Estimating Dengue Outbreak Thresholds in West Africa: A Comprehensive Analysis of Climatic Influences in Burkina Faso, 2018–2024
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Study Design
2.3. Data Collection and Management
2.3.1. Case Definition and Surveillance Data
2.3.2. Data Structure and Processing
2.3.3. Climatic Data
2.4. Analytical Approaches
2.4.1. Threshold Determination
2.4.2. Climate–Disease Analysis
2.5. Statistical Software and Validation
3. Results
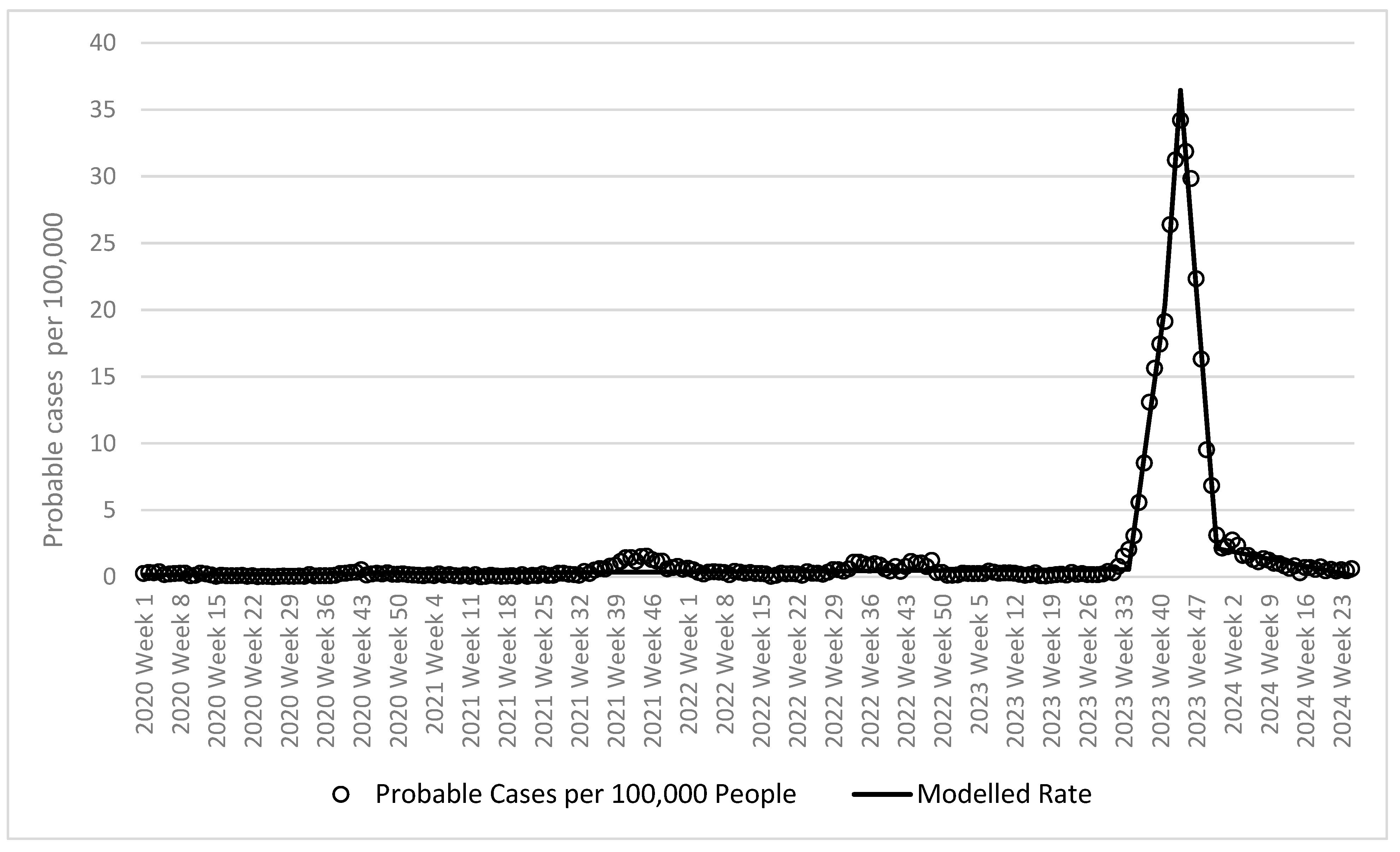
3.1. Joinpoint Regression Analysis
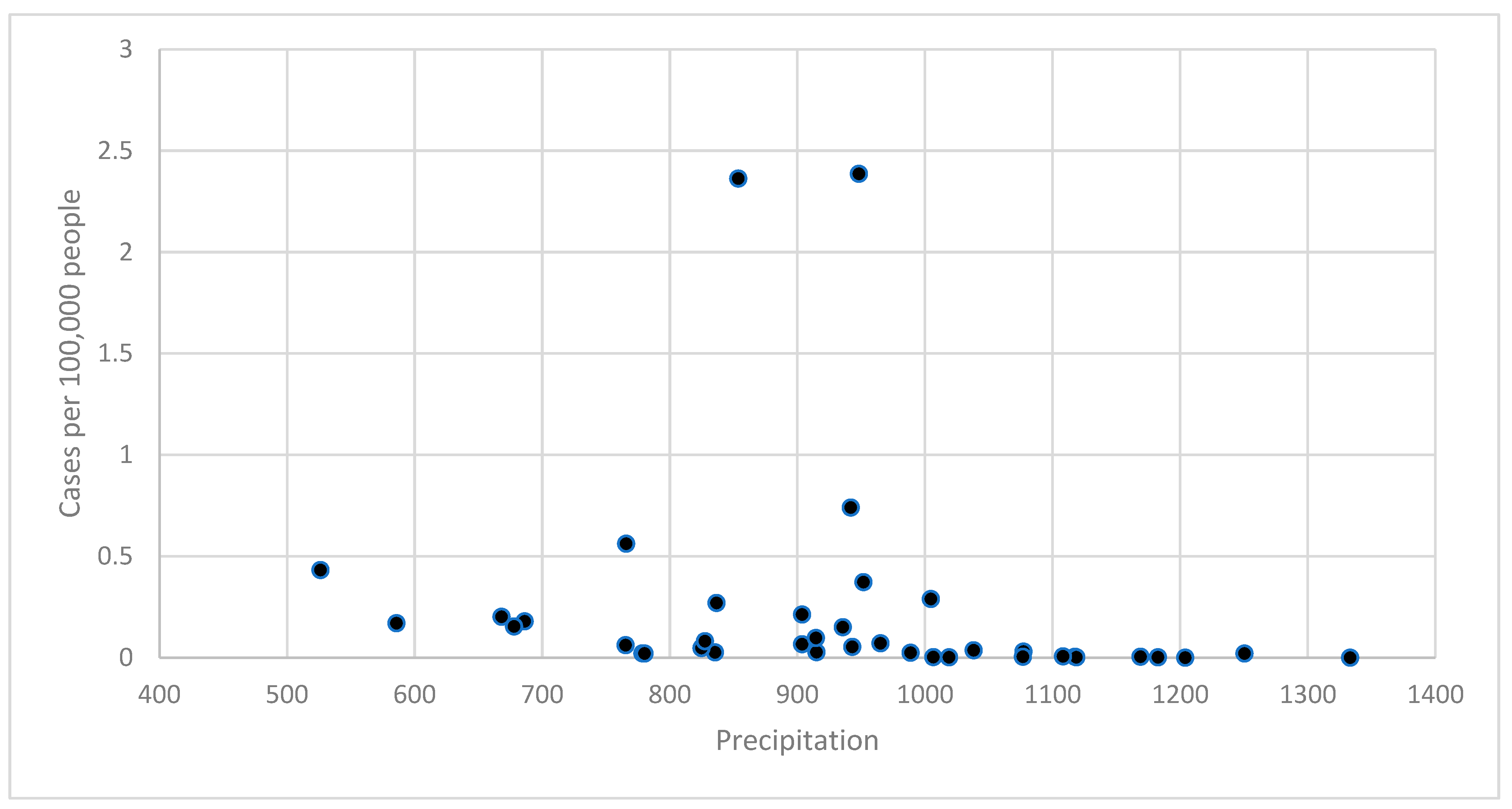
3.2. Climate–Disease Associations
3.3. Mortality Analysis
3.4. Outbreak Threshold Validation
4. Discussion
4.1. Public Health Implications
4.2. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue and Severe Dengue Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 26 November 2024).
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Africa. Dengue in the WHO African Region: Situation Report 02 (14 January 2024); World Health Organization: Brazzaville, Republic of Congo, 2024; Available online: https://www.afro.who.int/countries/burkina-faso/publication/dengue-who-african-region-situation-report-02-14-january-2024 (accessed on 18 November 2024).
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Zhang, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.-G. Urbanization Increases Aedes albopictus Larval Habitats and Accelerates Mosquito Development and Survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Tatem, A.J.; Rogers, D.J.; Hay, S.I. Global Transport Networks and Infectious Disease Spread. Adv. Parasitol. 2006, 62, 293–343. [Google Scholar] [CrossRef]
- Jaenisch, T.; Junghanss, T.; Wills, B.; Brady, O.J.; Eckerle, I.; Farlow, A.; Hay, S.I.; McCall, P.J.; Messina, J.P.; Ofula, V.; et al. Dengue expansion in Africa—Not recognized or not happening? Emerg. Infect. Dis. 2014, 20, e140487. [Google Scholar] [CrossRef]
- United Nations Population Division. World Population Prospects. 2022. Available online: https://population.un.org/wpp/ (accessed on 26 November 2024).
- Ridde, V.; Agier, I.; Bonnet, E.; Carabali, M.; Dabiré, K.R.; Fournet, F.; Ly, A.; Meda, I.B.; Parra, B. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): Research and public health implications. Infect. Dis. Poverty 2016, 5, 23. [Google Scholar] [CrossRef]
- Xu, Z.; Bambrick, H.; Frentiu, F.D.; Devine, G.; Yakob, L.; Williams, G.; Hu, W. Projecting the future of dengue under climate change scenarios: Progress, uncertainties and research needs. PLoS Negl. Trop. Dis. 2020, 14, e0008118. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is Dengue Vector Control Deficient in Effectiveness or Evidence?: Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef]
- Brady, O.J.; Smith, D.L.; Scott, T.W.; Hay, S.I. Dengue disease outbreak definitions are implicitly variable. Epidemics 2015, 11, 92–102. [Google Scholar] [CrossRef]
- Johansson, M.A.; Reich, N.G.; Hota, A.; Brownstein, J.S.; Santillana, M. Evaluating the performance of infectious disease forecasts: A comparison of climate-driven and seasonal dengue forecasts for Mexico. Sci. Rep. 2016, 6, 33707. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Ethical Issues in Public Health Surveillance [Internet]; World Health Organization: Geneva, Switzerland, 2017; 56p, ISBN 9789241512657. Available online: https://www.who.int/publications/i/item/9789241512657 (accessed on 26 November 2024).
- World Health Organization. Technical Handbook for Dengue Surveillance, Outbreak Prediction/Detection and Outbreak Response; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-154973-8. Available online: https://apps.who.int/iris/handle/10665/250240 (accessed on 18 November 2024).
- World Bank Group. Climate Change Knowledge Portal: Burkina Faso Country Profile [Internet]; World Bank: Washington, DC, USA, 2024; Available online: https://climateknowledgeportal.worldbank.org/country/burkina-faso (accessed on 29 January 2025).
- Pantolla, H.; Gonzaga, A. Proposed Econometric Strategy in Determining Thresholds of Levels of Diseases with Applications to Dengue Cases of a Highly Urbanized City. SciEnggJ 2023, 16, 346–358. [Google Scholar] [CrossRef]
- Thayer, M.B.; Marzan-Rodriguez, M.; TorresAponte, J.; Rivera, A.; Rodriguez, D.M.; Madewell, Z.J.; Rysava, K.; Paz-Bailey, G.; Adams, L.E.; Johansson, M.A. Dengue epidemic alert thresholds: A tool for surveillance and epidemic detection. medRxiv 2024. [Google Scholar] [CrossRef]
- Hossain, S. Generalized Linear Regression Model to Determine the Threshold Effects of Climate Variables on Dengue Fever: A Case Study on Bangladesh. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 2131801. [Google Scholar] [CrossRef]
- Schlesinger, M.; Alvarado, F.E.P.; Ramos, M.E.B.; Sewe, M.O.; Merle, C.S.; Kroeger, A.; Hussain-Alkhateeb, L. Enabling countries to manage outbreaks: Statistical, operational, and contextual analysis of the early warning and response system (EWARS-csd) for dengue outbreaks. Front. Public Health 2024, 12, 1323618. [Google Scholar] [CrossRef] [PubMed]
- Firdaust, M.; Yudhastuti, R.; Mahmudah, M.; Notobroto, H.B. Predicting dengue incidence using panel data analysis. J. Public Health Afr. 2023, 14, 2570. [Google Scholar] [CrossRef]
- Mwanyika, G.O.; Moir, M.; Musa, A.O.; Poongavanan, J.; Dor, G.; Wilkinson, E.; Baxter, C.; de Oliveira, T.; Tegally, H. A decade of dengue disease burden in Africa (2013–2023): A systematic review. medRxiv 2024, 2024010822. [Google Scholar] [CrossRef]
- Mercy, K.; Youm, E.; Aliddeki, D.; Faria, N.R.; Kebede, Y.; Ndembi, N. The looming threat of dengue fever: The Africa context. Open Forum Infect. Dis. 2024, 11, ofae362. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Carabali, M.; Edwards, T.; Barro, A.; Lee, J.-S.; Dahourou, D.; Lee, K.S.; Nikiema, T.; Shin, M.Y.; Bonnet, E.; et al. Estimating the Force of Infection for Dengue Virus Using Repeated Serosurveys, Ouagadougou, Burkina Faso. Emerg. Infect. Dis. 2021, 27, 130–139. [Google Scholar] [CrossRef]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef]
- Ouattara, C.A.; Traore, T.I.; Ouedraogo, B.; Sylla, B.; Traore, S.; Meda, C.Z.; Sangare, I.; Savadogo, L.B.G. Spatio-Temporal Determinants of Dengue Epidemics in the Central Region of Burkina Faso. Trop. Med. Infect. Dis. 2023, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial Capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Lowe, R.; Gasparrini, A.; Van Meerbeeck, C.J.; Lippi, C.A.; Mahon, R.; Trotman, A.R.; Rollock, L.; Hinds, A.Q.J.; Ryan, S.J.; Stewart-Ibarra, A.M. Nonlinear and delayed impacts of climate on dengue risk in Barbados: A modelling study. PLoS Med. 2018, 15, e1002613. [Google Scholar] [CrossRef]
- Wilastonegoro, N.N.; Andriani, S.; Sebong, P.H.; Agarwal-Harding, P.; Shepard, D.S. Estimating Dengue Disease and Economic Burden to Inform Municipal-Level Policymakers. Gates Open Res. 2024, 8, 15. [Google Scholar] [CrossRef]
- Soto-Rojas, C.; Garita, C.; Abdalah, M.; Calvo, J.G.; Sánchez, F.; Meneses, E. Preliminary Analysis of Socioeconomic Variable Correlation with Geospatial Modeling in Costa Rica Dengue Epidemics. Rev. Tecnol. Marcha 2024, 37, 7292. [Google Scholar] [CrossRef]

| Slope | Probable Cases Per 100,000 People Around Inflection Points | ||||||
|---|---|---|---|---|---|---|---|
| 95% CI | |||||||
| Trend Time Period | Value | Lower | Upper | p-Value | One Week Before Inflection Point | Week of Inflection Point | One Week After Inflection Point |
| Week 1 of 2020 to week 34 of 2023 | 0.002 | 0.001 | 0.003 | 0.0001 | N/A | ||
| Week 34 of 2023 to week 41 of 2023 | 2.832 | 2.620 | 3.045 | <0.0001 | 1.6 | 2.1 | 3.1 |
| Week 41 of 2023 to week 44 of 2023 | 5.358 | 4.013 | 6.703 | <0.0001 | 17.4 | 19.1 | 26.4 |
| Week 44 of 2023 to week 51 of 2023 | −4.904 | −5.117 | −4.692 | <0.0001 | 31.2 | 34.2 | 31.9 |
| Week 51 of 2023 to week 25 of 2024 | −0.077 | −0.098 | −0.057 | <0.0001 | 6.8 | 3.1 | 2.2 |
| 95% CI | |||||
|---|---|---|---|---|---|
| Rate Ratio | Lower | Upper | p-Value | AIC | |
| Average annual mean surface temperature | 2.764 | 0.990 | 7.716 | 0.0523 | 758.4 |
| Controlling for year | 2.653 | 0.946 | 7.438 | 0.0636 | 759.3 |
| Precipitation | 0.551 | 0.393 | 0.773 | 0.0006 | 751.3 |
| Controlling for year | 0.551 | 0.393 | 0.773 | 0.0007 | 751.9 |
| Number (n) | Average Probable Cases of Dengue Per 100,000 People | Standard Deviation | p-Value (Compared to <29.0 Category) | |
|---|---|---|---|---|
| Temperature | ||||
| <29.0 | 6 | 0.01 | 0.01 | |
| 29.0–29.9 | 22 | 0.26 | 0.52 | 0.0047 |
| 30.0+ | 11 | 0.34 | 0.68 | 0.0011 |
| p-value (compared to <900) | ||||
| Precipitation | ||||
| <900 | 14 | 0.58 | 0.93 | |
| 900–999 | 11 | 0.91 | 1.76 | 0.9362 |
| 1000+ | 14 | 0.07 | 0.01 | 0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otshudiema, J.O.; Diao, W.R.; Ouedraogo, S.M.W.-K.; Kapete, A.N.; Moyenga, L.; Chanda, E.; Traore, T.; Ramadan, O.P.; Zumla, A. Estimating Dengue Outbreak Thresholds in West Africa: A Comprehensive Analysis of Climatic Influences in Burkina Faso, 2018–2024. Trop. Med. Infect. Dis. 2025, 10, 66. https://doi.org/10.3390/tropicalmed10030066
Otshudiema JO, Diao WR, Ouedraogo SMW-K, Kapete AN, Moyenga L, Chanda E, Traore T, Ramadan OP, Zumla A. Estimating Dengue Outbreak Thresholds in West Africa: A Comprehensive Analysis of Climatic Influences in Burkina Faso, 2018–2024. Tropical Medicine and Infectious Disease. 2025; 10(3):66. https://doi.org/10.3390/tropicalmed10030066
Chicago/Turabian StyleOtshudiema, John Otokoye, Watton R. Diao, Sonia Marie Wend-Kuuni Ouedraogo, Alain Ngoy Kapete, Laurent Moyenga, Emmanuel Chanda, Tieble Traore, Otim Patrick Ramadan, and Alimuddin Zumla. 2025. "Estimating Dengue Outbreak Thresholds in West Africa: A Comprehensive Analysis of Climatic Influences in Burkina Faso, 2018–2024" Tropical Medicine and Infectious Disease 10, no. 3: 66. https://doi.org/10.3390/tropicalmed10030066
APA StyleOtshudiema, J. O., Diao, W. R., Ouedraogo, S. M. W.-K., Kapete, A. N., Moyenga, L., Chanda, E., Traore, T., Ramadan, O. P., & Zumla, A. (2025). Estimating Dengue Outbreak Thresholds in West Africa: A Comprehensive Analysis of Climatic Influences in Burkina Faso, 2018–2024. Tropical Medicine and Infectious Disease, 10(3), 66. https://doi.org/10.3390/tropicalmed10030066









