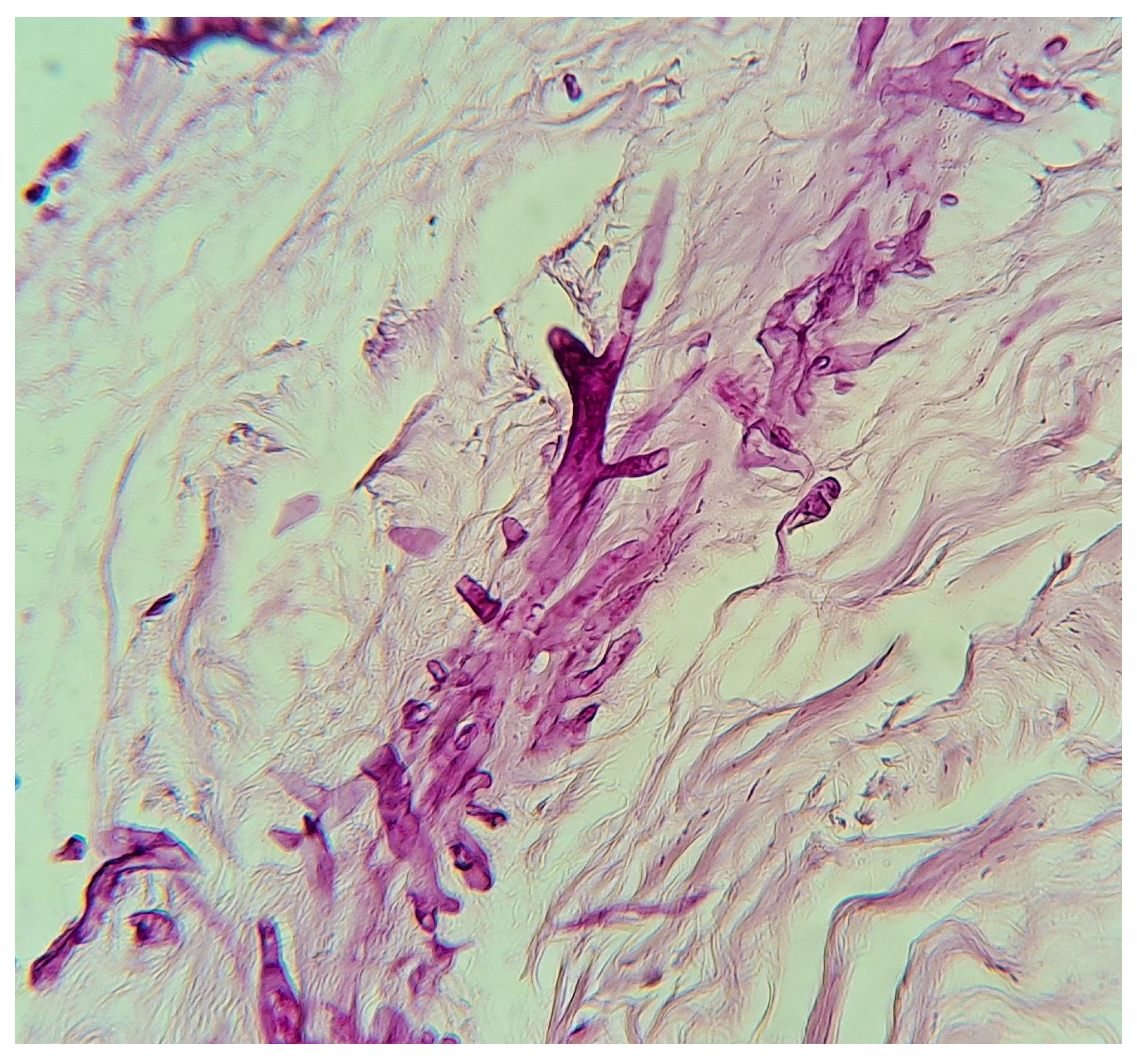
| Year | Country | Topography | Sex | Onset Age | Gestational Age (Weeks) | Birth Weight (kg) | Respiratory Distress | Oxygen | Diagnostic Method | Etiological Agent | Antibiotics | Surgical Treatment | Antifungal Therapy | Survival | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | Austria | Gastrointestinal | M | 21 | 36 | NA | Yes | Yes | Biopsy, culture | Mucor spp. | Yes | Yes | AmB | Yes | [11] |
| 2004 | China | Gastrointestinal | M | 15 | 26.4 | 0.839 | Yes | Yes | Biopsy, GMS | Mucor spp. | Yes | Yes | AmB | Yes | [12] |
| 2020 | Colombia | Cutaneous | F | 20 | 24.42 | 0.645 | Yes | Yes | Biopsy, culture | Rhizopus spp. | Yes | No | AmB | No | [13] |
| 2008 | Czech Republic | Gastrointestinal | F | 1 | 26 | 0.85 | Yes | Yes | Biopsy | Mucor spp. | Yes | No | NO | No | [14] |
| 2008 | Gastrointestinal | NA | 5 | 26 | 0.78 | Yes | Yes | Biopsy | Mucor spp. | No | Yes | NO | No | ||
| 2008 | Gastrointestinal | M | 3 | 27 | 0.96 | Yes | Yes | Biopsy | Mucor spp. | No | Yes | NO | No | ||
| 2008 | Gastrointestinal | F | 8 | 30 | 1.1 | Yes | Yes | Biopsy | Mucor spp. | Yes | No | NO | No | ||
| 2008 | Gastrointestinal | NA | 6 | NA | 0.98 | Yes | Yes | Biopsy | Mucor spp. | No | No | NO | No | ||
| 2008 | Gastrointestinal | M | 7 | NA | 1.9 | Yes | Yes | Biopsy | Mucor spp. | No | Yes | NO | No | ||
| 2005 | India | Cutaneous | M | 10 | 35 | 1.6 | Yes | Yes | Biopsy, culture | Mucor spp. | Yes | Yes | AmB | Yes | [15] |
| 2006 | Cutaneous | M | 2 | 34 | 2 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | AmB | Yes | [16] | |
| 2007 | Gastrointestinal | M | 2 | 37 | 2.1 | No | No | Biopsy, GMS | Mucor spp. | No | Yes | YES * | Yes | [17] | |
| 2012 | Gastrointestinal | M | 8 | 32 | NA | No | No | Biopsy, GMS | Mucor spp. | No | Yes | AmB | No | [18] | |
| 2012 | Gastrointestinal | F | 22 | 32 | NA | No | No | Biopsy, GMS | Mucor spp. | Yes | Yes | AmB | Yes | ||
| 2012 | Gastrointestinal | F | 7 | 33 | NA | No | No | Biopsy, GMS | Mucor spp. | No | Yes | AmB | No | ||
| 2012 | Gastrointestinal | F | 10 | 33 | 1.6 | No | No | Biopsy, GMS | Mucor spp. | Yes | Yes | AmB | Yes | ||
| 2012 | Gastrointestinal | M | 18 | 36 | NA | No | No | Biopsy, GMS | Mucor spp. | Yes | Yes | AmB | Yes | ||
| 2013 | Gastrointestinal | M | 2 | 37 | 1.5 | No | No | Biopsy | Mucor spp. | No | Yes | YES * | Yes | [19] | |
| 2016 | Gastrointestinal | F | 13 | 33 | NA | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | NO | No | [20] | |
| 2018 | Gastrointestinal | M | 3 | NA | NA | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | AmB | No | [21] | |
| 2018 | Gastrointestinal | M | 5 | NA | NA | Yes | Yes | Biopsy | Mucor spp. | No | Yes | AmB | No | ||
| 2018 | Cutaneous | M | 5 | 25 | 0.5 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | AmB | No | [22] | |
| 2022 | Cutaneous | M | 11 | 30 | 1.8 | Yes | Yes | Culture, KOH, biopsy | Lichtheimia ramosa | Yes | Yes | AmB | Yes | [23] | |
| 2022 | Gastrointestinal | M | 10 | 36 | 1.5 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | NO | Yes | [24] | |
| 2011 | Japan | Gastrointestinal | M | 1 | 26 | 0.59 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | FLC | No | [25] |
| 2017 | Disseminated infection | NA | 7 | 22 | 0.452 | No | No | Blood culture | Mucor spp. | No | No | MCFG | No | [26] | |
| 2017 | Gastrointestinal | M | 9 | 24.71 | 0.58 | No | No | Biopsy, GMS | Rhizopus spp. | No | Yes | AmB | No | [27] | |
| 2014 | Lithuania | Cutaneous | F | 14 | 24 | 0.584 | Yes | Yes | KOH, culture | Syncephalastrum spp. | Yes | No | FLC, CLO | Yes | [28] |
| 2004 | Mexico | Cutaneous | F | 18 | 36 | 1.6 | No | No | Culture, biopsy | Absidia corymbifera | Yes | Yes | AmB | Yes | [29] |
| 2010 | Saudi Arabia | Cutaneous | M | 4 | 26 | 0.715 | Yes | Yes | Biopsy | Mucor spp. | Yes | No | AmB | Yes | [30] |
| 2011 | Türkiye | Gastrointestinal | M | 15 | 27 | 0.9 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | NO | No | [31] |
| 2020 | UK | Cutaneous | NA | 6 | 23.28 | 0.521 | No | No | Biopsy | Rhizopus microsporus | No | No | AmB | Yes | [32] |
| 2022 | Cutaneous | F | 7 | 23.57 | 0.46 | Yes | Yes | Biopsy | Mucor spp. | Yes | No | YES * | No | [33] | |
| 2004 | USA | Gastrointestinal | F | 15 | 32 | 2.94 | Yes | Yes | GMS | Mucor spp. | Yes | Yes | NO | Yes | [34] |
| 2004 | Gastrointestinal | F | 7 | 25 | 0.704 | Yes | Yes | Biopsy, GMS | Absidia corymbifera | Yes | Yes | AmB | No | [35] | |
| 2014 | Gastrointestinal | NA | 7 | 29 | 1.4 | No | No | Biopsy | Mucor spp. | No | Yes | NO | No | [36] | |
| 2017 | Cutaneous | M | 7 | 23.57 | 0.45 | No | No | Biopsy, culture | Rhizopus spp. | Yes | No | AmB | Yes | [37] | |
| 2018 | Cutaneous | M | 6 | 24 | NA | No | No | Culture | Rhizopus spp. | No | No | NO | No | [38] | |
| 2018 | Cutaneous | F | 6 | 25 | 0.806 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | FLC, VOR, MCFG, AmB | Yes | [39] | |
| 2019 | Cutaneous | F | 4 | 23.57 | 0.605 | Yes | Yes | Culture, KOH, biopsy | Rhizopus spp. | Yes | Yes | FLC, AmB | No | [40] | |
| 2020 | Cutaneous | M | 14 | 24.57 | 0.585 | Yes | No | Biopsy, culture | Rhizopus spp. | Yes | Yes | AmB, MCFG, PSZ | Yes | [41] | |
| 2022 | Cutaneous | M | 6 | 24.85 | 0.75 | Yes | No | Culture, biopsy | Rhizopus spp. | Yes | No | AmB, FLC | Yes | [42] | |
| 2005 | Venezuela | Cutaneous | F | 5 | 34 | 2.7 | Yes | Yes | Biopsy, KOH, culture | Rhizopus spp. | Yes | Yes | AmB | Yes | [43] |
| Year | Country | Topography | Sex | Onset Age | Gestational Age (Weeks) | Birth Weight (kg) | Respiratory Distress | Oxygen | Diagnostic Method | Etiological Agent | Antibiotics | Surgical Treatment | Antifungal Therapy | Survival | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | Austria | Gastrointestinal | M | 21 | 38 | Na | Yes | Yes | Biopsy, culture | Rhizopus | No | Yes | NO | No | [11] |
| 2009 | Germany | Gastrointestinal | M | 13 | 38 | 2.2 | No | No | Biopsy | Mucor spp. | Yes | Yes | AmB | Yes | [44] |
| 2004 | India | Gastrointestinal | F | 7 | >38 | Na | No | No | Biopsy | Mucor spp. | Na | Yes | FLC | Yes | [45] |
| 2004 | Gastrointestinal | M | 16 | >38 | Na | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | NO | No | [45] | |
| 2004 | Gastrointestinal | M | 4 | >38 | Na | No | No | Biopsy | Mucor spp. | No | Yes | AmB | No | [45] | |
| 2010 | Gastrointestinal | M | 6 | >38 | 2.75 | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | LAmB, FLC | Yes | [46] | |
| 2011 | Gastrointestinal | M | 1 | 41 | 1.7 | Na | No | Biopsy | Mucor spp. | Yes | Yes | LAmB | Yes | [47] | |
| 2012 | Gastrointestinal | M | 8 | >38 | Na | No | No | Biopsy, GMS | Mucor spp. | Yes | Yes | AmB | Yes | [18] | |
| 2015 | Gastrointestinal | M | 4 | >38 | 1.75 | No | No | Biopsy, GMS | Mucor spp. | No | Yes | AmB | Yes | [48] | |
| 2016 | Cutaneous | NA | 7 | >38 | 2.3 | Yes | Yes | Biopsy, GMS | Mucor spp. | No | Yes | LAmB, CASP | Yes | [49] | |
| 2018 | Gastrointestinal | M | 2 | 39 | 2.7 | No | No | Biopsy | Mucor spp. | Yes | Yes | AmB, CASP | Yes | [50] | |
| 2018 | Gastrointestinal | M | 13 | >38 | Na | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | AmB, FLC | Yes | [21] | |
| 2018 | Gastrointestinal | F | 6 | >38 | Na | Yes | Yes | Biopsy | Mucor spp. | Yes | Yes | FLC, AmB | Yes | [21] | |
| 2018 | Gastrointestinal | M | 5 | >38 | Na | Yes | No | Biopsy | Mucor spp. | Yes | Yes | AmB, PSZ | No | [21] | |
| 2021 | Rhino-cerebral | M | 22 | >38 | 2.2 | Yes | Yes | KOH | Rhizopus spp. | No | No | LAmB, PSZ | Yes | [51] | |
| 2023 | Rhino-cerebral | F | 23 | >38 | 2 | Yes | Yes | Culture, KOH, biopsy | Rhizopus spp. | Yes | No | LAmB | No | [52] | |
| 2018 | Oman | Cutaneous | F | 5 | >38 | 1.95 | Yes | Yes | Biopsy, culture | Mucor spp. | Yes | Yes | LAmB | No | [53] |
| 2019 | Pakistan | Gastrointestinal | M | 10 | >38 | Na | No | No | Biopsy | Mucor spp. | Yes | Yes | Yes * | No | [54] |
| Univariate Analysis | Multivariate Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total N = 61 | Mortality N = 29 (47.5%) | Survival N = 32 (52.5%) | Χ2 | OR | CI 95% | p | OR | CI 95% | p | |
| Birth stage Missing n = 4 | Term | 18 (100%) | 7 (38.89%) | 11 (61.11%) | |||||||
| Preterm | 39 (100%) | 18 (46.15%) | 21 (53.85%) | 0.264 | 1.347 | 0.4269 to 4.046 | 0.607 | 0.9835 | 0.1697 to 6.072 | 0.9851 | |
| Preterm | 12 (100%) | 2 (16.67%) | 10 (83.33%) | ||||||||
| Very premature | 6 (100%) | 3 (50.00%) | 3 (50.00%) | ||||||||
| Preterm extreme | 21 (100%) | 13 (61.91%) | 8 (38.09%) | 6.064 | 7.273 | 1.301 to 36.39 | 0.014 * | 0.436 | 0.06086 to 2.758 | 0.3851 | |
| Sex Missing n = 6 | Female | 19 (100%) | 10 (52.64%) | 9 (47.36%) | |||||||
| Male | 36 (100%) | 15 (41.67%) | 21 (58.33%) | 0.570 | 0.643 | 0.2188 to 2.025 | 0.571 | 0.506 | 0.1207 to 2.022 | 0.335 | |
| Topography Missing n = 1 | Gastrointestinal | 39 (100%) | 21 (53.84%) | 18 (46.16%) | |||||||
| Cutaneous | 19 (100%) | 6 (31.58%) | 13 (68.42%) | 2.546 | 0.396 | 0.1150 to 1.225 | 0.111 | 2.112 | 0.3590 to 16.61 | 0.432 | |
| Rhino-cerebral | 2 (100%) | 1 (50.00%) | 1 (50.00%) | ||||||||
| Antifungal Treatment | Yes | 47 (100%) | 17 (36.18%) | 30 (63.82%) | |||||||
| No | 14 (100%) | 12 (85.71%) | 2 (14.29%) | 10.62 | 10.59 | 2.406 to 50.02 | 0.002 * | 0.171 | 0.01943 to 0.9974 | 0.068 | |
| Surgical Intervention | No | 14 (100%) | 8 (57.14%) | 6 (42.86%) | |||||||
| Yes | 47 (100%) | 21 (44.69%) | 26 (55.31%) | 0.672 | 1.651 | 0.5355 to 5.639 | 0.545 | 0.838 | 0.07576 to 8.367 | 0.880 | |
| Pathogen Missing n = 4 | Mucor spp. | 45 (100%) | 22 (48.89%) | 23 (51.11%) | |||||||
| Rhizopus spp. | 12 (100%) | 6 (50.00%) | 6 (50.00%) | 0.005 | 1.045 | 0.3081 to 3.536 | >0.999 | 0.699 | 0.08821 to 4.827 | 0.375 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Martinez, A.; Santoyo-Alejandre, M.I.; Arenas, R.; Fuentes-Venado, C.E.; Ramírez-Lozada, T.; Bastida-González, F.; Calzada-Mendoza, C.C.; Martínez-Herrera, E.; Pinto-Almazán, R. Neonatal Mucormycosis: A Rare but Highly Lethal Fungal Infection in Term and Preterm Newborns—A 20-Year Systematic Review. Trop. Med. Infect. Dis. 2025, 10, 86. https://doi.org/10.3390/tropicalmed10040086
Valdez-Martinez A, Santoyo-Alejandre MI, Arenas R, Fuentes-Venado CE, Ramírez-Lozada T, Bastida-González F, Calzada-Mendoza CC, Martínez-Herrera E, Pinto-Almazán R. Neonatal Mucormycosis: A Rare but Highly Lethal Fungal Infection in Term and Preterm Newborns—A 20-Year Systematic Review. Tropical Medicine and Infectious Disease. 2025; 10(4):86. https://doi.org/10.3390/tropicalmed10040086
Chicago/Turabian StyleValdez-Martinez, Alfredo, Mónica Ingrid Santoyo-Alejandre, Roberto Arenas, Claudia Erika Fuentes-Venado, Tito Ramírez-Lozada, Fernando Bastida-González, Claudia Camelia Calzada-Mendoza, Erick Martínez-Herrera, and Rodolfo Pinto-Almazán. 2025. "Neonatal Mucormycosis: A Rare but Highly Lethal Fungal Infection in Term and Preterm Newborns—A 20-Year Systematic Review" Tropical Medicine and Infectious Disease 10, no. 4: 86. https://doi.org/10.3390/tropicalmed10040086
APA StyleValdez-Martinez, A., Santoyo-Alejandre, M. I., Arenas, R., Fuentes-Venado, C. E., Ramírez-Lozada, T., Bastida-González, F., Calzada-Mendoza, C. C., Martínez-Herrera, E., & Pinto-Almazán, R. (2025). Neonatal Mucormycosis: A Rare but Highly Lethal Fungal Infection in Term and Preterm Newborns—A 20-Year Systematic Review. Tropical Medicine and Infectious Disease, 10(4), 86. https://doi.org/10.3390/tropicalmed10040086








