Treatment Adherence and Outcomes in Patients with Tuberculosis Treated with Telemedicine: A Scoping Review
Abstract
1. Introduction
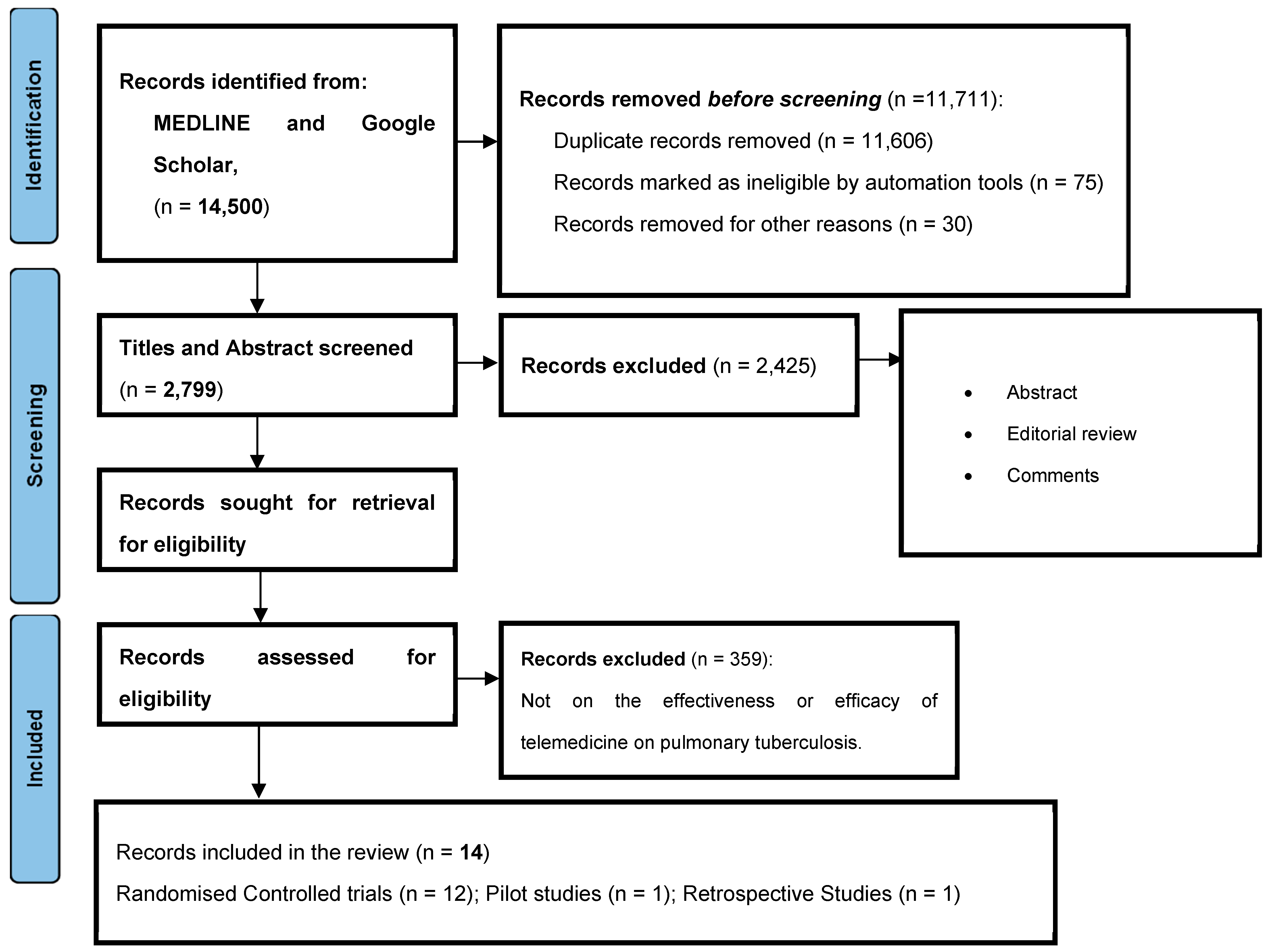
2. Methodology
2.1. Literature Search
2.2. Search Strategy and Selection Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction, Critical Appraisal, and Synthesis
3. Results
| SN | Author/ Year/Country | Title | Goal/Objective | Methodology | Sample Size | Intervention | Result/Findings |
|---|---|---|---|---|---|---|---|
| 1. | Lam et al. [26] (2018) USA | “Using video technology to increase treatment completion for patients with latent tuberculosis infection on 3-month isoniazid and rifapentine: an implementation study” | Assessing the use of video technology on improving completion of treatment in patients undergoing a 3-month rifapentine and isoniazid regimen for latent tuberculosis infection (LTBI). | Randomized controlled trial | 116 | Scheduled VDOT session | Use of VDOT significantly improved treatment completion rates compared to historical controls. |
| 2. | Burzvnski et al. [20] (2022) USA | “In-person vs. electronic directly observed therapy for tuberculosis treatment Adherence: A Randomized Noninferiority” | To determine if electronic directly observed therapy (DOT) for monitoring tuberculosis treatment can achieve a comparable treatment level to in-person DOT. | Randomized crossover | 216 | Monitoring treatment of tuberculosis with electronic directly observed therapy (DOT) | This trial demonstrated that electronic DOT was non-inferior to in-person DOT in terms of the percentage of completed doses. |
| 4. | Garfein et al. [35] (2018) USA | “Tuberculosis treatment monitoring by video directly observed therapy in 5 health districts, California, USA” | Examines the implementation and effectiveness of video directly observed therapy (VDOT) in five health districts in California, USA for monitoring tuberculosis (TB) treatment. | Retrospective study | 467 | Video directly observed therapy (VDOT) | VDOT was particularly effective in improving treatment adherence among specific patient groups, including those with substance use disorders, homeless individuals, and patients with previous treatment non-adherence. |
| 5. | Guo et al. [32] (2020) | “A comprehensive app that improves tuberculosis treatment management through video-observed therapy: usability study” | To evaluate the cost and clinical benefits of video directly observed therapy (VDOT) in comparison to traditional directly observed therapy (DOT) for tuberculosis (TB) treatment. | Randomized controlled trial | 405 | Video directly observed therapy (VDOT) | High rates of treatment completion in both the VDOT and DOT groups, with no statistical differences between the two methods. VDOT was associated with a significantly shorter time per observed dose compared to DOT, as well as lower costs. Patients in the VDOT group reported better experiences and higher levels of satisfaction compared to those in the DOT group. They found VDOT to be convenient and comfortable and expressed a willingness to recommend the method to other patients. |
| 6. | Holzman et al. [23] (2019) India | “Use of smartphone-based video directly observed therapy (VDOT) in tuberculosis care: a single-arm, prospective feasibility study” | To assess its feasibility and acceptability for TB treatment monitoring. | Pilot study | 25 | Video directly observed therapy (VDOT) | More than 90% of patients find it easy to make and upload videos. These findings suggest that vDOT is an acceptable and feasible method for the monitoring of TB treatment in India, expanding the evidence base for VDOT in resource-limited settings and first documenting the use of VDOT in India. |
| 7. | Manyasewal et al. [24] (2022) Ethiopia | “Effect of digital medication event reminder and monitor-observed therapy vs. standard directly observed therapy on health-related quality of life and catastrophic costs in patients with tuberculosis: A secondary analysis of a randomized clinical Trial” | Investigating the impact of a digital medication event reminder monitor (MERM)-observed therapy compared to traditional directly observed therapy (DOT) on catastrophic costs and health-related quality of life (HRQoL) in patients with tuberculosis (TB) in a resource-constrained setting. | Randomized clinical trial | 109 | Digital medication event reminder monitor (MERM) | The median index value for EQ-5D-5L and HRQoL was significantly higher in the MERM-observed therapy group compared to the control group. Additionally, the intervention group had significantly lower median costs compared to the control group, resulting in potential cost savings. |
| 8. | Chuck et al. [25] (2016) USA | “Enhancing management of tuberculosis treatment with video directly observed therapy in New York City” | To evaluate the effectiveness of video directly observed therapy (VDOT) in improving treatment outcomes for tuberculosis (TB) patients in New York City. | Randomized controlled trial | 201 | Video directly observed therapy (VDOT) | High levels of satisfaction were reported by patients in the VDOT group with the technology, and VDOT was found to be convenient and user-friendly. Healthcare providers also reported positive experiences with the VDOT system, finding it to be an effective tool for monitoring patients’ medication adherence. |
| 9. | Browne et al. [27] (2019) | “Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence” | To compare wirelessly observed therapy (WOT) with directly observed therapy (DOT) in confirming and supporting adherence to tuberculosis (TB) treatment. | Randomized controlled trial | 175 | Smartphone that was equipped with a video-based adherence system | Both WOT and DOT groups had high levels of treatment adherence, with mean adherence rates of 90.7% and 90.6%, respectively. There was no significant difference in adherence between the two groups. Treatment completion rates were also similar between the WOT and DOT groups, with 85.5% and 82.8% of participants completing their treatment, respectively. Clinical response and adverse events did not differ significantly between the groups. |
| 10. | Liu et al. [28] (2015) China | “Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients” | To estimate the effectiveness of electronic reminders in improving medication adherence among tuberculosis (TB) patients. | Randomized controlled trial | 4173 | Patients received electronic reminders in the form of phone calls or texts in short message service (SMS) | The intervention group, receiving electronic reminders, had a higher significance in treatment completion rate when compared to the normal control. |
| 11. | Chen et al. [29] (2020) | “Advantage in privacy protection by using synchronous video-observed treatment enhances treatment adherence among patients with latent tuberculosis infection” | To investigate the impact of synchronous video-observed treatment (SVOT) on adherence to treatment among latent tuberculosis infection (LTBI) patients. | Randomized controlled trial | 200 | Video call application to connect with healthcare providers | The SVOT group had significantly higher treatment adherence (98.2%) compared to the SAT group (89.0%). Furthermore, group SVOT had a higher treatment completion rate (92.0%) compared to the SAT group (81.0%). Patient satisfaction was also higher in the SVOT group, with 96.0% of participants expressing satisfaction with the SVOT intervention. |
| 12. | Mohammed et al. [30] (2016) | “Impact of a daily SMS medication reminder system on tuberculosis treatment outcomes” | To assess how effective a daily SMS medication reminder would improve treatment outcomes in tuberculosis (TB) patients. | Randomized controlled trial | 200 | Daily SMS reminders | The intervention group had higher significance in treatment success (92.0%) and treatment adherence, with 94.0% of participants reporting good adherence when compared to patients in the control group, with 78.0% and 79.0%, respectively. Additionally, the intervention group had a lower treatment failure rate and a lower loss to follow-up rate compared to the control group. |
| 13. | Farooqi et al. [31] (2017) | “The role of mobile SMS reminders in improving drug compliance in patients receiving anti-TB treatment from DOTS program” | To assess the effectiveness of mobile SMS reminders in improving medication compliance among patients receiving anti-tuberculosis (TB) treatment through the directly observed treatment short-course (DOTS) program. | Randomized controlled trial | 300 | Mobile SMS reminders | The intervention group had significantly higher medication compliance when compared to patients in the control group. A lower proportion of missed doses was observed in the intervention group, indicating better adherence to the regime of prescribed medication. Furthermore, a higher treatment success rate and treatment completion rate were recorded in the intervention compared to the control group. Patient satisfaction with the SMS reminder system was also reported to be high. |
| 14. | Johnston et al. [34] (2018) | “The effect of text messaging on latent tuberculosis treatment adherence: a randomized controlled trial” | To investigate the influence of text messaging on adherence to treatment for latent tuberculosis infection (LTBI). | Randomized controlled trial | 133 | Text message reminders | The group that received text messages had significantly higher adherence to LTBI treatment compared to the standard care group. Adherence rates were 87.5% in the text message group and 75.0% in the standard care group. The text message intervention also resulted in a higher treatment completion rate (92.3% vs. 81.3%) and greater participant satisfaction. When the occurrence of adverse events between the two groups was observed, there were no significant differences, indicating that text messaging did not pose any additional risks. |
| 15. | Guo et al. [22] 2020 | Telemedicine technologies and tuberculosis management: a randomized controlled trial | To assess the clinical and cost-benefit of video directly observed therapy (VDOT), compared with DOT service. | Randomized controlled trial | 405 participants from each study arm | Video directly observed therapy (VDOT) | VDOT enabled meaningful direct observation for TB patients through mobile devices, which was highly acceptable to patients and health care providers. It also saves time and is a cost-effective method, enabling the use of the saved money in other much-needed areas for TB. |
4. Discussion
5. Conclusions
6. Limitations of the Review
- Geographical Bias: The majority of the studies included in this review were conducted in developed nations, leading to potential geographical bias. Limited representation from resource-constrained settings, particularly in Africa and other low-income regions, may restrict the generalizability of findings to diverse healthcare contexts.
- Study Design Variability: The studies encompassed a variety of designs, including randomized controlled trials (RCTs), pilot studies, and retrospective analyses. While each design offers unique insights, the variability in methodologies makes it challenging to directly compare findings and draw definitive conclusions.
- Population Heterogeneity: The patient populations across the reviewed studies exhibited considerable heterogeneity in terms of demographic characteristics, TB severity, comorbidities, and socioeconomic status. This heterogeneity may introduce confounding variables that could influence treatment outcomes and adherence rates.
- Intervention Diversity: The telemedicine interventions evaluated in the studies varied widely, encompassing video directly observed therapy (VDOT), text message reminders (TMRs), and other telecommunication technologies. While this diversity reflects the evolving landscape of telemedicine, it also complicates the synthesis of results and limits the ability to identify optimal intervention strategies.
- Limited Longitudinal Data: Many of the reviewed studies provided short-term outcomes, such as treatment completion rates and adherence metrics. Longitudinal data on sustained treatment outcomes, relapse rates, and long-term patient follow-up were often lacking, limiting the assessment of telemedicine’s durability and effectiveness over time.
7. Recommendations
- Clear guidelines and protocols need to be developed and implemented to ensure standardized and effective telemedicine practices in TB treatment. These guidelines should address issues such as patient selection criteria for telemedicine, data privacy, and security measures, as well as ethical considerations specific to telemedicine practice.
- Healthcare professionals, including nurses, should receive comprehensive training on telemedicine technologies, platforms, and best practices. Continued professional development opportunities and training programs should also be provided to ensure healthcare professionals remain updated on evolving telemedicine practices.
- Expanding internet access and ensuring reliable connectivity in remote regions will enable effective telemedicine implementation and bridge the digital divide, ensuring equal access to TB care, most especially in developing countries.
- Further research is needed to evaluate the long-term impact and effectiveness of telemedicine in TB treatment. Studies should focus on outcomes such as treatment adherence, treatment success rates, cost-effectiveness, patient satisfaction, and healthcare provider experiences. The findings from such studies will provide valuable insights and evidence to guide future telemedicine interventions and inform policy decisions.
- Collaboration among healthcare institutions, professional associations, and researchers is crucial for sharing best practices and experiences related to telemedicine in TB treatment.
- Efforts should be made to ensure equitable access to telemedicine services for all TB patients with special attention to vulnerable populations, such as those in rural or underserved areas, individuals with low socioeconomic status, and marginalized communities. Strategies like subsidized internet access or mobile data plans can help address access barriers and ensure inclusivity.
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Wang, T.; Du, J.; Sun, L.; Wang, G.; Ni, R.; An, Y.; Fan, X.; Li, Y.; Guo, R.; et al. Decoding the WHO Global Tuberculosis Report 2024: A Critical Analysis of Global and Chinese Key Data. Zoonoses 2025, 5, 999. [Google Scholar] [CrossRef]
- Liebenberg, D.; Gordhan, B.G.; Kana, B.D. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front. Cell. Infect. Microbiol. 2022, 12, 943545. [Google Scholar] [CrossRef]
- Global Tuberculosis Programme. Available online: https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy (accessed on 15 June 2024).
- Adejumo, O.A.; Daniel, O.J.; Otesanya, A.F.; Salisu-Olatunj, S.O.; Abdur-Razzaq, H.A. Evaluation of outcomes of tuberculosis management in private for profit and private-not-for profit directly observed treatment short course facilities in Lagos State, Nigeria. Niger. Med. J. 2017, 58, 44. [Google Scholar] [CrossRef]
- Atif, M.; Anwar, Z.; Fatima, R.K.; Malik, I.; Asghar, S.; Scahill, S. Analysis of tuberculosis treatment outcomes among pulmonary tuberculosis patients in Bahawalpur, Pakistan. BMC Res. Notes 2018, 11, 370. [Google Scholar] [CrossRef]
- Rabinovich, L.; Molton, J.S.; Ooi, W.T.; Paton, N.I.; Batra, S.; Yoong, J. Perceptions and Acceptability of Digital Interventions Among Tuberculosis Patients in Cambodia: Qualitative Study of Video-Based Directly Observed Therapy. J. Med. Internet Res. 2020, 22, e16856. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, M.; van Aken, I.; Niguse, S.; Hailekiros, H.; Spigt, M. Treatment outcomes and their trend among tuberculosis patients treated at peripheral health settings of Northern Ethiopia between 2009 and 2014: A registry-based retrospective analysis. BMC Res. Notes 2019, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Tola, A.; Minshore, K.M.; Ayele, Y.; Mekuria, A.N. Tuberculosis treatment outcomes and associated factors among TB patients attending public hospitals in Harar town, Eastern Ethiopia: A five-year retrospective study. Tuberc. Res. Treat. 2019, 2019, 1503219. [Google Scholar] [CrossRef]
- Adejumo, O.A.; Daniel, O.J.; Adepoju, V.A.; Femi-Adebayo, T.; Adebayo, B.I.; Airauhi, A.O. Challenges of tuberculosis control in Lagos state, Nigeria: A qualitative study of health-care Providers’ perspectives. Niger. Med. J. 2020, 61, 37. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Digital Health 2020–2025. Geneva. 2021. Available online: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf (accessed on 20 February 2025).
- Parajuli, R.; Doneys, P. Exploring the role of telemedicine in improving access to healthcare services by women and girls in rural Nepal. Telemat. Inform. 2017, 34, 1166–1176. [Google Scholar] [CrossRef]
- Brauchli, K. Telemedicine for Improving Access to Health Care in Resource-Constrained Areas: From Individual Diagnosis to Strengthening Health Systems. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2006. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Mustopa, R.; Damris, D.; Syamsurizal, S.; Emawati, M. Evaluation of M-Health On Medication Adherence In Tuberculosis Patients: A Systematic Review. Evaluation 2023, 3, 1–29. [Google Scholar] [CrossRef]
- Eisenstein, E.; Kopacek, C.; Cavalcante, S.S.; Neves, A.C.; Fraga, G.P.; Messina, L.A. Telemedicine: A bridge over knowledge gaps in healthcare. Curr. Pediatr. Rep. 2020, 8, 93–98. [Google Scholar] [CrossRef] [PubMed]
- O’Cathail, M.; Sivanandan, M.A.; Diver, C.; Patel, P.; Christian, J. The use of patient-facing teleconsultations in the national health service: Scoping review. JMIR Med. Inform. 2020, 8, e15380. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, M.J.; Schmidt, H. mHealth for tuberculosis treatment adherence: A framework to guide ethical planning, implementation, and evaluation. Glob. Health Sci. Pract. 2016, 4, 211–221. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Garfein, R.S.; Collins, K.; Muñoz, F.; Moser, K.; Cerecer-Callu, P.; Raab, F.; Rios, P.; Flick, A.; Zúñiga, M.L.; Cuevas-Mota, J. Feasibility of tuberculosis treatment monitoring by video directly observed therapy: A binational pilot study. Int. J. Tuberc. Lung Dis. 2015, 19, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Burzynski, J.; Mangan, J.M.; Lam, C.K.; Macaraig, M.; Salerno, M.M.; deCastro, B.R.; Goswami, N.D.; Lin, C.Y.; Schluger, N.W.; Vernon, A. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: A randomized noninferiority trial. JAMA Netw. Open 2022, 5, e2144210. [Google Scholar] [CrossRef]
- DeMaio, J.; Schwartz, L.; Cooley, P.; Tice, A. The application of telemedicine technology to a directly observed therapy program for tuberculosis: A pilot project. Clin. Infect. Dis. 2001, 33, 2082–2084. [Google Scholar] [CrossRef]
- Guo, P.; Qiao, W.; Sun, Y.; Liu, F.; Wang, C. Telemedicine technologies and tuberculosis management: A randomized controlled trial. Telemed. e-Health 2020, 26, 1150–1156. [Google Scholar] [CrossRef]
- Holzman, S.B.; Atre, S.; Sahasrabudhe, T.; Ambike, S.; Jagtap, D.; Sayyad, Y.; Kakrani, A.L.; Gupta, A.; Mave, V.; Shah, M. Use of Smartphone-Based Video Directly Observed Therapy (vDOT) in Tuberculosis Care: Single-Arm, Prospective Feasibility Study. JMIR Form. Res. 2019, 3, e13411. [Google Scholar] [CrossRef]
- Manyazewal, T.; Woldeamanuel, Y.; Fekadu, A.; Holland, D.P.; Marconi, V.C. Effect of digital medication event reminder and monitor-observed therapy vs standard directly observed therapy on health-related quality of life and catastrophic costs in patients with tuberculosis: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2022, 5, e2230509. [Google Scholar] [CrossRef] [PubMed]
- Chuck, C.; Robinson, E.; Macaraig, M.; Alexander, M.; Burzynski, J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int. J. Tuberc. Lung Dis. 2016, 20, 588–593. [Google Scholar] [CrossRef]
- Lam, C.K.; Pilote, K.M.; Haque, A.; Burzynski, J.; Chuck, C.; Macaraig, M. Using video technology to increase treatment completion for patients with latent tuberculosis infection on 3-month isoniazid and rifapentine: An implementation study. J. Med. Internet Res. 2018, 20, e9825. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.H.; Umlauf, A.; Tucker, A.J.; Low, J.; Moser, K.; Gonzalez Garcia, J.; Peloquin, C.A.; Blaschke, T.; Vaida, F.; Benson, C.A. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: A randomized controlled trial. PLoS Med. 2019, 16, e1002891. [Google Scholar] [CrossRef]
- Liu, X.; Lewis, J.J.; Zhang, H.; Lu, W.; Zhang, S.; Zheng, G.; Bai, L.; Li, J.; Li, X.; Chen, H. Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients: A cluster-randomised trial. PLoS Med. 2015, 12, e1001876. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Wang, I.; Hsu, H.-L.; Huang, C.-C.; Liu, Y.-J.; Putri, D.U.; Lee, C.-H. Advantage in privacy protection by using synchronous video observed treatment enhances treatment adherence among patients with latent tuberculosis infection. J. Infect. Public Health 2020, 13, 1354–1359. [Google Scholar] [CrossRef]
- Mohammed, S.; Glennerster, R.; Khan, A.J. Impact of a daily SMS medication reminder system on tuberculosis treatment outcomes: A randomized controlled trial. PLoS ONE 2016, 11, e0162944. [Google Scholar] [CrossRef]
- Farooqi, R.J.; Ashraf, S.; Zaman, M. The role of mobile SMS-reminders in improving drugs compliance in patients receiving anti-TB treatment from DOTS program. J. Postgrad. Med. Inst. 2017, 31, 156–162. [Google Scholar]
- Guo, X.; Yang, Y.; Takiff, H.E.; Zhu, M.; Ma, J.; Zhong, T.; Liu, S. A comprehensive app that improves tuberculosis treatment management through video-observed therapy: Usability study. JMIR Mhealth Uhealth 2020, 8, e17658. [Google Scholar] [CrossRef]
- Belknap, R.; Holland, D.; Feng, P.J.; Millet, J.P.; Caylà, J.A.; Martinson, N.A.; Wright, A.; Chen, M.P.; Moro, R.N.; Scott, N.A.; et al. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: A randomized trial. Ann. Intern. Med. 2017, 167, 689–697. [Google Scholar] [CrossRef]
- Johnston, J.C.; van der Kop, M.L.; Smillie, K.; Ogilvie, G.; Marra, F.; Sadatsafavi, M.; Romanowski, K.; Budd, M.A.; Hajek, J.; Cook, V. The effect of text messaging on latent tuberculosis treatment adherence: A randomised controlled trial. Eur. Respir. J. 2018, 51, 1701488. [Google Scholar] [CrossRef] [PubMed]
- Garfein, R.S.; Liu, L.; Cuevas-Mota, J.; Collins, K.; Muñoz, F.; Catanzaro, D.G.; Moser, K.; Higashi, J.; Al-Samarrai, T.; Kriner, P.; et al. Tuberculosis treatment monitoring by video directly observed therapy in 5 health districts, California, USA. Emerg. Infect. Dis. 2018, 24, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis Fact Sheet. Available online: https://www.afro.who.int/health-topics/tuberculosis-tb (accessed on 20 February 2025).
- Sekandi, J.N.; Buregyeya, E.; Zalwango, S.; Dobbin, K.K.; Atuyambe, L.; Nakkonde, D.; Turinawe, J.; Tucker, E.G.; Olowookere, S.; Turyahabwe, S.; et al. Video directly observed therapy for supporting and monitoring adherence to tuberculosis treatment in Uganda: A pilot cohort study. ERJ Open Res. 2020, 6, 00175-2019. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olowoyo, K.S.; Esan, D.T.; Olowoyo, P.; Oyinloye, B.E.; Fawole, I.O.; Aderibigbe, S.; Adigun, M.O.; Olawade, D.B.; Esan, T.O.; Adeyanju, B.T. Treatment Adherence and Outcomes in Patients with Tuberculosis Treated with Telemedicine: A Scoping Review. Trop. Med. Infect. Dis. 2025, 10, 78. https://doi.org/10.3390/tropicalmed10030078
Olowoyo KS, Esan DT, Olowoyo P, Oyinloye BE, Fawole IO, Aderibigbe S, Adigun MO, Olawade DB, Esan TO, Adeyanju BT. Treatment Adherence and Outcomes in Patients with Tuberculosis Treated with Telemedicine: A Scoping Review. Tropical Medicine and Infectious Disease. 2025; 10(3):78. https://doi.org/10.3390/tropicalmed10030078
Chicago/Turabian StyleOlowoyo, Kikelomo Sabainah, Deborah Tolulope Esan, Paul Olowoyo, Babatunji Emmanuel Oyinloye, Israel Opeyemi Fawole, Segun Aderibigbe, Mary Opeyemi Adigun, David Bamidele Olawade, Theophilus Olaide Esan, and Benedict Tolulope Adeyanju. 2025. "Treatment Adherence and Outcomes in Patients with Tuberculosis Treated with Telemedicine: A Scoping Review" Tropical Medicine and Infectious Disease 10, no. 3: 78. https://doi.org/10.3390/tropicalmed10030078
APA StyleOlowoyo, K. S., Esan, D. T., Olowoyo, P., Oyinloye, B. E., Fawole, I. O., Aderibigbe, S., Adigun, M. O., Olawade, D. B., Esan, T. O., & Adeyanju, B. T. (2025). Treatment Adherence and Outcomes in Patients with Tuberculosis Treated with Telemedicine: A Scoping Review. Tropical Medicine and Infectious Disease, 10(3), 78. https://doi.org/10.3390/tropicalmed10030078





