1. Introduction
Snakebite is a neglected public health crisis that results in high mortality and morbidity rates worldwide, with exacerbated severity in rural areas of tropical and subtropical regions [
1]. The cause of complications and death from snakebite is due to the composition of the enzymes in snake venom. Venom is a complex biochemical mixture, comprising various biologically active components, including hydrolytic enzymes, non-enzymatic proteins or peptides, and smaller amounts of organic and inorganic molecules [
2]. Together, these toxins cause local tissue damage or result in systemic toxicity, resulting in symptoms including necrosis, coagulation disorders, and even death [
3]. Snake venoms contain multiple toxin families, primarily causing net cytotoxic, neurotoxic and/or hemotoxic effects. Envenomation from vipers (family Viperidae) primarily results in hemotoxic effects due to the presence of serine proteases (SVSPs), metalloproteinases (SVMPs), and phospholipase A
2 (PLA
2), typically leading to a pathology characterized by overt or internal bleeding and degradative effects on tissues [
4].
The only currently accepted standard and efficacious treatment for snakebite envenoming is antivenom. Antivenoms are highly purified therapeutic antibody formulations derived from the plasma of animals, typically horses or sheep, that have been immunized with snake venoms [
5]. These preparations consist of polyclonal antibodies, encompassing a wide variety of immunoglobulins with differing titers and affinities that target various venom components. When immunization on an antibody-producing animal is performed using snake venom sourced from a single species, the resulting product is a monovalent antivenom; however, it still comprises a polyclonal antibody mixture due to the natural immune response [
6]. In contrast, polyvalent antivenoms are produced by immunizing animals with multiple venoms, or by blending several monovalent antivenoms produced as indicated previously. Typically, antibodies are isolated from the hyperimmune plasma via the protein precipitation method, and depending on the subsequent processing methods for the antibodies (e.g., enzymatic digestion), antivenom can be formulated as whole IgG molecules, F(ab’)
2 fragments, or Fab fragments [
5].
Although antivenom is considered an effective therapy for snakebite envenoming, several limitations accompany it. Due to its high cost and limited availability, antivenom may be incredibly difficult for snakebite patients to access in remote areas with inadequate healthcare services. Additionally, the effectiveness of antivenom may be significantly reduced is administration is delayed. Furthermore, only 10–20% of the antibodies in an antivenom product may target the toxin(s) of medical relevance, whereas the remaining antibodies may bind nonspecifically to host molecules, potentially decreasing venom neutralization while increasing the risk of adverse effects via hypersensitivity reactions [
7]. Finally, and perhaps most importantly, variation in snake venom composition associated with geography, ontogeny, or local adaptation—both among and within species—can reduce the efficacy of antivenoms produced for geographically distant populations [
8]. Therefore, it is essential to develop and produce region-specific antivenoms to ensure optimal venom neutralization efficacy and to make these products widely available and accessible.
The therapeutic efficacy of antivenoms has been demonstrated in numerous clinical cases, with favorable outcomes commonly observed following administration. However, in the case we reported highlights significant issues associated with antivenom supply, timely administration and the potential limitations of non-region-specific antivenom efficacy. This report case highlights the urgent need for C. rhodostoma antivenom supply across Vietnam and provides insights into the molecular mechanisms underlying variation in the efficacy of C. rhodostoma antivenoms. Although numerous in vivo studies have investigated venom composition and antivenom efficacy, the molecular mechanisms underlying differences in antivenom performance in clinical cases remain poorly understood. Based on the presented clinical case results, our study aims to clarify the molecular mechanisms of antivenom efficacy variation through SDS-PAGE profiling and enzyme activity assays. Our findings underscore the importance of developing regionally specific snakebite therapy and ensuring that these products are available and accessible.
2. Material and Methods
2.1. Clinical Presentation
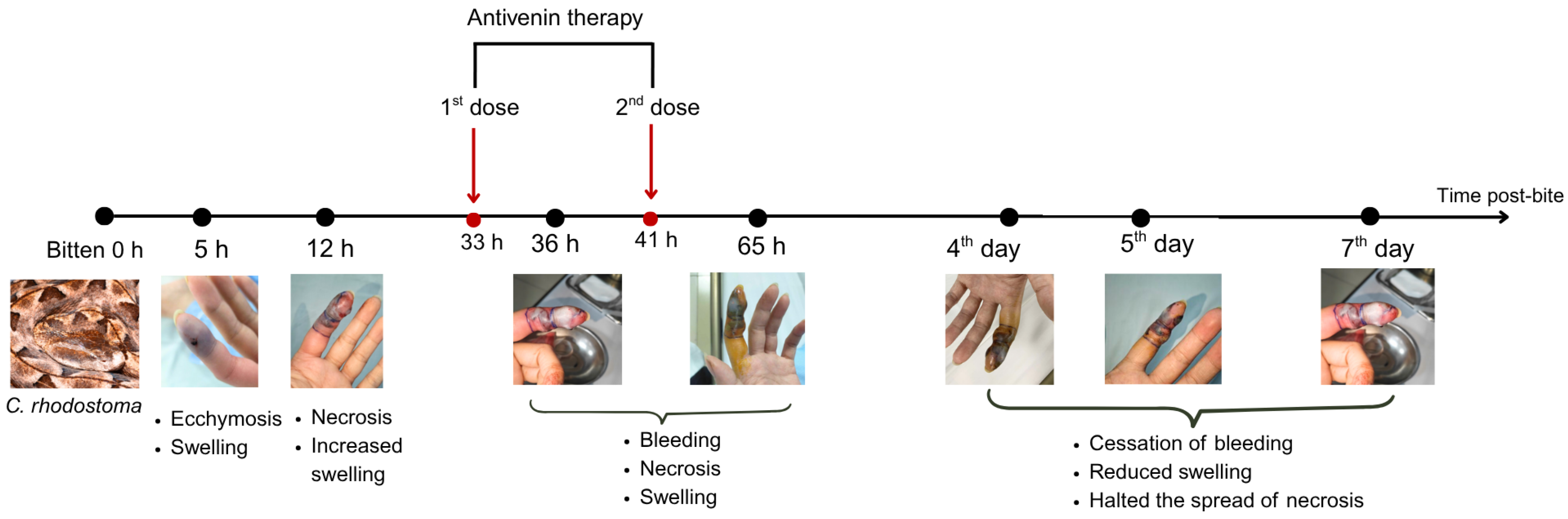
A 22-year-old male patient with a history of bronchial asthma was bitten on the distal phalanx of the second finger of the left hand by a captive C. rhodostoma originating from Dak Lak Province, Vietnam. The individual brought the live snake with him to the hospital, where the species identity was verified. One hour following the bite, the patient experienced intense pain, along with swelling and bruising at the distal phalanx of the left index finger, but no bleeding or vomiting of blood occurred. The patient was admitted to the Poison Control Center at Bach Mai Hospital, Hanoi. The patient agreed and signed a consent form for the publication of their clinical information and images.
The patient was conscious at the time of admission with vital signs as follows: blood pressure 130/80 mmHg, pulse 103 beats/min, respiratory rate 20 breaths/min, SpO
2 99% on room air, and temperature: 37 °C. A fang puncture wound was visible on the distal phalanx of the second finger on the left hand, with swelling and mild bruising around the wound covering an area of approximately 1 cm × 2 cm. The patient’s test results are presented in detail in
Table 1.
2.2. Venom Sample Collection
The
C. rhodostoma specimen responsible for the envenoming was collected from the patient and housed alive in the Institute of Biology (IB) collections at the Vietnam Academy of Science and Technology (VAST) in Hanoi, Vietnam. The snake venom extraction procedure was approved by the Ethics Committee of the Institutional Ethics Committee of the Institute of Genome Research (currently the Institute of Biology), Vietnam Academy of Science and Technology (VAST), which approved the work as part of its internal research program. The specimen (Cr.2025.01) was identified as a female with a total length of 55 cm, snout–vent length of 35.5 cm, and mass of approximately 200 g. For venom collection, the snake’s mouth was rinsed with water to remove debris, and the snake was then induced to bite a parafilm membrane extended over a glass container. Once firmly latched onto the membrane, the venom glands on both sides of the jaw were gently massaged and pressed, facilitating the expression of venom into the glass container. Following collection, the venom was gently centrifuged to pellet debris and frozen at −20 °C; the sample was not subjected to lyophilization (freeze-drying). Protein concentration of the collected venom sample (SV6; snake venom 6) was 174.38 mg/mL, and was determined using the Bradford assay [
10].
We tested the efficacy of two different antivenoms raised against
C. rhodostoma: a Vietnamese product (AV. Cr. VN) obtained from Pasteur Institute in Nha Trang (Khanh Hoa Province, Vietnam, and a Thai product (AV. Cr. TL) obtained from Ho Chi Minh City, Vietnam. Protein concentrations were determined for AV. Cr. VN (74.825 mg/mL) and AV. Cr. TL (75.625 mg/mL) using the Bradford assay [
10].
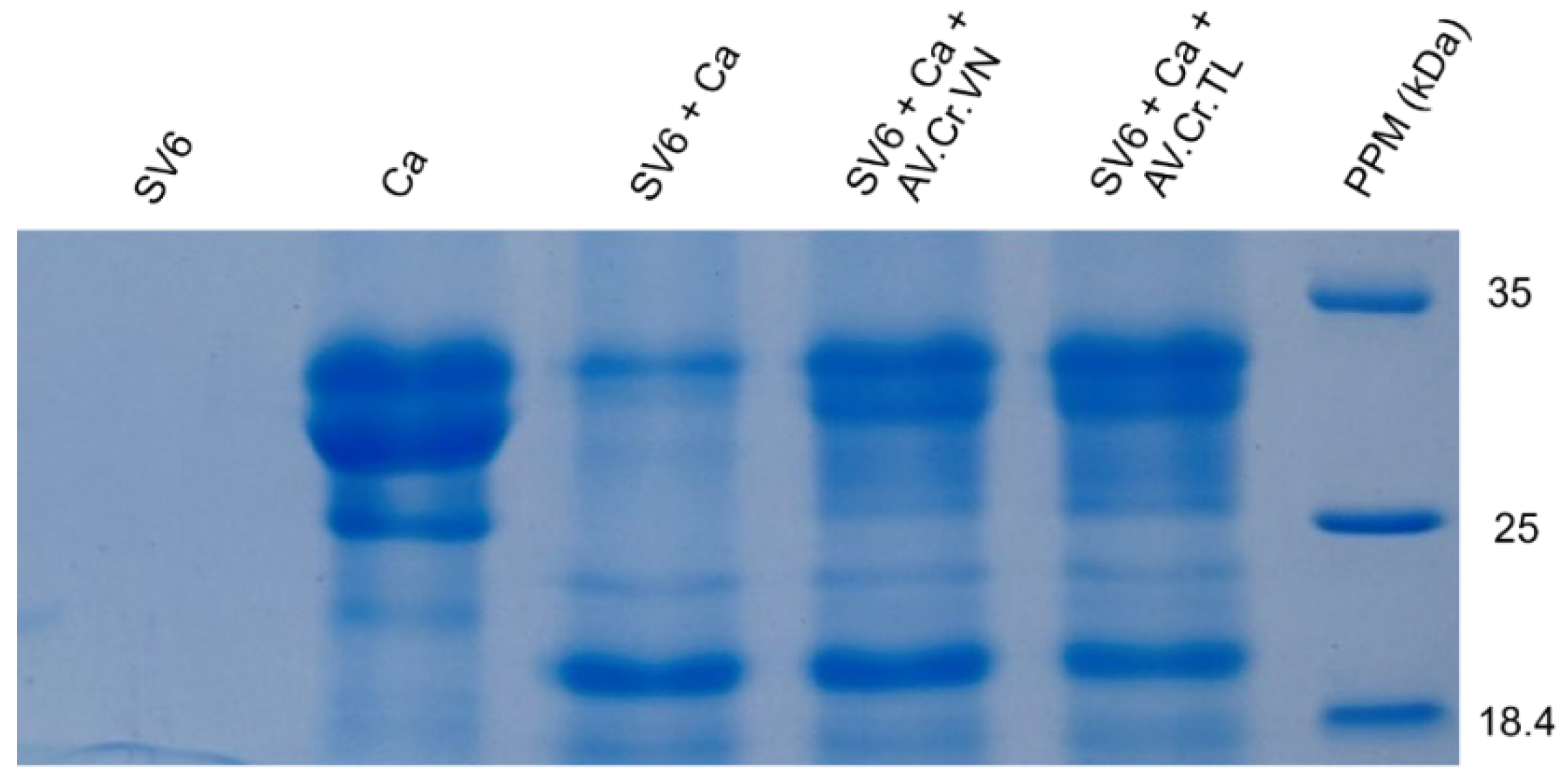
2.3. SDS−PAGE Analysis
To produce a molecular fingerprint of the protein components in the C. rhodostoma venom, crude SV6 venom (174.38 mg/mL) was diluted with 1X phosphate-buffered saline (PBS), pH 7.4, to a working concentration of 10 mg/mL. A 10 µg aliquot of this 10 mg/mL solution was then subjected to gel electrophoresis. We used a casein substrate to assess the proteolytic activity of snake venom proteinases, and a 1% casein solution (0.01 g/mL) prepared in phosphate-buffered saline (PBS) pH 7.4 was used as a negative control. The SV6 sample was adjusted to the same amount (0.16 µg, 0.08 µg/µL) and incubated with 1% casein (0.01 g/mL) for 10 min at 37 °C, after which the percentage activity was measured. To evaluate the inhibitory capacity of the C. rhodostoma antivenoms produced in Vietnam (AV. Cr. VN) and Thailand (AV. Cr. TL) against our venom sample, the SV6 sample was adjusted to the same amount (0.16 µg, 0.08 µg/µL) and incubated with each antivenom (0.16 µg, 226.2 µg/µL) for 10 min at 37 °C, after which percent activity was measured.
For gel electrophoresis, we prepared a SDS-PAGE gel using the following protocol: 4 mL of 15% resolving gel (1000 µL H2O, 1000 µL Tris-HCl pH 8.8 containing SDS, 2000 µL 30% bis-acrylamide, 30 µL 10% APS, and 7 µL TEMED); 1.01 mL of 4% stacking gel (610 µL H2O, 250 µL Tris-HCl pH 6.8, 130 µL 30% bis-acrylamide containing SDS, 12.5 µL 10% APS, and 3 µL TEMED). Each sample (9 µL) was mixed with a reducing buffer solution (0.032 M Tris-HCl, 51% glycerol, 5.1% SDS, 0.1% (v/v) saturated bromophenol blue, 4.08% β−mercaptoethanol, pH 6.8) in a 1:3 ratio and heated for 10 min at 99 °C. Samples were loaded onto the gel and run in two stages using the Mini-PROTEAN electrophoresis system (Bio-Rad, Hertfordshire, UK): stage (1) 90 volts for 15 min, and stage (2) 140 volts for 60 min. The resulting gel was stained using a buffer containing 30% Coomassie Brilliant Blue R-250 and destained for at least one hour at room temperature. Gel imaging was performed using an imaging station to capture and characterize protein band patterns.
2.4. Analysis of the Relative Band Intensity from the Electrophoresis Gel
The band intensity of the SDS-PAGE gel was analyzed using ImageJ Densitogram Suite version 1.54p after gel imaging software [
11]. Each lane was isolated and compared, generating a densitometric plot where band intensities were represented as peaks. The area under each peak, corresponding to individual bands, was quantified using built−in measurement tools, and intensity ratios were calculated using Microsoft Excel.
2.5. Phospholipase Activity Assay
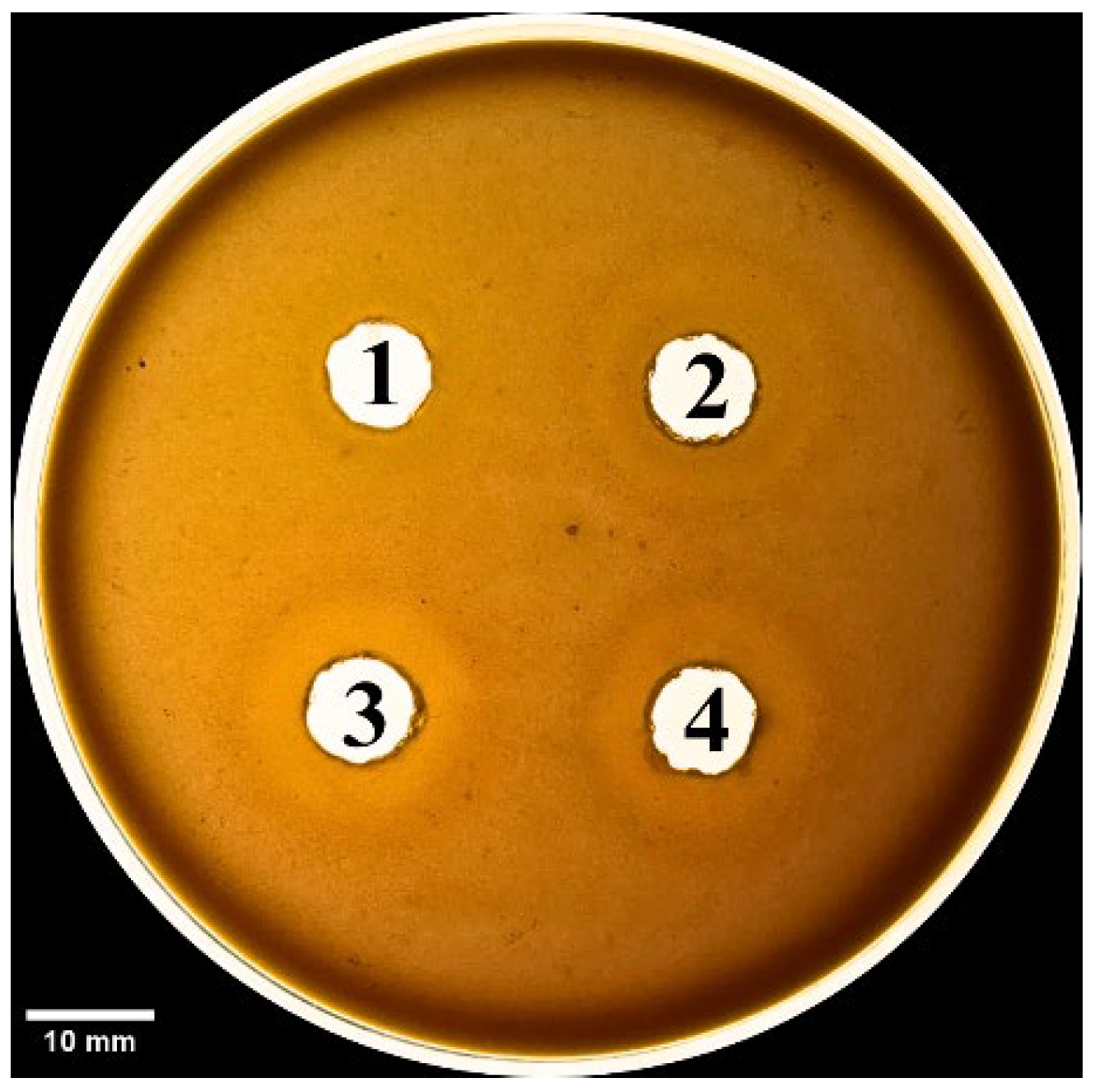
Agar plates were prepared as described by Suji et al. [
12] with modifications. Briefly, 4% defibrinated sheep blood, 1.2% egg yolk as a source of lecithin, 10 mM CaCl
2, and 1% agar in PBS pH 8.1 were solidified in a Petri dish, and wells of approximately 9 mm diameter were made in the gel. Samples included PBS (negative control), SV6 venom (15 µg), and mixtures of AV. Cr. VN. (15 µg) or AV. Cr. TL. (15 µg) antivenoms with SV6 venom, pre−incubated for 30 min at 37 °C, before introduction to the wells. Plates were incubated at the respective temperatures for 24 h, and the experiment was replicated three times. Hemolytic zone diameters were measured in ImageJ, and the following formula was used for the calculation of neutralization:
d1: diameter of SV6 in antivenom treatment;
d2: diameter of SV6.
2.6. Statistical Analysis
We statistically analyzed the phospholipase activity of SV6 snake venom after antivenom addition in Microsoft Office Excel 2021. We used a paired-samples t-test, with a significance threshold of p = 0.05.
4. Discussion
This clinical case presented here was novel, as it involved a patient bitten by a Malayan pit viper (
C. rhodostoma) under unusual circumstances. Epidemiologically, bites from this species are primarily associated with wild snakes from within the native geographic distribution in Southern Vietnam, as the range of this species does not extend into Northern Vietnam [
14]. The case presented herein, received at the Poison Control Center, Bach Mai Hospital, Hanoi, is thus considered anomalous, and consequently, species-specific antivenom was not available for immediate administration. The envenomation occurred because the patient (a veterinarian) owned the captive snake and was bitten on the distal phalanx of the second finger of the left hand during a venom extraction. The patient brought the snake to the Poison Control Center, facilitating accurate species verification and therefore allowing sourcing of appropriate antivenom. The bite could additionally be confirmed by one fang mark puncture visible on the individual’s left second finger, corroborating the need for monitoring of envenomation symptoms. When species responsible for snakebites cannot be identified, diagnoses become reliant on bite wound characteristics, local symptoms (including the presence of one fang mark, swelling, blistering, or necrosis), and laboratory tests. These tests may include prolonged clotting times (Lee White, PT, aPTT), platelet counts (<20.000/mm
3), decreased (sometimes undetectable) fibrinogen levels, elevated D-dimer levels, and non-clotting blood [
15]. ELISA can also be used for species identification and can additionally quantify venom doses based on venom antigens [
16]. However, these diagnostic methods (i.e., ELISA) have significant limitations, such as conserved signals across different venomous snake species, and the risk of cross-reactivity with venoms from closely related snake species producing incorrect identifications. The limit detection of ELISA for venoms and toxins ranges from 0.1 to 20 µg/L, and it requires a long antibody incubation period [
17,
18], Researchers are developing rapid detection methods to overcome these shortcomings, such as using bite swabs that can generate effective results through PCR testing [
19,
20].
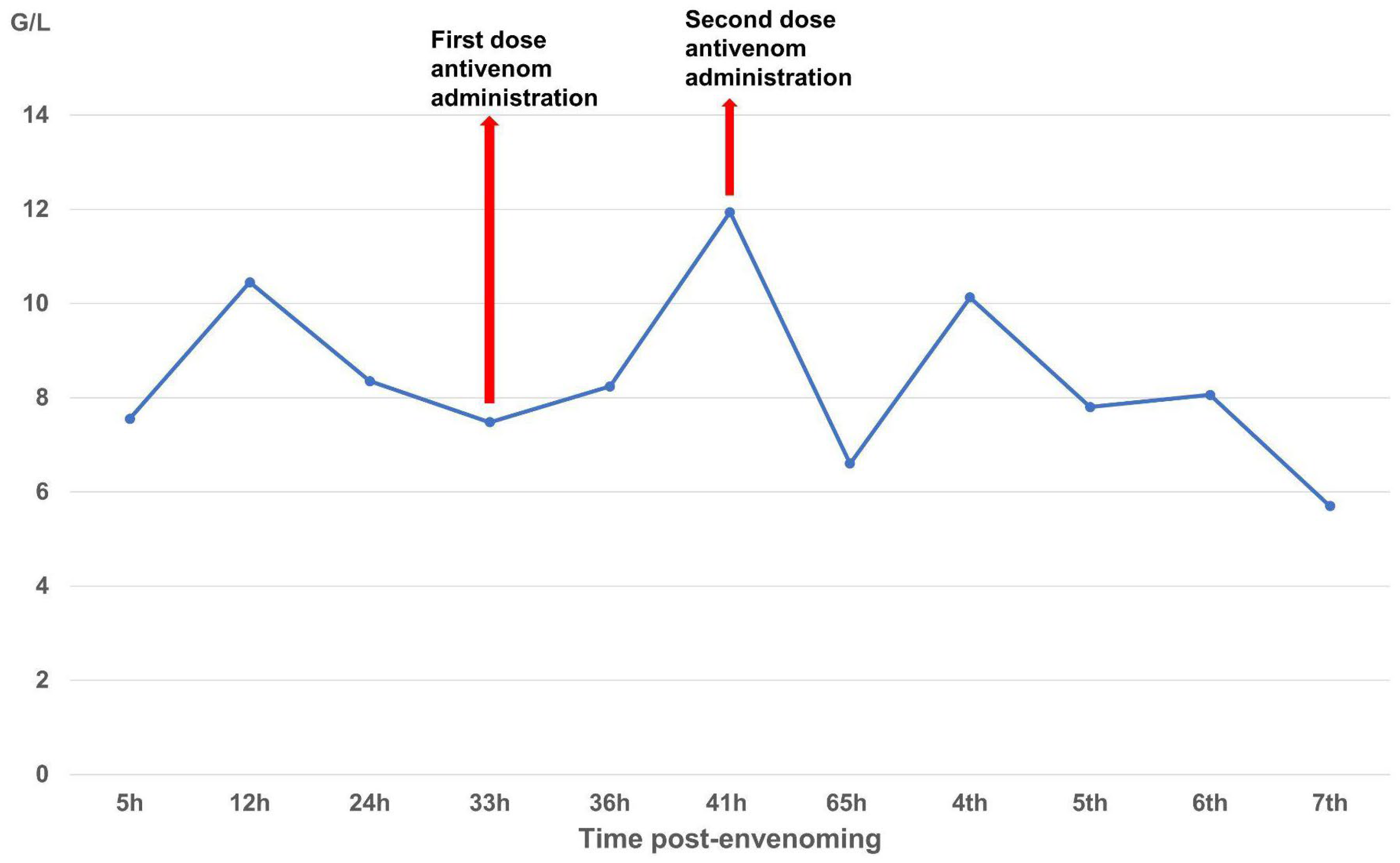
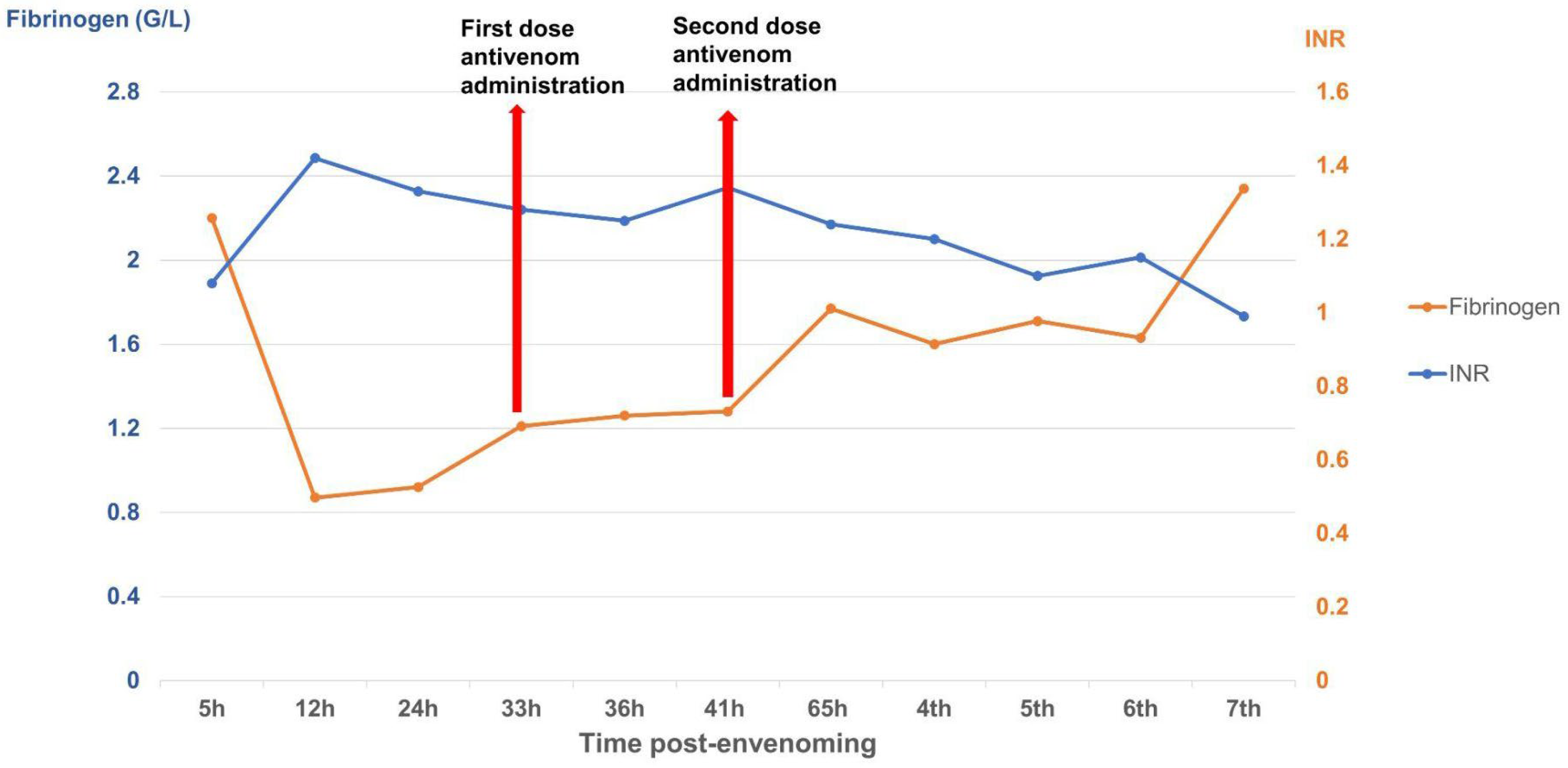
The case described here met conditions for antivenom administration due to local necrosis and coagulopathy immediately evident in paraclinical results. However, antivenoms were unavailable locally and required transport from Ho Chi Minh City to Hanoi, resulting in major delays with the first dosage being administered 33 h and the second dosage 39 h post-bite. Antivenom should be administered as soon as possible following a snakebite, ideally within 4 h as per manufacturer guidelines, though it can still be effective for as long as 24 h post-bite. Considering the delayed administration of antivenom in our case, we observed rapid improvement in the patient’s symptoms and laboratory results. Three important parameters for evaluating the impact of snake envenomation and antivenom on the immune system include WBC (normal range 4–10 G/L) for inflammation, INR (normal range 0.8–1.2), and fibrinogen (normal range 2–4 G/L) for coagulation. After the envenomation, the venom caused tissue damage and triggered an inflammatory response, resulting in WBC increasing to 10.45 G/L by day 2 post-bite. The venom also caused clotting disorders and bleeding, evidenced by the depletion of the clotting factor fibrinogen to 0.87 G/L and extending the coagulation INR to 1.42. These parameters tended to increase slightly for fibrinogen and decrease for INR and WBC to normal levels as the patient was treated with supportive medicines, including analgesics and antibiotics. Following antivenom administrations, these parameters gradually improved to normal levels in the subsequent days of treatment. Interestingly, WBC initially increased above normal to 11.94 G/L after antivenom administrations, most likely due to an immune response to the antivenom acting as an antigen; however, these levels rapidly normalized (<10 G/L), indicating no adverse reactions. Our clinical results demonstrate that the timing of antivenom administration is crucial for effectively reducing venom toxicity, but support the use of antivenom even when administration is delayed.
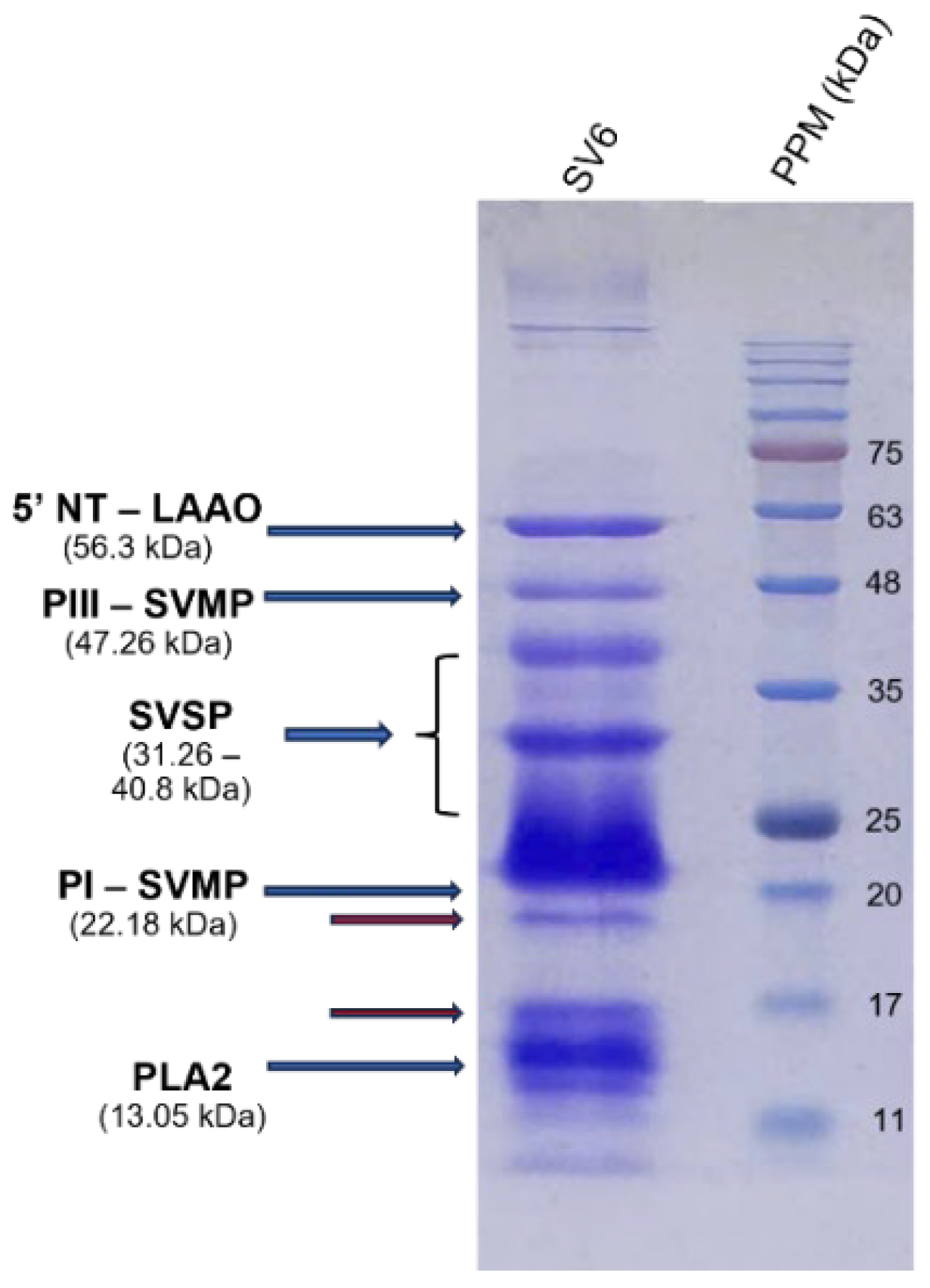
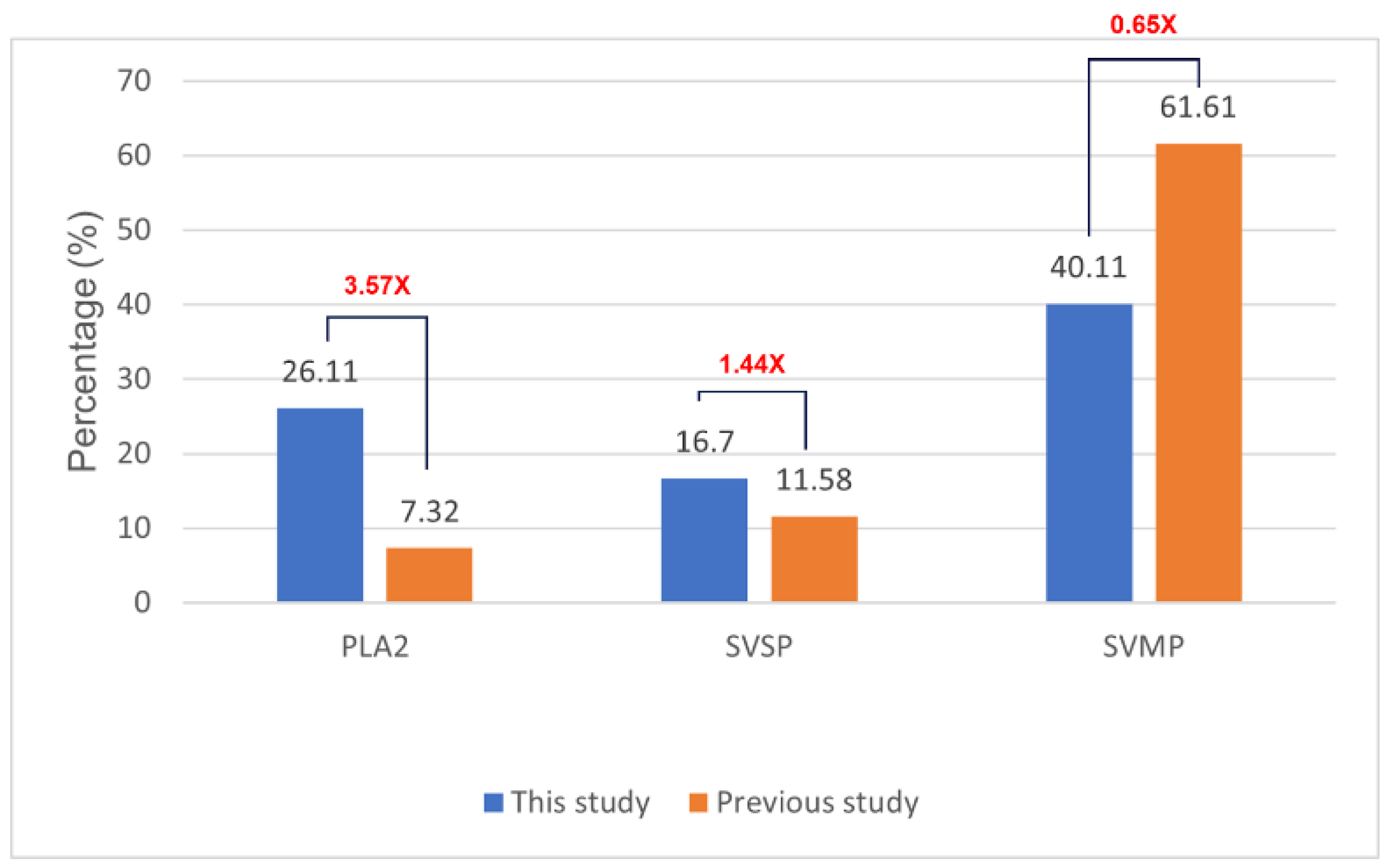
The effectiveness of antivenom largely depends on the toxin composition profile of the respective snake venom. To elucidate the molecular mechanisms of antivenom activity, we constructed a protein profile of the
C. rhodostoma responsible for the envenomation described in this case. We provided important insights into the molecular mechanisms that caused clinical symptoms, as well as evaluated the efficacy of two different antivenom products in neutralizing toxin activity. We identified three major enzyme families, including SVMPs, SVSP, and PLA
2, which are related to the clinical presentation of
C. rhodostoma envenomed patients. Specifically, SVMPs contribute to extracellular matrix protein degradation, leading to tissue breakdown and necrosis [
21]. SVSPs are thrombin-like enzymes, cleaving fibrinogen and causing coagulation disorders [
22]. The proteolytic activities of both SVSPs and SVMPs can activate inflammatory responses and cause hypotension [
23,
24]. Meanwhile, PLA
2 hydrolyzes the phospholipids in red blood cell membranes, resulting in hemorrhage and swelling. The severity of the effects induced by these toxic components is associated with their respective relative abundances in snake venoms. We found significant differences in proportions of toxin components of SV6 venom when compared to the previous reports of
C. rhodostoma venom from Surat Thani, Thailand, by Adisakwattana et al. [
13]. These differences may be explained by geographic variation, diet, age, sex, and physiological differences among individuals. Additional research on geographic venom variation represents a vital first step in improving and progressing the effectiveness of species-specific antivenom products.
We also evaluated the efficacy of antivenoms at the molecular level. In our case, the patient was treated with antivenom produced in Thailand (AV. Cr. TL), which was transported from Ho Chi Minh to Hanoi. Although C. rhodostoma antivenom in Vietnam (AV. Cr. VN) has been researched and tested, it remains uncommercialized and unavailable. Our neutralization experiments showed that both Vietnamese (AV. Cr. VN) and Thai (AV. Cr. TL) antivenoms had equivocal efficacies in inhibiting proteases from our sampled Vietnamese individual of C. rhodostoma. Evidencing this, when venom was combined with each antivenom on the 15% SDS-PAGE, the number and intensity of casein protein bands were greater than those of antivenom-free treatments. The zones of phospholipid degradation (diameter, measured in mm) on a blood agar plate were reduced in treatments with either of the two antivenom products compared to treatments lacking antivenom. Furthermore, quantitative analyses also demonstrated that AV. Cr. VN (Vietnam) was more efficacious in neutralizing proteinase activity than AV. Cr. TL (Thailand), further emphasizing the importance of using regionally derived antivenoms to optimize neutralization of activities.
Our case report highlights the reliance on antivenoms produced in other countries and the limitations imposed by domestic transportation and distribution of antivenom in Vietnam, inhibiting the rapid administration of antivenom and reducing the effectiveness of treatment. WHO data on global antivenom production reveal heterogeneity among manufacturers in terms of production scale and antivenom characteristics [
1]. Some manufacturers produce antivenoms only for their own country, while others distribute products regionally or globally [
21]. Furthermore, geographical variation in snake venom composition can lead to reduced specificity and efficacy when using antivenoms produced in neighboring countries [
22]. With these challenges in mind, promoting research and establishing industrial-scale production of specific antivenoms for local Vietnamese snake species is a promising direction for future efforts. Simultaneously, exploring alternative therapies to traditional antivenoms is a crucial step, especially when antivenoms are unavailable or ineffective in abating clinical symptoms. This proposed approach could shorten waiting times, enhance treatment efficacy, and reduce the burden of severe snakebite complications in Vietnam.
This study has several limitations, including reporting a single case and presenting an in vitro scope. The main geographical distribution of this snake species is in the Southern provinces, and this was the first case reported in the Northern area. The clinical presentation, however, was consistent with the multiple case study in Malaysia, with most of the abnormalities occurring after 6 h post-bite [
25]. For further investigation, in vivo studies need to be carried out using animal models.