A Review of the Efficacy, Safety, and Feasibility of Rifamycin-Based Post-Exposure Chemoprophylaxis for Leprosy
Abstract
1. Introduction
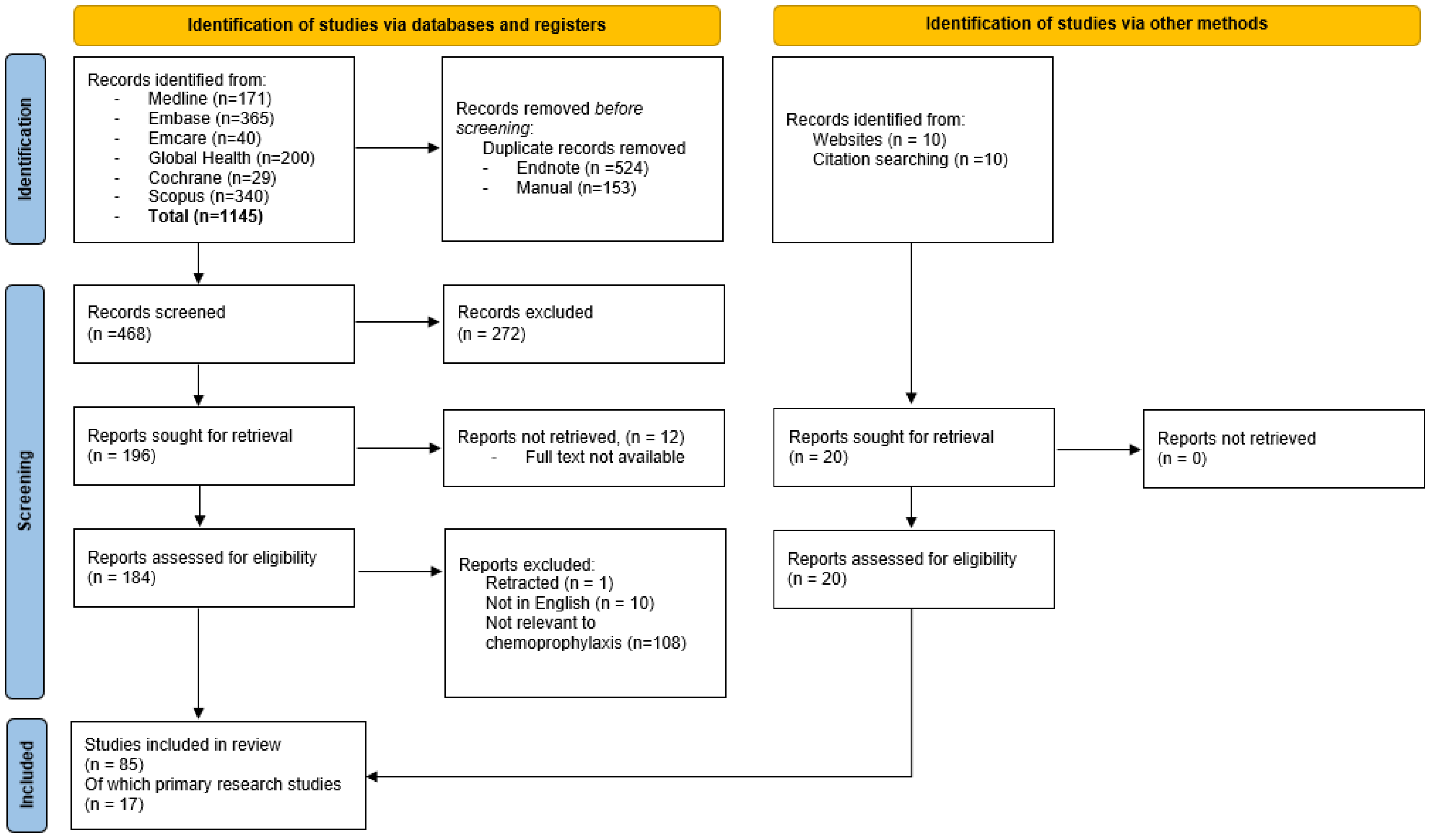
2. Materials and Methods
2.1. Literature Search
2.2. Data Presentation
3. Results
3.1. Literature Review
3.2. Post-Exposure Chemoprophylaxis
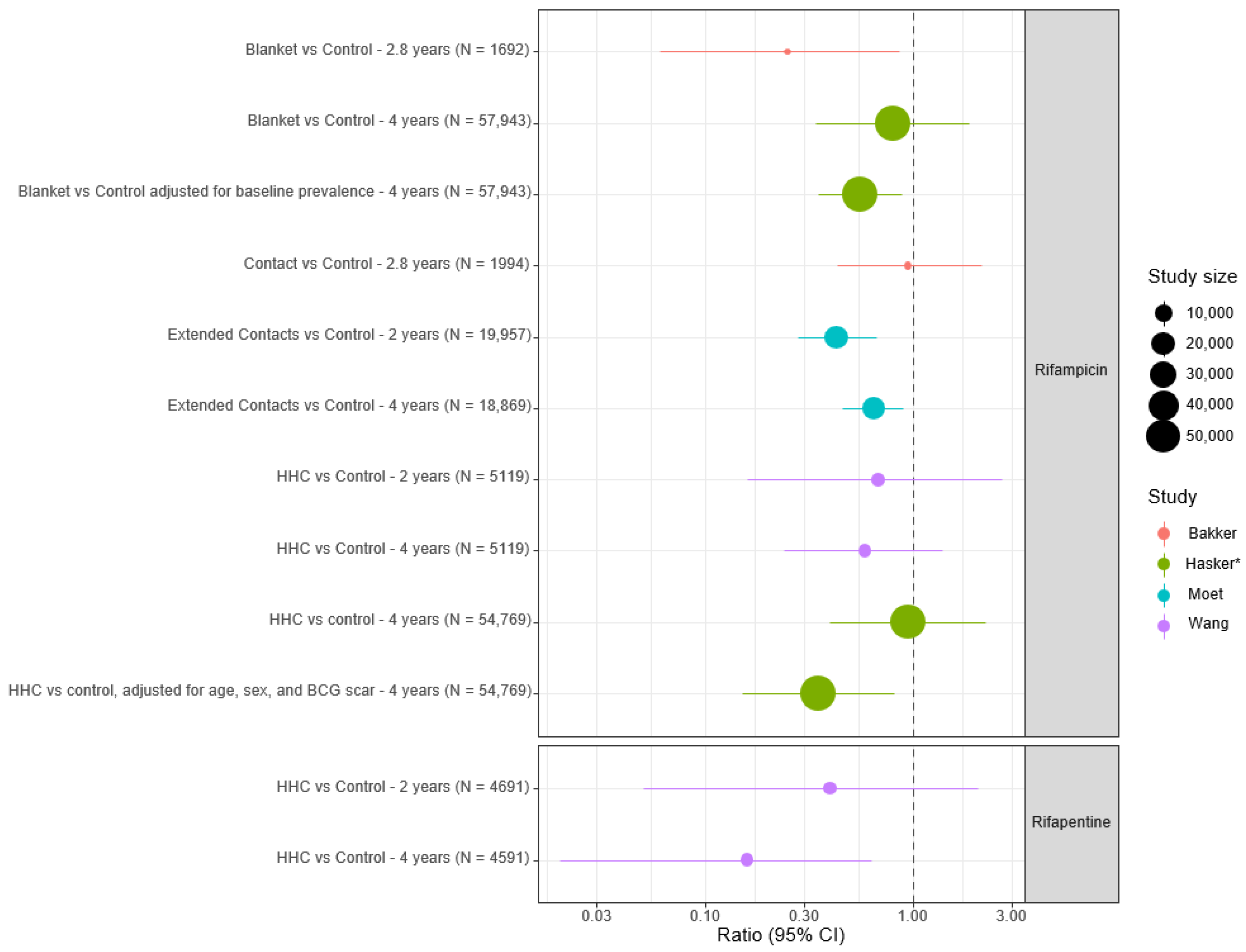
3.2.1. Evidence for Efficacy of Single Agent Rifamycin-Based Chemoprophylaxis Regimens
| First Author | Year Published | Location | Study Design | Study Population | Chemoprophylaxis Regimen | No. of Contacts Included | Duration of Follow-Up | Estimated Efficacy | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Completed rifamycin monotherapy studies | |||||||||
| Cartel [21] | 1992 | Marquesas Islands | Uncontrolled trial | Endemic population | SDR
| 5895 | 4 years | 40–50% | Small case numbers Susceptible to confounding |
| Nguyen [22] | 2000 | 10 years | 35–40% | ||||||
| Bakker [17] | 2005 | Indonesia | Controlled clinical trial | Endemic population (contact group, blanket group + control group) | Two doses of rifampicin 3.5 months apart
| 3965 | 33.5 months | HR 0.25 (95% CI 0.068–0.95) in blanket group compared to control group (p = 0.031) | No difference between contact and control groups (p = 0.93) |
| Moet [7] | 2008 | India | Cluster randomised controlled trial | Household + neighbour contacts | SDR (weight-based)
| 28,092 | 4 years | 57% (95% CI 32.9%–71.9%) overall reduction in incidence at 2 years (p = 0.0002) | NNT = 265 (95% CI 176–537) Benefit less in HHC + contacts of MB cases |
| Khoudri [24] | 2018 | Morocco | Retrospective time series analysis | Household contacts | SDR (age/weight-based dosing)
| 4019 screened 3704 received SDR | 12 years | 16% per year reduction in NCDR following SDR-PEP introduction (p = 0.05) | Multiple limitations Possible confounding |
| Wang [25] | 2023 | China | Cluster randomised controlled trial | Household contacts | Single-dose rifapentine or rifampicin
| 2331 Rifapentine 2760 Rifampicin 2359 Control | 4 years | Cumulative incidence ratio 0.16 (95% CI 0.03–0.87) in rifapentine group compared to control group (p = 0.02) | No significant difference in cumulative incidence between rifampicin and control group (p = 0.23). No adverse events recorded |
| Hasker [26] | 2024 | Madagascar and Comoros | Cluster randomised trial |
| Single double-dose rifampicin (20 mg/kg) | 110,666 | Madagascar: 2 years Comoros: 3 years | Arm 2 (IRR 0.95, 95% CI 0.4–2.23) Arm 3 (IRR 0.80, 95% CI 0.34–1.87) Arm 4 (IRR 0.58, 95% CI 0.22–1.56)
| IRR 0.35 (95% CI 0.15–0.82) in HHC on subgroup analysis NNT = 82 for HHC compared to 870 for study population as a whole |
| Active rifamycin monotherapy studies | |||||||||
| Schoenmakers [27] | Protocol published 2021 | Multiple countries | Cluster randomised trial | Endemic districts within countries
| Single-dose rifampicin (150–600 mg) | Calculated sample size:
| 2 years | - | |
| Coleman [28] | Protocol published 2023 | Kiribati | Implementation study | Endemic population
| SDR (Age/weight-based dosing)
| 64,439 | 3 years | - | |
| Completed studies of combination rifamycin-based regimens | |||||||||
| Diletto [29] | 1999 | Federated States of Micronesia | Uncontrolled trial | Endemic population
| Rifampicin 600 mg, ofloxacin 400 mg + Minocycline 100 mg | 105,506 total
| 2 years | Impact of chemoprophylaxis on incidence not determined as part of this study | Reduction of cases between subsequent rounds but not possible to separate effects of case finding/treatment from screening/prophylaxis. Relative risk of clinical disease lower in chemoprophylaxis group |
| Daulako [30] | 1999 | Kiribati | Implementation study | Endemic population
| Rifampicin 600 mg, ofloxacin 400 mg + minocycline 100 mg | 32,645 total for two islands
| 1 year | Impact of chemoprophylaxis on incidence not determined as part of this study. | Good safety profile of regimen with low rates of adverse events. |
| Tin [31] | 1999 | Marshall Islands | Implementation study | Household contacts | Rifampicin 600 mg, ofloxacin 400 mg + Minocycline 100 mg | 2831
| - | Impact of chemoprophylaxis on incidence not determined as part of this study | Good safety profile of regimen with low rates of adverse events. |
| Khaing [32] | 2009 | Myanmar | Randomised trial
| Household contacts
|
| 152 contacts
| 6 months | Efficacy of chemoprophylaxis not assessed as part of this study | Significant reduction in PGL-1 titres in adults but not in children. Non-significant reduction seen overall. |
| Oo [33] | 2008 | Myanmar | Randomised controlled trial
| Household and neighbour contacts in endemic community |
| 829 extended contacts | 2 years | Efficacy of chemoprophylaxis not assessed as part of this study | Significant reduction in mean antibody titres in adults but not in children 2 years post chemoprophylaxis |
| Astari [34] | 2021 | Indonesia | Cohort study | Elementary school children
|
| 2548 screened
| 5 years | No progression to leprosy at 5 years | Significant reduction in PGL-1 antibody titres over study period No comparator group |
| Active studies of combination rifamycin-based regimen | |||||||||
| Hinders [35] | Protocol published 2023 | Multiple countries | PEP ++ Cluster randomised controlled trial | Endemic populations
| Intervention arm:
| Calculated required sample size of 202,360 | 2 years | - | |
| Younoussa [36] | Protocol published 2024 | Madagascar + Comoros | BE-PEOPLE cluster randomised controlled trial | Endemic population
| Intervention arm
Rifampicin 150–600 mg | Calculated required sample size of 124,000 | 3 years | - | |
3.2.2. Combination/Enhanced Rifamycin-Based Chemoprophylaxis Regimens
3.2.3. Safety
3.2.4. Promotion of Antimicrobial Resistance
3.2.5. Feasibility and Acceptability of Rifamycin-Based Chemoprophylaxis
3.2.6. Modelling
3.2.7. Implementation and Cost Effectiveness of Chemoprophylaxis Programmes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hatta, M.; van Beers, S.M.; Madjid, B.; Djumadi, A.; de Wit, M.Y.; Klatser, P.R. Distribution and persistence of Mycobacterium leprae nasal carriage among a population in which leprosy is endemic in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 381–385. [Google Scholar]
- Kumar, B.; Kar, H.K. IAL Textbook of Leprosy; Jaypee Brothers Medical Publishers Pvt. Limited: New Delhi, India, 2015. [Google Scholar]
- Marpaung, Y.M.; Ernawati, E.; Dwivania, A.T. Stigma towards leprosy across seven life domains in Indonesia: A qualitative systematic review. BMJ Open 2022, 12, e062372. [Google Scholar] [CrossRef]
- WHO. Global Leprosy (Hansen Disease) Update, 2023: Elimiation of Leprosy Disease Is Possible—Time to Act! Weekly Epidemiological Record; World Health Organisation: Geneva, Switzerland, 2024. [Google Scholar]
- WHO. Towards Zero Leprosy. Global Leprosy (Hansen’s Disease) Strategy 2021–2030; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Moet, F.J.; Pahan, D.; Schuring, R.P.; Oskam, L.; Richardus, J.H. Physical Distance, Genetic Relationship, Age, and Leprosy Classification Are Independent Risk Factors for Leprosy in Contacts of Patients with Leprosy. J. Infect. Dis. 2006, 193, 346–353. [Google Scholar] [CrossRef]
- Moet, F.J.; Pahan, D.; Oskam, L.; Richardus, J.H. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: Cluster randomised controlled trial. BMJ Clin. Res. Ed. 2008, 336, 761–764. [Google Scholar] [CrossRef]
- Steinmann, P.; Reed, S.G.; Mirza, F.; Hollingsworth, T.D.; Richardus, J.H. Innovative tools and approaches to end the transmission of Mycobacterium leprae. Lancet Infect. Dis. 2017, 17, e298–e305. [Google Scholar] [CrossRef]
- Dharmendra, M.P.; Nordeen, S.K.; Ramanujam, K. Prophylactic value of DDS against leprosy—An interim report. Lepr. India 1965, 37, 447–467. [Google Scholar]
- Wardekar, R. DDS prophylaxis against leprosy. Lepr. India. 1967, 39, 155–159. [Google Scholar]
- Nordeen, S.K. Chemoprophylaxis in leprosy. Lepr. India 1969, 41, 247–254. [Google Scholar]
- Nordeen, S.K. Long term effects of chemoprophylaxis among contacts of lepromatous cases. Results of 8 1/2 years follow-up. Lepr. India 1977, 49, 504. [Google Scholar]
- Neelan, P.N.; Sirumban, P.; Sivaprasad, N. Limited duration acedapsone prophylaxis in leprosy. Indian J. Lepr. 1986, 58, 251–256. [Google Scholar]
- WHO. Report of the Scientific Working Group Meeting on Leprosy, Geneva, 26–28 November, 2002; World Health Organisation: Geneva, Switzerland, 2003. [Google Scholar]
- Vijayakumaran, P. Does MDT arrest transmission of leprosy to household contacts. Int. J. Lepr. 1998, 66, 125–130. [Google Scholar]
- Cartel, J.L.; Chanteau, S.; Boutin, J.P.; Taylor, R.; Plichart, R.; Roux, J.; Celerier, P.; Grosset, J.H. Implementation of chemoprophylaxis of leprosy in the Southern Marquesas with a single dose of 25 mg per kg rifampin. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1989, 57, 810–816. [Google Scholar]
- Bakker, M.I.; Hatta, M.; Kwenang, A.; Van Benthem, B.H.B.; Van Beers, S.M.; Klatser, P.R.; Oskam, L. Prevention of leprosy using rifampicin as chemoprophylaxis. Am. J. Trop. Med. Hyg. 2005, 72, 443–448. [Google Scholar] [CrossRef]
- Richardus, J.H.; Cavaliero, A.; Steinmann, P. Feasibility and impact of leprosy post-exposure prophylaxis: Evidence from LPEP, a multi-country, 5-year implementation research program. Trans. R. Soc. Trop. Med. Hyg. 2019, 113 (Suppl. S1), S71. [Google Scholar] [CrossRef]
- Kumar, B. Response to ‘Elimination of leprosy in India: An analysis’. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 443–444. [Google Scholar] [CrossRef]
- Lockwood, D.N.J.; Krishnamurthy, P.; Kumar, B.; Penna, G. Single-dose rifampicin chemoprophylaxis protects those who need it least and is not a cost-effective intervention. PLoS Negl. Trop. Dis. 2018, 12, e0006403. [Google Scholar] [CrossRef]
- Cartel, J.L.; Chanteau, S.; Moulia-Pelat, J.P.; Plichart, R.; Glaziou, P.; Boutin, J.P.; Roux, J.F.; Grosset, J.H. Chemoprophylaxis of leprosy with a single dose of 25 mg per kg rifampin in the southern Marquesas; results after four years. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1992, 60, 416–420. [Google Scholar]
- Nguyen, L.N.; Cartel, J.L.; Grosset, J.H. Chemoprophylaxis of leprosy in the Southern Marquesas with a single 25 mg/kg dose of rifampicin. Results after 10 years. Lepr. Rev. 2000, 71, S33–S35. [Google Scholar] [CrossRef]
- Otsyula, Y.; Ibworo, C.; Chum, H.J. Four years experience with dapsone as prophylaxis against leprosy. Lepr. Rev. 1971, 42, 98–100. [Google Scholar]
- Khoudri, I.; Elyoussfi, Z.; Mourchid, Y.; Youbi, M.; Bennani Mechita, N.; Abouqal, R.; Maaroufi, A. Trend analysis of leprosy in Morocco between 2000 and 2017: Evidence on the single dose rifampicin chemoprophylaxis. PLoS Negl. Trop. Dis. 2018, 12, e0006910. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Yan, L.; Yu, M.; Yang, J.; Li, J.; Li, J.; Ning, Y.; Jiang, H.; Shi, Y.; et al. Single-Dose Rifapentine in Household Contacts of Patients with Leprosy. N. Engl. J. Med. 2023, 388, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Hasker, E.; Assoumani, Y.; Randrianantoandro, A.; Ramboarina, S.; Braet, S.M.; Cauchoix, B.; Baco, A.; Mzembaba, A.; Salim, Z.; Amidy, M.; et al. Post-exposure prophylaxis in leprosy (PEOPLE): A cluster randomised trial. Lancet Glob. Health 2024, 12, e1017–e1026. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, A.; Hambridge, T.; van Wijk, R.; Kasang, C.; Richardus, J.H.; Bobosha, K.; Mitano, F.; Mshana, S.E.; Mamo, E.; Marega, A.; et al. PEP4LEP study protocol: Integrated skin screening and SDR-PEP administration for leprosy prevention: Comparing the effectiveness and feasibility of a community-based intervention to a health centre-based intervention in Ethiopia, Mozambique and Tanzania. BMJ Open 2021, 11, e046125. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.; Hill, J.; Timeon, E.; Rimon, E.; Bauro, T.; Ioteba, N.; Cunanan, A.; Douglas, N.M.; Islam, T.; Tomlinson, J.; et al. Effectiveness of population-wide screening and mass drug administration for leprosy control in Kiribati: The COMBINE protocol. BMJ Open 2023, 13, e065369. [Google Scholar] [CrossRef]
- Diletto, C. Elimination of leprosy in the federated states of micronesia by intensive case finding, treatment with WHO/MDT and administration of chemoprophylaxis. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1999, 67 (Suppl. S4), S10–S13. [Google Scholar]
- Daulako, E.C. Population screening and mass chemoprophylaxis in Kiribati. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1999, 67 (Suppl. S4), S23–S25. [Google Scholar]
- Tin, K. Population screening and chemoprophylaxis for household contacts of leprosy patients in the Republic of the Marshall Islands. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1999, 67 (Suppl. S4), S26–S29. [Google Scholar]
- Khaing Win, H.; Khin Nwe, O. Serological response of chemoprophylaxis on high risk contacts of new leprosy cases in Nyaungdon Township. Myanmar Health Sci. Res. J. 2009, 21, 7–11. [Google Scholar]
- Oo, K.N.; Yin, N.N.; Han, T.T.; Wai, K.T.; Myint, K.; Gyi, M.M. Serological response to chemoprophylaxis in extended contacts in leprosy—A randomized controlled trial. Nihon Hansen. Gakkai Zasshi Jpn. J. Lepr. Off. Organ Jpn. Lepr. Assoc. 2008, 77, 3–10. [Google Scholar] [CrossRef]
- Astari, L.; Sari, N.I.P.; Nanang, K.; Cahyono, E.; Herwanto, N.; Listiawan, M.Y.; Adriaty, D.; Iswahyudi, I.; Wahyuni, R.; Alinda, M.D.; et al. A 5-year evaluation of chemoprophylactic treatment in elementary school children with subclinical leprosy. Biomed. Rep. 2021, 15, 88. [Google Scholar] [CrossRef]
- Hinders, D.C.; Taal, A.T.; Lisam, S.; Rocha, A.M.d.; Banstola, N.L.; Bhandari, P.; Saha, A.; Kishore, J.; Fernandes, V.O.; Chowdhury, A.S.; et al. The pep++ study protocol: A cluster-randomised controlled trial on the effectiveness of an enhanced regimen of post-exposure prophylaxis for close contacts of persons affected by leprosy to prevent disease transmission. BMC Infect. Dis. 2024, 24, 226. [Google Scholar] [CrossRef]
- Younoussa, A.; Samidine, S.N.; Bergeman, A.T.; Piubello, A.; Attoumani, N.; Grillone, S.H.; Braet, S.M.; Tsoumanis, A.; Baco, A.; Mzembaba, A.; et al. Protocol, rationale and design of BE-PEOPLE (Bedaquiline enhanced exposure prophylaxis for LEprosy in the Comoros): A cluster randomized trial on effectiveness of rifampicin and bedaquiline as post-exposure prophylaxis of leprosy contacts. BMC Infect. Dis. 2023, 23, 310. [Google Scholar] [CrossRef]
- Tiwari, A.; Dandel, S.; Mieras, L.; Richardus, J.H. Population-wide administration of single dose rifampicin for leprosy prevention in isolated communities: A feasibility study in Indonesia. Trop. Med. Int. Health 2017, 22 (Suppl. S1), 235. [Google Scholar]
- Tiwari, A.; Dandel, S.; Djupuri, R.; Mieras, L.; Richardus, J.H. Population-wide administration of single dose rifampicin for leprosy prevention in isolated communities: A three year follow-up feasibility study in Indonesia. BMC Infect. Dis. 2018, 18, 324. [Google Scholar] [CrossRef]
- Richardus, J.H.; Cairns, S.S.W. Three common misinterpretations of the COLEP trial. Lepr. Rev. 2018, 89, 173–175. [Google Scholar]
- Schuring, R.P.; Richardus, J.H.; Pahan, D.; Oskam, L. Protective effect of the combination BCG vaccination and rifampicin prophylaxis in leprosy prevention. Vaccine 2009, 27, 7125–7128. [Google Scholar] [CrossRef]
- Ji, B.; Chauffour, A.L.; Andries, K.; Jarlier, V. Bactericidal Activities of R207910 and Other Newer Antimicrobial Agents against Mycobacterium leprae in Mice. Antimicrob. Agents Chemother. 2006, 50, 1558–1560. [Google Scholar] [CrossRef]
- Scollard, D.M. A New Step in Postexposure Prophylaxis for Leprosy. N. Engl. J. Med. 2023, 388, 1904–1905. [Google Scholar] [CrossRef]
- Blanc, L.J.; Brennan, P.J.; Cho, S.N.; Daulako, E.C.; Diletto, C.; Farrugia, R.; Fine, P.E.M.; Gupte, M.D.; Iehsi-Keller, E.; Izumi, S.; et al. Workshop on the prevention of leprosy, Pohnpei, Federated States of Micronesia, 25–27 May 1999. Int. J. Lepr. Other Mycobact. Dis. 1999, 67 (Suppl. S4), S1–S82. [Google Scholar]
- Noordeen, S.K. Prophylaxis-Scope and limitations. Lepr. Rev. 2000, 71, S16–S20. [Google Scholar] [CrossRef]
- Oo, K.; Wai, K.T.; Myint, K.; Gyi, M.M. Ineffectiveness of a single dose of rifampicin, ofloxacin and minocycline (ROM) chemoprophylaxis in preventing leprosy. Myanmar Health Sci. Res. J. 2010, 22, 1–7. [Google Scholar]
- Lenz, S.M.; Collins, J.H.; Ray, N.A.; Hagge, D.A.; Lahiri, R.; Adams, L.B. Post-exposure prophylaxis (PEP) efficacy of rifampin, rifapentine, moxifloxacin, minocycline, and clarithromycin in a susceptible-subclinical model of leprosy. PLoS Negl. Trop. Dis. 2020, 14, e0008583. [Google Scholar] [CrossRef]
- Mieras, L.; Anthony, R.; Van Brakel, W.; Bratschi, M.W.; Van Den Broek, J.; Cambau, E.; Cavaliero, A.; Kasang, C.; Perera, G.; Reichman, L.; et al. Negligible risk of inducing resistance in Mycobacterium tuberculosis with single-dose rifampicin as post-exposure prophylaxis for leprosy. Infect. Dis. Poverty 2016, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Braet, S.M.; Jouet, A.; Aubry, A.; Dyck-Lippens, M.V.; Lenoir, E.; Assoumani, Y.; Baco, A.; Mzembaba, A.; Cambau, E.; Vasconcellos, S.E.G.; et al. Investigating drug resistance of Mycobacterium leprae in the Comoros: An observational deep-sequencing study. Lancet Microbe 2022, 3, E693–E700. [Google Scholar] [CrossRef]
- Feenstra, S.G.; Nahar, Q.; Pahan, D.; Oskam, L.; Richardus, J.H. Acceptability of chemoprophylaxis for household contacts of leprosy patients in Bangladesh: A qualitative study. Lepr. Rev. 2011, 82, 178–187. [Google Scholar] [CrossRef]
- Peters, R.; Mieras, L.; Subedi, M.; Apte, H.; Koesbardiati, T.; Banstola, N.L.; Das, S.; Van Brakel, W. A single dose of rifampicin to prevent leprosy: Qualitative analysis of perceptions of persons affected, contacts, community members and health professionals towards chemoprophylaxis and the impact on their attitudes in India, Nepal and Indonesia. Lepr. Rev. 2018, 89, 335–352. [Google Scholar]
- Apte, H.; Chitale, M.; Das, S.; Manglani, P.R.; Mieras, L.F. Acceptability of contact screening and single dose rifampicin as chemoprophylaxis for leprosy in Dadra and Nagar Haveli, India. Lepr. Rev. 2019, 90, 31–45. [Google Scholar] [CrossRef]
- Espiridion-Calma, A.D.V.; Dofitas, B.L.; Sison, M.E.G.Q. Acceptability of immunoprophylaxis and/or chemoprophylaxis for household contacts of patients with Hansen’s disease: A prospective, single-center, mixed methods study. Acta Medica Philipp. 2020, 54, 278–288. [Google Scholar]
- Richardus, J.H.; Tiwari, A.; Barth-Jaeggi, T.; Arif, M.A.; Banstola, N.L.; Baskota, R.; Blaney, D.; Blok, D.J.; Bonenberger, M.; Budiawan, T.; et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): An international feasibility programme. Lancet Glob. Health 2021, 9, e81–e90. [Google Scholar] [CrossRef]
- Cavaliero, A.; Ay, S.S.; Aerts, A.; Lay, S.; So, V.; Robijn, J.; Steinmann, P. Preventing leprosy with retrospective active case finding combined with single-dose rifampicin for contacts in a low endemic setting: Results of the Leprosy Post-Exposure Prophylaxis program in Cambodia. Acta Trop. 2021, 224, 106138. [Google Scholar] [CrossRef]
- Campbell, P.O.; Bauro, T.; Rimon, E.; Timeon, E.; Bland, C.; Ioteba, N.; Douglas, N.M.; Cunanan, A.; Chambers, S.T. Single-Dose Rifampicin Leprosy Chemoprophylaxis for Household Contacts in Kiribati: An Audit of a Combined Retrospective and Prospective Approach. Trop. Med. Infect. Dis. 2024, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Cavaliero, A.; Lay, S.; Dayer, C.; Chan, S.; Smrekar, A.; So, V.; Barth-Jaeggi, T.; Steinmann, P. Retrospective active case finding in Cambodia: An innovative approach to leprosy control in a low-endemic country. Acta Trop. 2018, 180, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Mieras, L. PEP4LEP research and its contribution to capacity building. Lepr. Rev. 2022, 93, 180–183. [Google Scholar] [CrossRef]
- Cavaliero, A.; Greter, H.; Furst, T.; Lay, S.; Sao Ay, S.; Robijn, J.; Steinmann, P. An innovative approach to screening and chemoprophylaxis among contacts of leprosy patients in low endemic settings: Experiences from Cambodia. PLoS Negl. Trop. Dis. 2019, 13, e0007039. [Google Scholar] [CrossRef]
- Mwageni, N. Leprosy epidemiological trends and diagnosis delay in three districts in Tanzania: A PEP4LEP baseline study. Trop. Med. Int. Health 2021, 26, 84. [Google Scholar] [CrossRef]
- Fischer, E.A.J.; de Vlas, S.J.; Habbema, J.D.F.; Richardus, J.H. The long term effect of current and new interventions on the new case detection of leprosy: A modeling study. PLoS Negl. Trop. Dis. 2011, 5, e1330. [Google Scholar] [CrossRef]
- De Matos, H.J.; Blok, D.J.; De Vlas, S.J.; Richardus, J.H. Leprosy new case detection trends and the effect of preventive interventions in Para State, Brazil: A modeling study. Am. J. Trop. Med. Hyg. 2015, 93 (Suppl. S4), 514. [Google Scholar]
- Gilkison, C.; Chambers, S.; Blok, D.J.; Richardus, J.H.; Timeon, E.; Rimon, E.; Priest, P. Predicting the impact of household contact and mass chemoprophylaxis on future new leprosy cases in South Tarawa, Kiribati: A modelling study. PLoS Negl. Trop. Dis. 2019, 13, e0007646. [Google Scholar] [CrossRef]
- Taal, A.T.; Blok, D.J.; van Brakel, W.H.; de Vlas, S.J.; Richardus, J.H. Number of people requiring post-exposure prophylaxis to end leprosy: A modeling study. PLoS Negl. Trop. Dis. 2021, 15, e0009146. [Google Scholar] [CrossRef]
- Blok, D.J.; Steinmann, P.; Tiwari, A.; Barth-Jaeggi, T.; Arif, M.A.; Banstola, N.L.; Baskota, R.; Blaney, D.; Bonenberger, M.; Budiawan, T.; et al. The long-term impact of the Leprosy Post-Exposure Prophylaxis (LPEP) program on leprosy incidence: A modelling study. PLoS Negl. Trop. Dis. 2021, 15, e0009279. [Google Scholar] [CrossRef]
- Machado, L.M.G.; Dos Santos, E.S.; Cavaliero, A.; Steinmann, P.; Ignotti, E. Spatio-temporal analysis of leprosy risks in a municipality in the state of Mato Grosso-Brazilian Amazon: Results from the leprosy post-exposure prophylaxis program in Brazil. Infect. Dis. Poverty 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Richardus, J.H.; Kasang, C.; Mieras, L.; Anand, S.; Bonenberger, M.; Ignotti, E.; Barth-Jaeggi, T.; Greter, H.; Tiwari, A.; Cavaliero, A.; et al. Minimal essential data to document contact tracing and single dose rifampicin (SDR) for leprosy control in routine settings: A practical guide. Lepr. Rev. 2018, 89, 2–12. [Google Scholar]
- Ter Ellen, F.; Tielens, K.; Fenenga, C.; Mieras, L.; Schoenmakers, A.; Arif, M.A.; Veldhuijzen, N.; Peters, R.; Ignotti, E.; Kasang, C.; et al. Implementation approaches for leprosy prevention with single-dose rifampicin: A support tool for decision making. PLoS Negl. Trop. Dis. 2022, 16, e0010792. [Google Scholar] [CrossRef]
- Moet, F.J.; Oskam, L.; Faber, R.; Pahan, D.; Richardus, J.H. A study on transmission and a trial of chemoprophylaxis in contacts of leprosy patients: Design, methodology and recruitment findings of COLEP. Lepr. Rev. 2004, 75, 376–388. [Google Scholar]
- Idema, W.J.; Majer, I.M.; Pahan, D.; Oskam, L.; Polinder, S.; Richardus, J.H. Cost-effectiveness of a chemoprophylactic intervention with single dose rifampicin in contacts of new leprosy patients. PLoS Negl. Trop. Dis. 2010, 4, e874. [Google Scholar] [CrossRef]
- Tiwari, A.; Blok, D.J.; Arif, M.; Richardus, J.H. Leprosy post-exposure prophylaxis in the Indian health system: A cost-effectiveness analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008521. [Google Scholar] [CrossRef]
- Msyamboza, K.P.; Mawaya, L.R.; Kubwalo, H.W.; Ng’Oma, D.; Liabunya, M.; Manjolo, S.; Msiska, P.P.; Somba, W.W. Burden of leprosy in Malawi: Community camp-based cross-sectional study. BMC Int. Health Hum. Rights 2012, 12, 12. [Google Scholar] [CrossRef]
- Lockwood, D.N.J.; de Barros, B.; Negera, E.; Goncalves, H.; Hay, R.J.; Kahawita, I.P.; Singh, R.K.; Kumar, B.; Lambert, S.M.; Pai, V.; et al. Leprosy post-exposure prophylaxis risks not adequately assessed. Lancet Glob. Health 2021, 9, e400–e401. [Google Scholar] [CrossRef]
- Addiss, D.G. Evidence, opportunity, ethics, and the allure of zero leprosy. Lepr. Rev. 2018, 89, 90–101. [Google Scholar]
- Feenstra, S.G.; Pahan, D.; Moet, F.J.; Oskam, L.; Richardus, J.H. Patient-related factors predicting the effectiveness of rifampicin chemoprophylaxis in contacts: 6 year follow up of the COLEP cohort in Bangladesh. Lepr. Rev. 2012, 83, 292–304. [Google Scholar]
- Cambau, E.; Saunderson, P.; Matsuoka, M.; Cole, S.T.; Kai, M.; Suffys, P.; Rosa, P.S.; Williams, D.; Gupta, U.D.; Lavania, M.; et al. Antimicrobial resistance in leprosy: Results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–2015. Clin. Microbiol. Infect. 2018, 24, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.P.; Lavania, M.; Singh, I.; Nashi, S.; Preethish-Kumar, V.; Vengalil, S.; Polavarapu, K.; Pradeep-Chandra-Reddy, C.; Keerthipriya, M.; Mahadevan, A.; et al. Evidence for Mycobacterium leprae Drug Resistance in a Large Cohort of Leprous Neuropathy Patients from India. Am. J. Trop. Med. Hyg. 2020, 102, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Chokkakula, S.; Chen, Z.; Wang, L.; Jiang, H.; Chen, Y.; Shi, Y.; Zhang, W.; Gao, W.; Yang, J.; Li, J.; et al. Molecular surveillance of antimicrobial resistance and transmission pattern of Mycobacterium leprae in Chinese leprosy patients. Emerg. Microbes Infect. 2019, 8, 1479–1489. [Google Scholar] [CrossRef]
- Steinmann, P.; Cavaliero, A.; Aerts, A.; Anand, S.; Arif, M.; Ay, S.S.; Aye, T.M.; Barth-Jaeggi, T.; Banstola, N.L.; Bhandari, C.M.; et al. The Leprosy Post-Exposure Prophylaxis (LPEP) programme: Update and interim analysis. Lepr. Rev. 2018, 89, 102–116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, P.O.; Douglas, N.M.; Chambers, S.T. A Review of the Efficacy, Safety, and Feasibility of Rifamycin-Based Post-Exposure Chemoprophylaxis for Leprosy. Trop. Med. Infect. Dis. 2025, 10, 84. https://doi.org/10.3390/tropicalmed10040084
Campbell PO, Douglas NM, Chambers ST. A Review of the Efficacy, Safety, and Feasibility of Rifamycin-Based Post-Exposure Chemoprophylaxis for Leprosy. Tropical Medicine and Infectious Disease. 2025; 10(4):84. https://doi.org/10.3390/tropicalmed10040084
Chicago/Turabian StyleCampbell, Patrick O., Nicholas M. Douglas, and Stephen T. Chambers. 2025. "A Review of the Efficacy, Safety, and Feasibility of Rifamycin-Based Post-Exposure Chemoprophylaxis for Leprosy" Tropical Medicine and Infectious Disease 10, no. 4: 84. https://doi.org/10.3390/tropicalmed10040084
APA StyleCampbell, P. O., Douglas, N. M., & Chambers, S. T. (2025). A Review of the Efficacy, Safety, and Feasibility of Rifamycin-Based Post-Exposure Chemoprophylaxis for Leprosy. Tropical Medicine and Infectious Disease, 10(4), 84. https://doi.org/10.3390/tropicalmed10040084






