Dengue and Acute Pancreatitis: A Systematic Review
Abstract
1. Introduction
1.1. Background: Dengue Virus
1.2. Current Epidemiology of Dengue
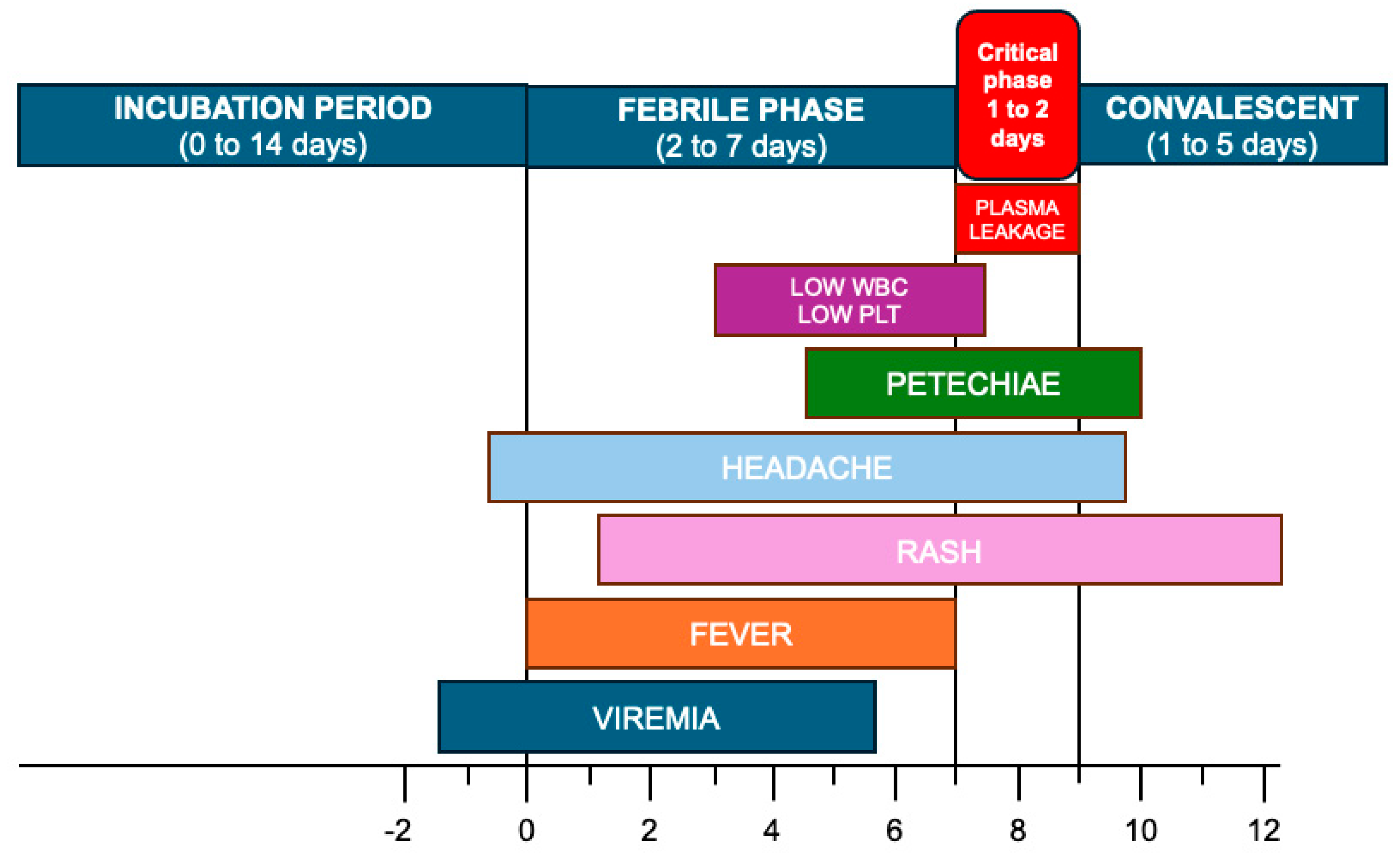
1.3. Clinical Features of Dengue
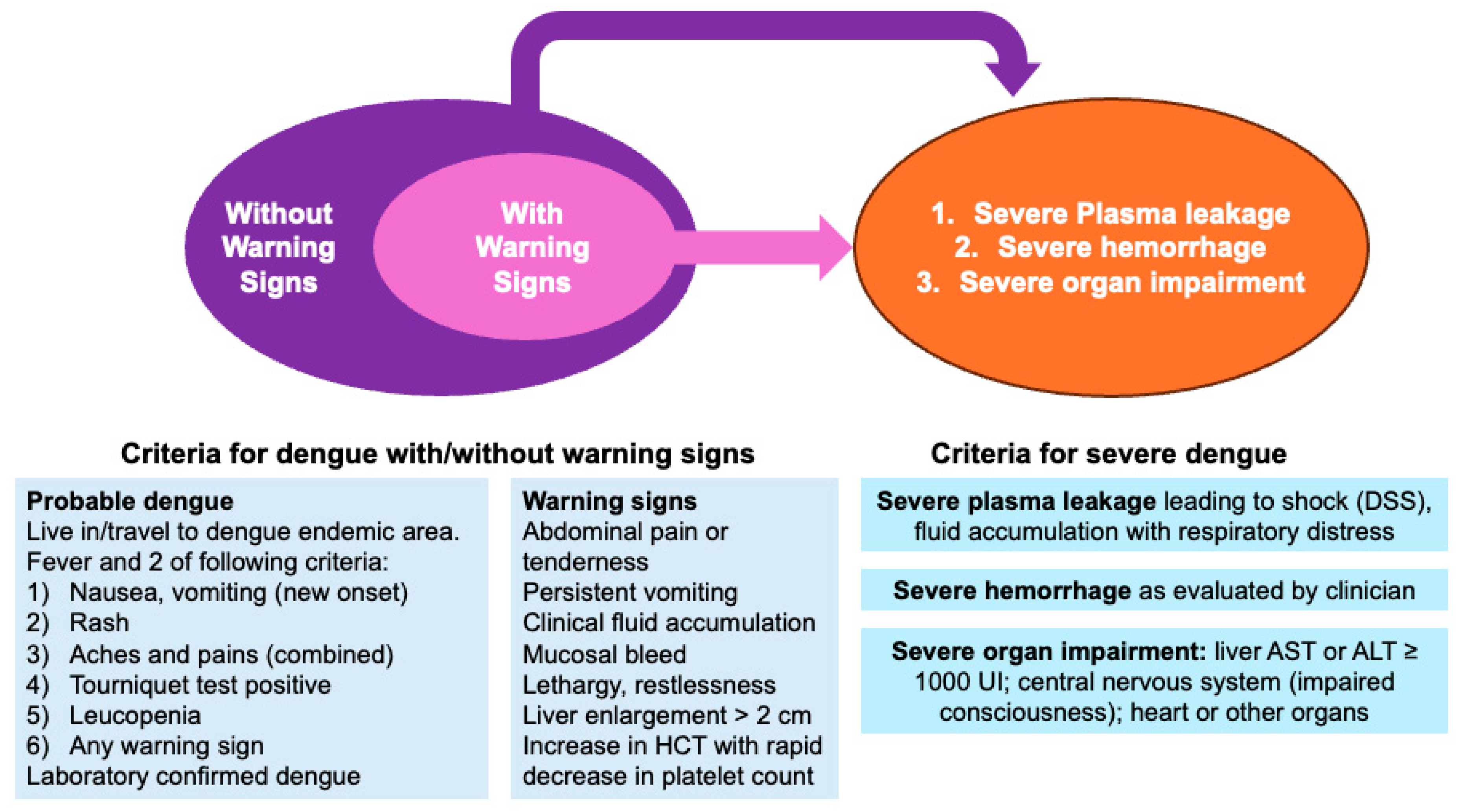
1.4. Dengue and Pancreatitis: What We Currently Know
2. Materials and Methods
2.1. Study Selection and Inclusion/Exclusion Criteria
- (1)
- Availability of detailed clinical and/or imaging features of the reported case.
- (2)
- Diagnosis of dengue virus infection via detection of IgG/IgM antibodies in a serum sample and/or detection of NS1 antigen and/or detection of viral RNA by means of polymerase chain reaction (PCR) assay and/or isolation of dengue virus from the index patient. Only for reports published before the widespread commercial availability of the enzyme-linked immunosorbent assay (ELISA) (i.e., mid-1980s), hemagglutination tests were used to document dengue virus infection; studies were deemed eligible for inclusion in the systematic review even when the specific diagnostic test was not explicitly reported, provided that the original source confirmed a laboratory-established diagnosis (i.e., “not provided”, NP).
- (3)
- Diagnosis of acute pancreatitis by means of two of the following features: (a) abdominal pain of acute onset, severe, affecting the epigastric region, and often radiating to the back; (b) serum lipase and/or amylase activity at least three times greater than the upper limit of the normal; (c) documented findings of acute pancreatis on contrast-enhanced computed tomography and/or magnetic resonance imaging and/or transabdominal ultrasonography [38,39,40,41].
- (a)
- Settings of the case: year, month or season, geographic region.
- (b)
- Age and gender of the reported cases.
- (c)
- Pre-existing clinical features, if any, and any of the following risk factors for acute pancreatitis [33,36,48,49,50,51,52,53]: alcohol abuse; smoking history; any previous diagnosis of gallstones; hypertriglyceridemia; endoscopic procedures; abdominal trauma; history of autoimmune disease; predisposing genetic mutations; any history of drugs associated with acute pancreatitis (angiotensin-converting enzyme (localized angioedema); statins (direct and accumulation toxicity); oral contraceptives or hormone replacement therapy, especially estrogen (hypertriglyceridemia, local arteriolar thrombosis); diuretics; antiviral therapy (HIV); valproic acid; and antidiabetic agents, such as GLP-1 mimetics).
- (d)
- Clinical characteristics: length of reported symptoms (days); fever (body temperature > 38 °C); abdominal pain; nausea; vomiting; any sign of altered consciousness; headache; skin rash; jaundice and scleral icterus; conjunctivitis; retro-orbital pain; pain; any sign of capillary refill disorder and peripheral edema; signs of minor bleedings (e.g., petechiae, purpura, subdermal hemorrhages, epistaxis, and gum bleeding); shortness of breath; hypotension (i.e., blood pressure < 90/60 mmHg); tachycardia (i.e., resting heart rate exceeding 100 beats per minute).
- (e)
- Diagnostic strategy for dengue virus infection (i.e., antigenic testing on viral NS1 protein, studies reporting IgM and IgG antibodies targeting DENV, polymerase chain reaction [PCR] studies).
- (f)
- Laboratory work-up results [54], specifically focusing on blood count values; blood sugar levels (BSLs); albuminemia; creatinine levels; liver function tests (i.e., serum aspartate transaminase [AST]; serum alanine aminotransferase [ALT]; alkaline phosphatase, gamma-glutamyl transferase [GGT]); lipases and amylases; values of proteins and/or lactate according to the normal range values of the parent institution.
- (g)
- (h)
- Features of all imaging studies, including ultrasonographic studies and CT scans.
- (i)
- (j)
- Outcomes: hospital stay (days); whether any respiratory support was required or not; whether any surgery was performed, including drainage of collection and/or necrotic areas; survival vs. death; and weeks of total survival time.
- (k)
- If a certain patient was cross-posted by different studies, reports were accurately analyzed to fill the knowledge gaps and provide an extensive description of the clinical case, as well as to eliminate duplicates.
2.2. Qualitative Assessment
2.3. Data Analysis
- -
- Model I, i.e., diagnosis of ANP vs. AIP, included the diagnosis of ANP as the outcome variable, and the time variable was represented by the age of the patient at the time of the onset of symptoms;
- -
- Model II, i.e., death of the patient during the follow-up, included the death of the patient as the outcome variable, and the time variable was represented by the time elapsed since the onset of the symptoms and the documented overall survival of the patient;
- -
- All other categorical variables that, in univariable analysis, were associated with the corresponding outcome variable with a p-value < 0.05 were also included in the stepwise binary logistic regression analysis model as explanatory variables.
3. Results
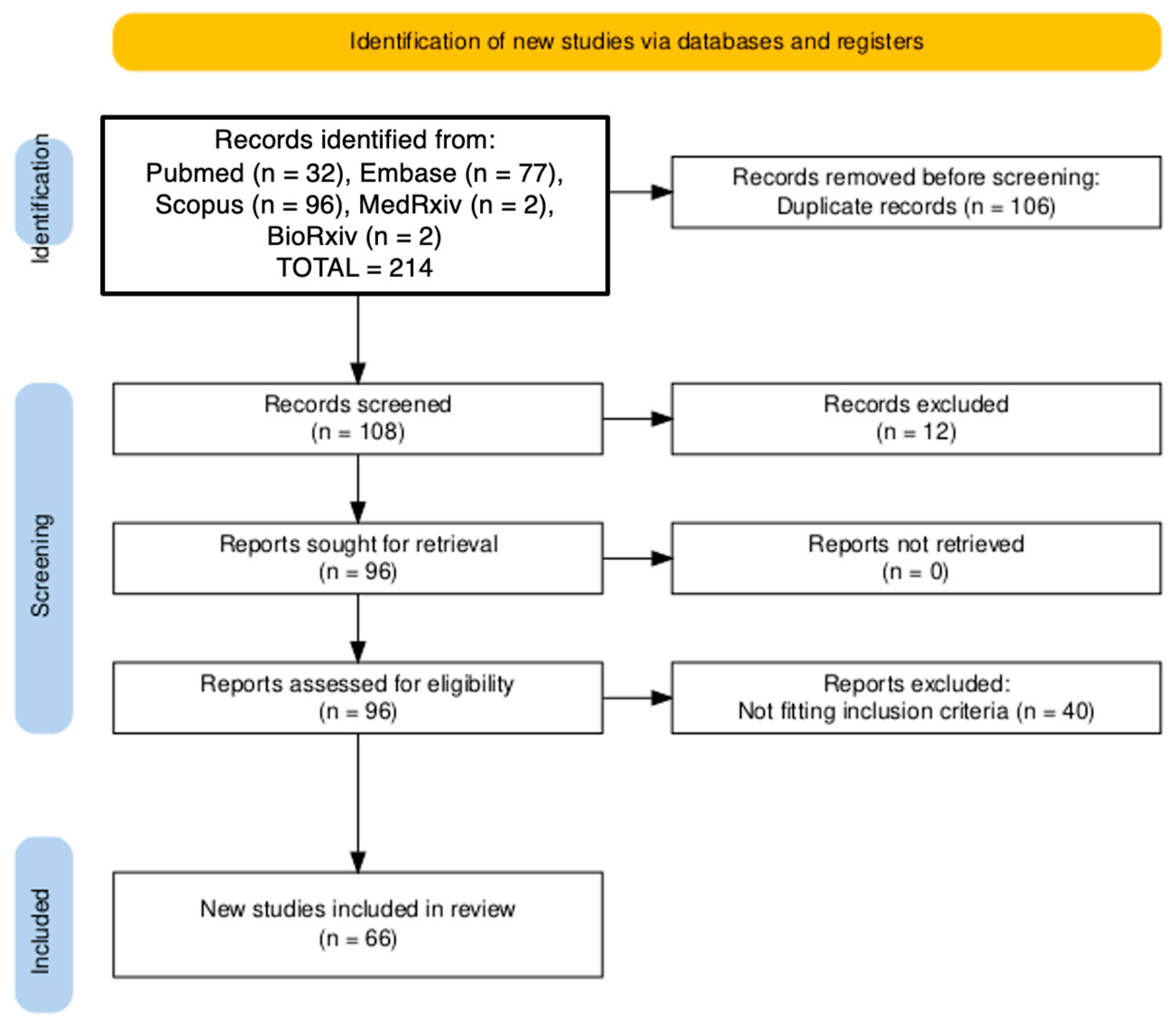
3.1. Results of the Query
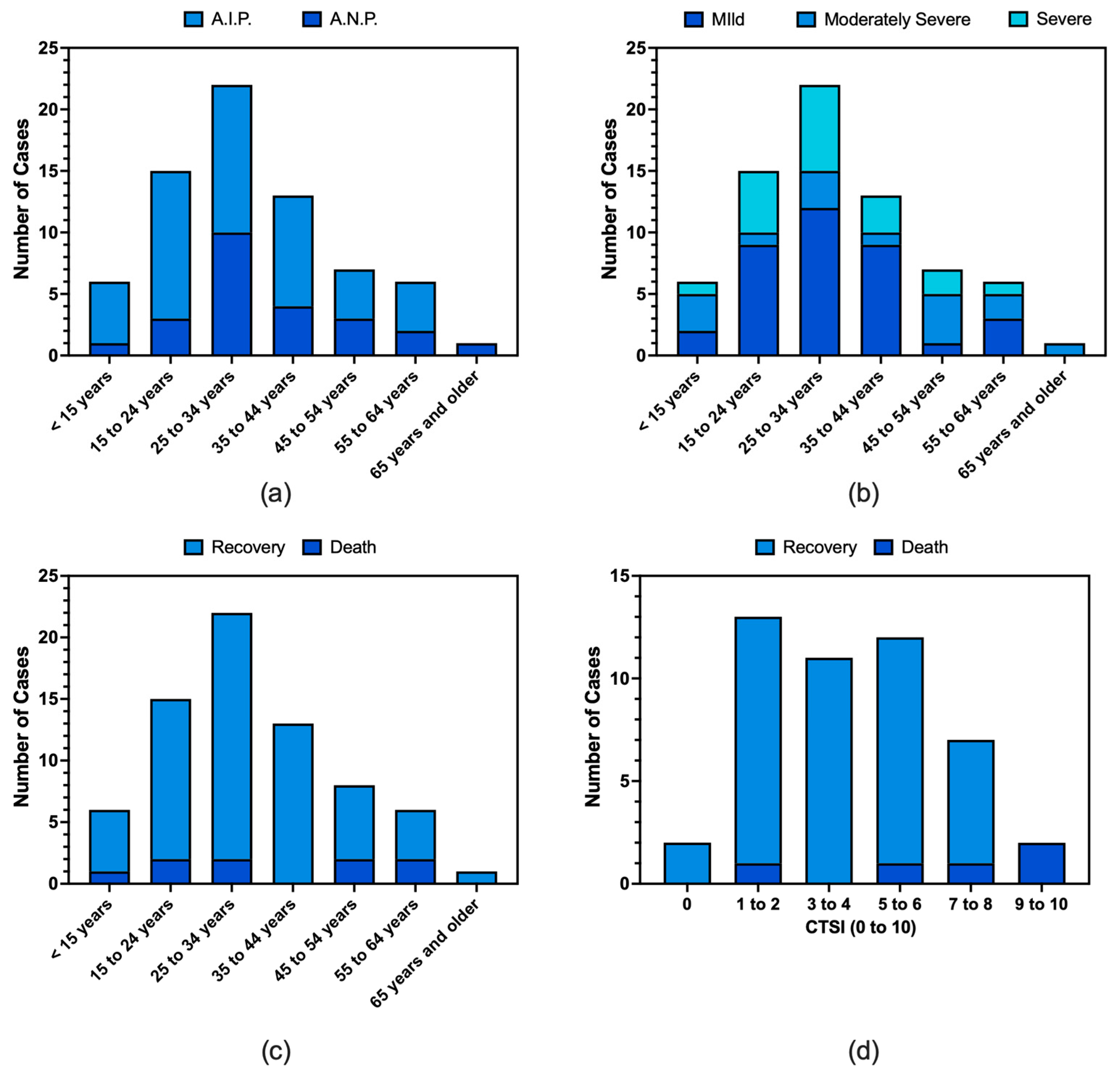
3.2. Demographics
3.3. Diagnosis of Dengue Fever
3.4. Clinical Features
3.5. Imaging
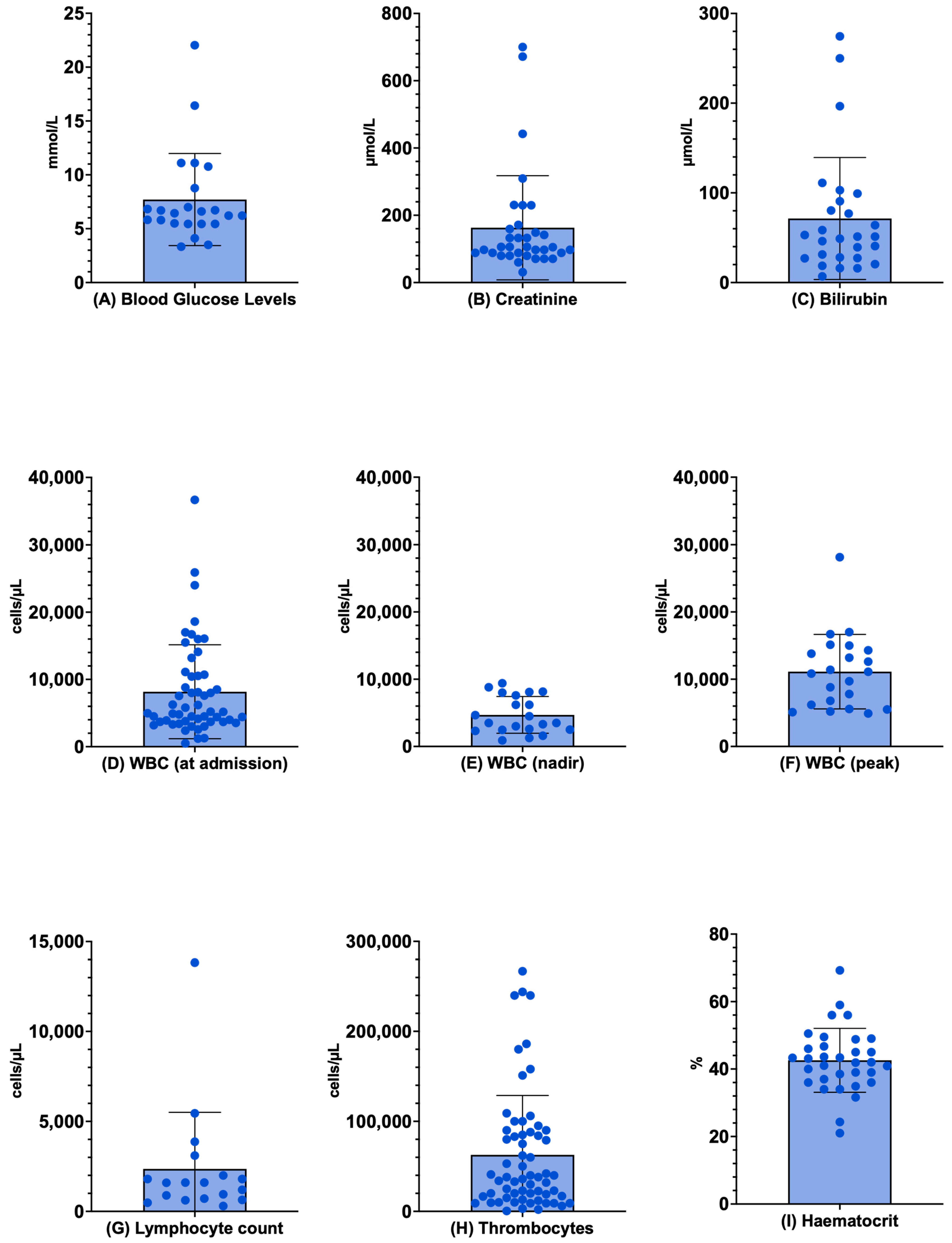
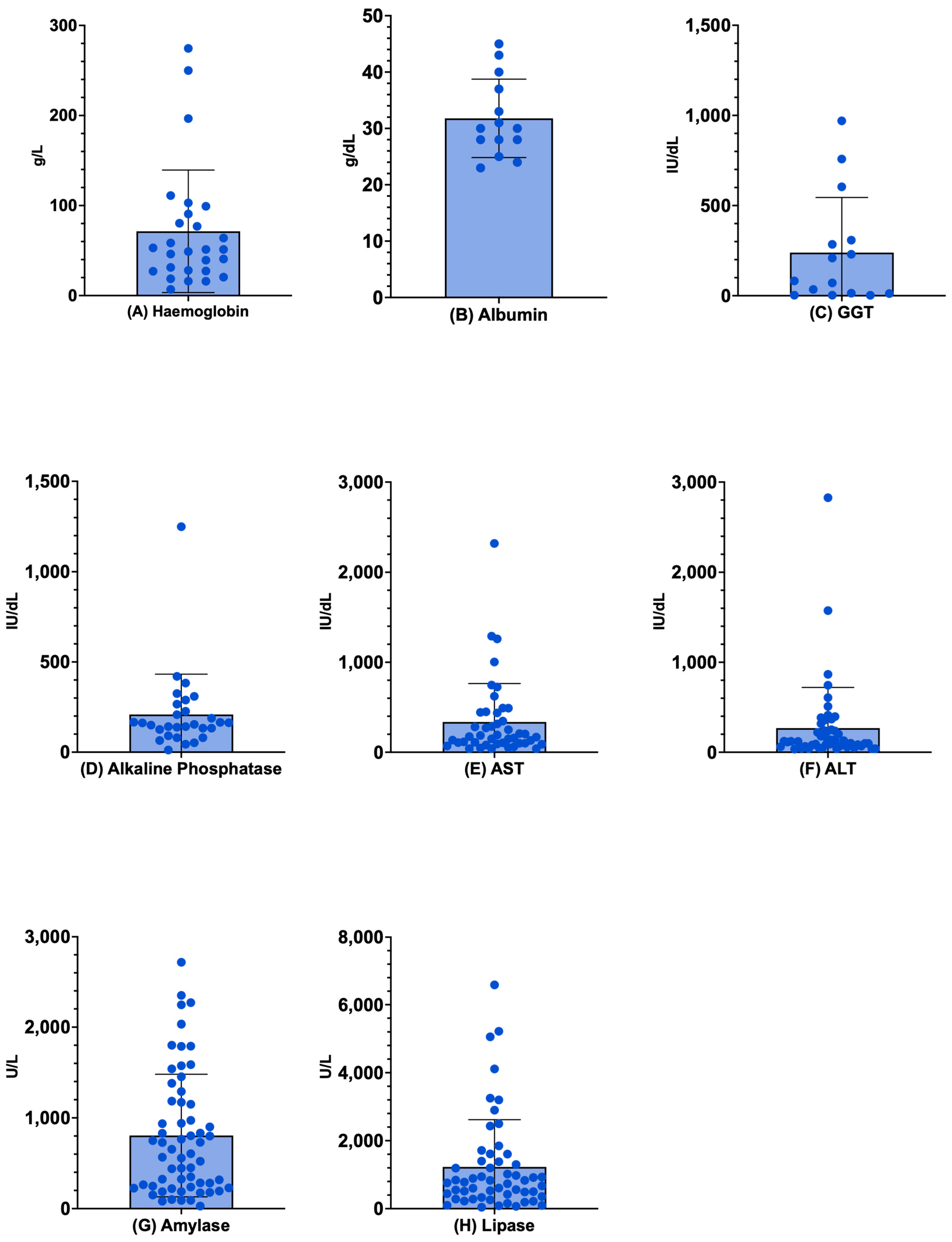
3.6. Laboratory Exams
3.7. Diagnosis and Characteristics of Pancreatitis
3.8. Clinical Course and Outcome
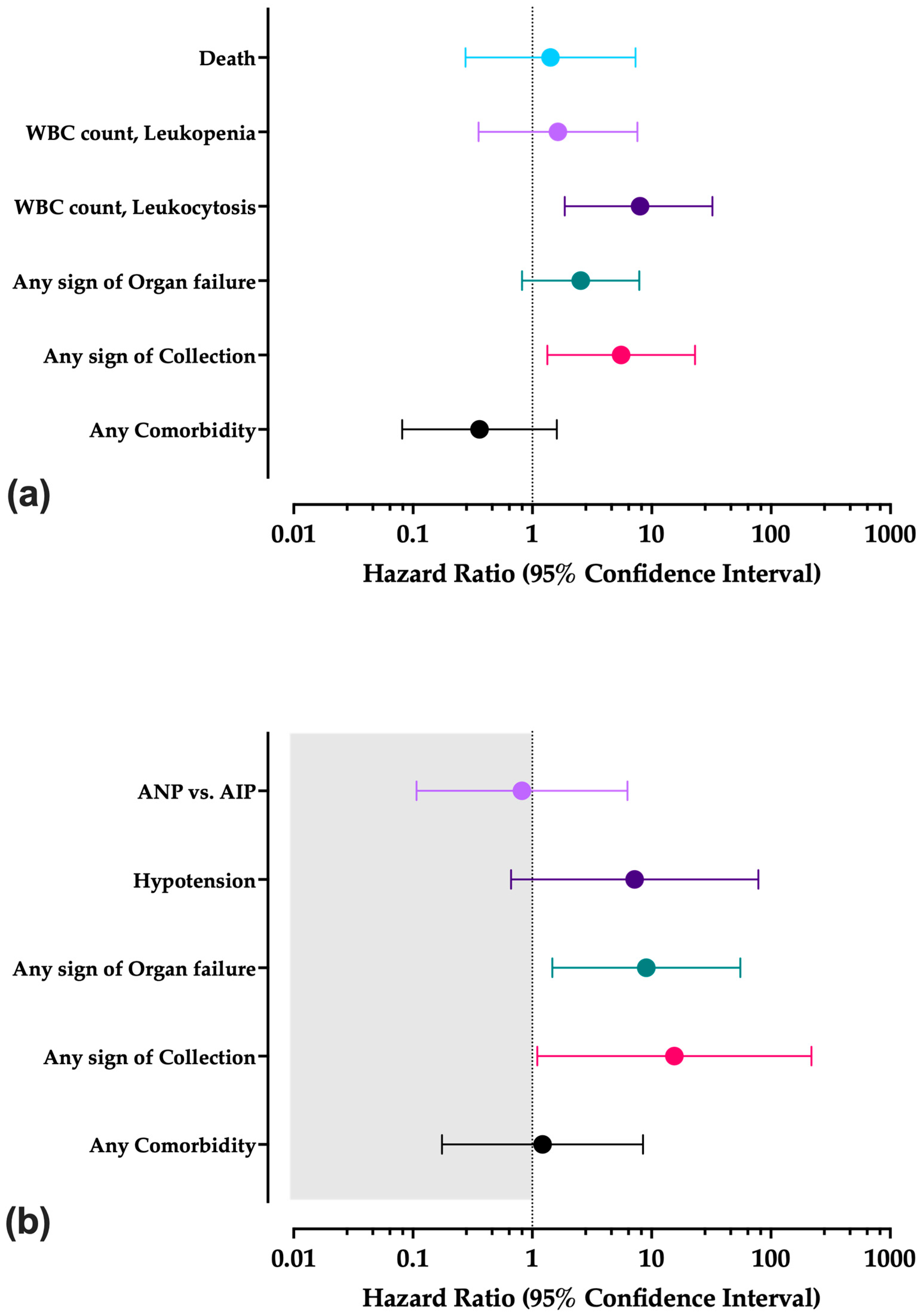
3.9. Univariate Analysis
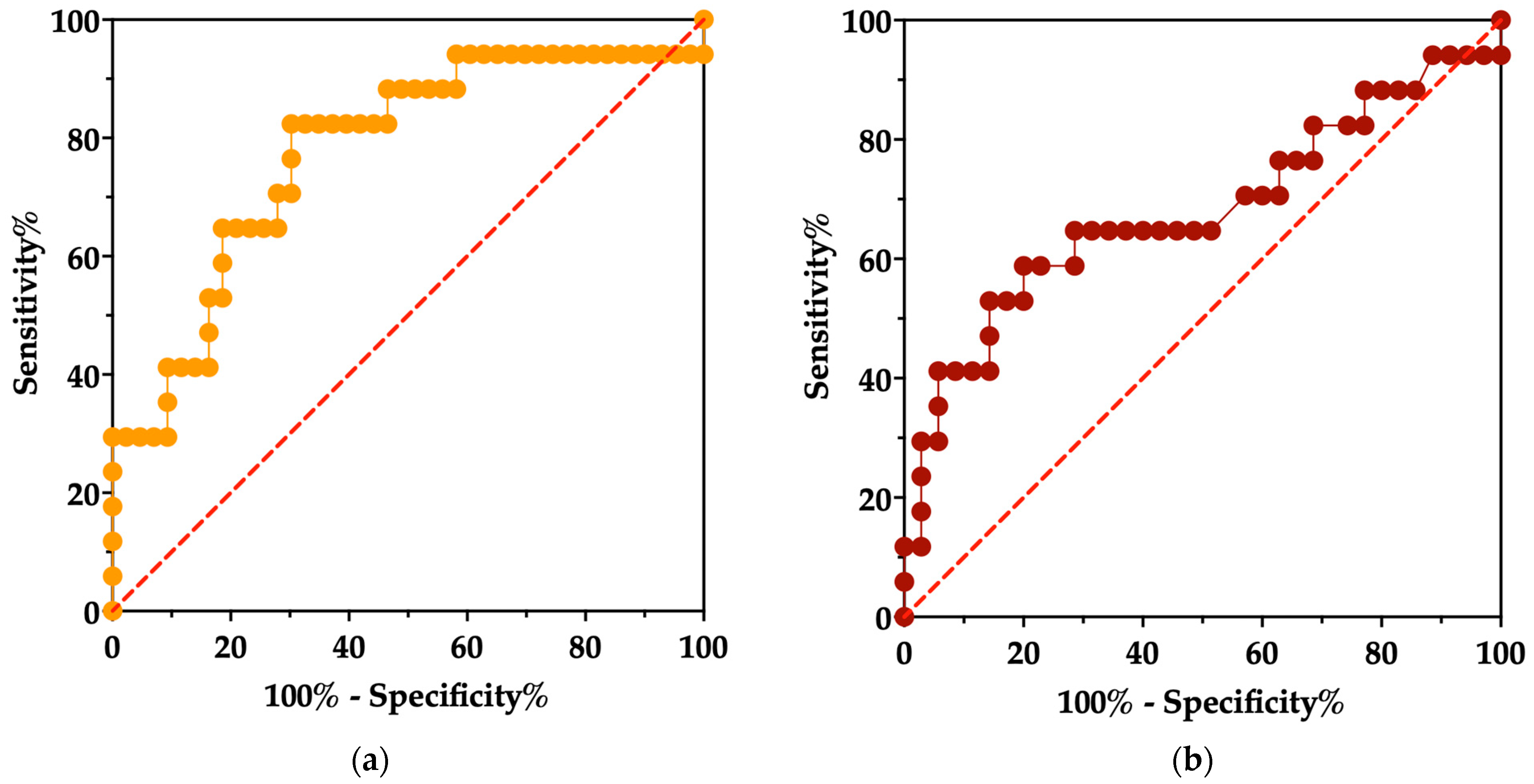
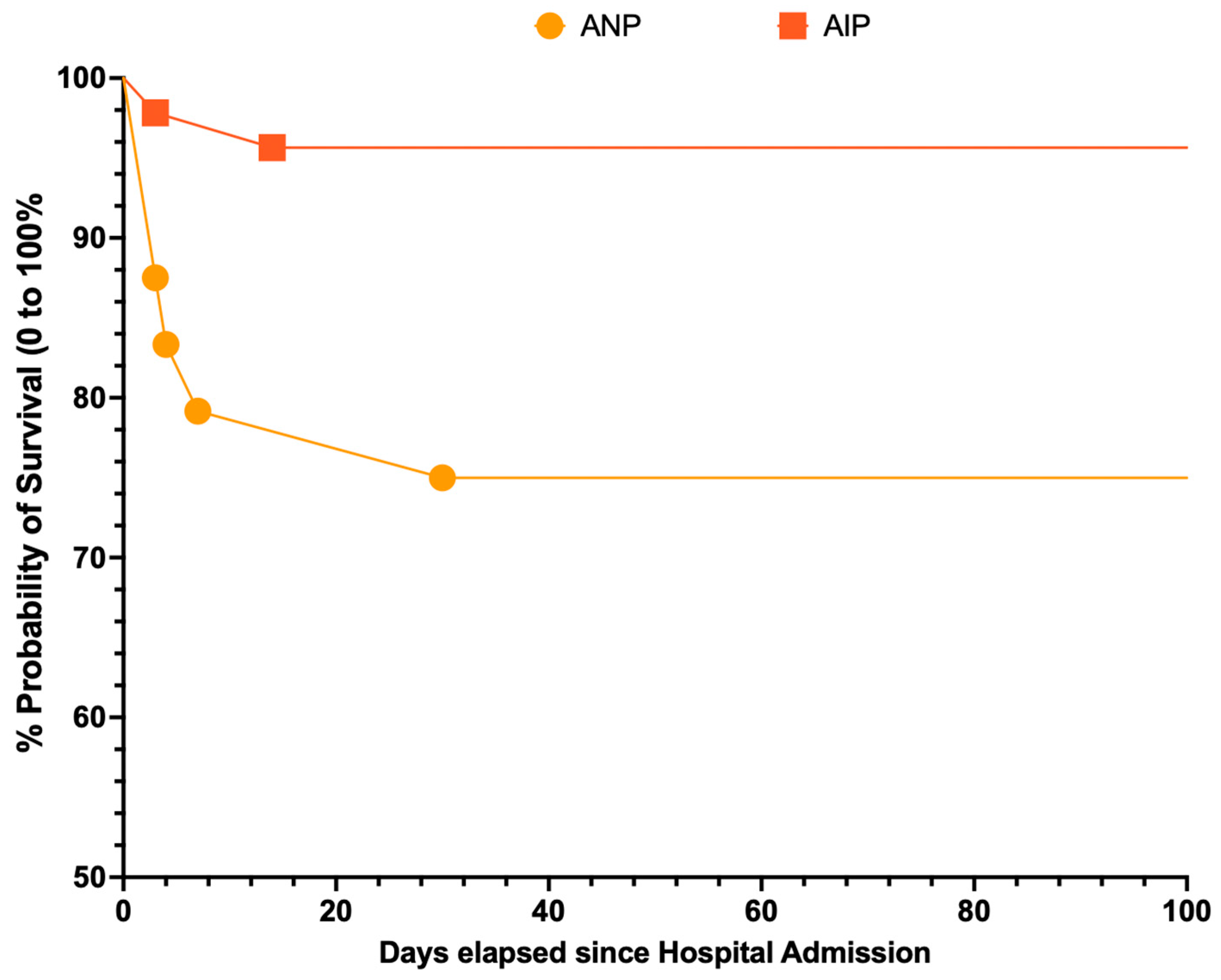
3.10. Survival Analysis
3.11. Multivariable Analysis
4. Discussion
4.1. Summary of Main Findings
4.2. Interpretation of Main Findings
4.3. Generalizability of Main Findings and Implications for Daily Practice
4.4. Limits and Implications for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95%CI | 95% Confidence Interval |
| ADE | Antibody-Dependent Enhancement |
| AIP | Acute Interstitial Pancreatitis |
| ALT | Alanine Aminotransferase |
| ANP | Acute Necrotizing Pancreatitis |
| AST | Aspartate Transaminase |
| CECT | Contrast-Enhanced Computed Tomography |
| CT | Computed Tomography |
| CTSI | Computed Tomography Severity Index |
| DENV | Dengue Virus |
| DHF | Dengue Hemorrhagic Fever |
| DF | Dengue Fever |
| DSS | Dengue Shock Syndrome |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GGT | Gamma-Glutamyl Transferase |
| HD | Home Discharge |
| HR | Hazard Ratio |
| PICO | Patient/Population/Problem; Investigated Results; Control/Comparator; Outcome |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction |
| SD | Standard Deviation |
| WBC | White Blood Cell |
| WHO | World Health Organization |
Appendix A
| Continent | Country | No./70, % | References |
|---|---|---|---|
| Asia | India | 28, 40.0% | [61,63,66,67,68,69,70,71,72,80,84,88,90,91,93,94,96,98,101,102,104,115,117] |
| Bangladesh | 7, 10.0% | [79,80,94,97,100,102,118] | |
| Sri Lanka | 4, 5.7% | [61,67,87,111] | |
| Nepal | 3, 4.3% | [99] | |
| Pakistan | 3, 4.3% | [76,107,115] | |
| Indonesia | 3, 4.3% | [58,60,83] | |
| Taiwan | 2, 2.9% | [63,66] | |
| United Arab Emirates | 1, 1.4% | [105] | |
| Brunei | 1, 1.4% | [88] | |
| Malaysia | 1, 1.4% | [120] | |
| Vietnam | 1, 1.4% | [114] | |
| People Republic of China | 1, 1.4% | [85] | |
| South America | Brazil | 5, 7.1% | [35,89,91,112] |
| Peru | 3, 4.3% | [109,113,116] | |
| Argentina | 1, 1.4% | [101] | |
| Colombia | 1, 1.4% | [64] | |
| Ecuador | 1, 1.4% | [110] | |
| Panama | 1, 1.4% | [84] | |
| North America | USA | 1, 1.4% | [59] |
| Oceania | New Caledonia | 1, 1.4% | [62] |
| Europe | France | 1, 1.4% | [49] |
| Reference | Year | D1 | D2 | D3 | D4 | D7 | D8 |
|---|---|---|---|---|---|---|---|
| Acherjya et al., 2023 [100] | 2022 | +1 | +1 | +1 | +1 | +1 | +1 |
| Agrawal et al., 2011 [73] | 2011 | +1 | +1 | +1 | +1 | +1 | +1 |
| Ahmed et al., 2024 [115] | 2024 | +1 | - | +1 | - | +1 | +1 |
| Alnuaimi et al., 2025 [108] | 2024 | +1 | +1 | +1 | +1 | +1 | +1 |
| Alvarez et al., 1985 [59] | 1981 | - | - | +1 | +1 | +1 | - |
| Anam et al., 2016 [80] | 2013 | +1 | - | +1 | +1 | +1 | +1 |
| Anam et al., 2017 [79] | 2015 | +1 | +1 | +1 | +1 | +1 | +1 |
| Arora et al., 2023 [92] | 2023 | +1 | - | +1 | +1 | +1 | - |
| 2023 | +1 | - | +1 | +1 | +1 | +1 | |
| 2023 | +1 | - | +1 | +1 | +1 | +1 | |
| Arredondo-Nontol et al., 2022 [113] | 2022 | +1 | +1 | +1 | +1 | +1 | +1 |
| Bashir Bhatti et al., 2015 [81] | 2015 | +1 | +1 | +1 | +1 | +1 | +1 |
| Beaussac et al., 2015 [49] | 2020 | - | - | +1 | +1 | +1 | - |
| Biswas et al., 2024 [103] | 2024 | +1 | +1 | +1 | +1 | +1 | +1 |
| Che Jusoh et al., 2016 [88] | 2015 | +1 | - | +1 | +1 | +1 | +1 |
| Chen et al., 2004 [63] | 2004 | +1 | - | +1 | +1 | - | - |
| Correa et al., 2019 [84] | 2018 | +1 | +1 | +1 | +1 | +1 | +1 |
| Dalugama et al., 2017 [87] | 2016 | +1 | - | +1 | +1 | +1 | +1 |
| de Andrade et al., 2022 [35] | 2002 | +1 | +1 | +1 | +1 | +1 | +1 |
| 2002 | +1 | - | +1 | - | +1 | +1 | |
| Derycke et al., 2005 [62] | 2005 | +1 | +1 | +1 | +1 | +1 | +1 |
| Devi et al., 2022 [76] | 2019 | +1 | - | +1 | - | +1 | +1 |
| dos Passos Cunha et al., 2021 [89] | 2019 | +1 | +1 | +1 | +1 | +1 | +1 |
| Duarte et al., 2024 [101] | 2023 | +1 | +1 | +1 | +1 | - | - |
| Dutta et al., 2025 [93] | 2025 | +1 | +1 | +1 | +1 | +1 | +1 |
| Flor et al., 2022 [110] | 2021 | +1 | +1 | +1 | +1 | +1 | +1 |
| Gomes et al., 2021 [94] | 2021 | +1 | +1 | +1 | +1 | +1 | +1 |
| Gonzalez-Fontal et al., 2011 [64] | 2009 | +1 | +1 | +1 | +1 | +1 | +1 |
| Iqbal et al., 2012 [82] | 2012 | +1 | +1 | +1 | +1 | +1 | +1 |
| Islam et al., 2023 [104] | 2023 | +1 | +1 | +1 | +1 | +1 | +1 |
| Jahan et al., 2020 [118] | 2020 | +1 | +1 | +1 | +1 | +1 | +1 |
| Jain et al., 2014 [71] | 2014 | +1 | +1 | +1 | +1 | +1 | +1 |
| Jusuf et al., 1998 [58] | 1997 | +1 | - | +1 | - | +1 | +1 |
| Karoli et al., 2012 [90] | 2012 | +1 | - | +1 | +1 | - | - |
| Kashyap et al., 2024 [105] | 2024 | +1 | +1 | +1 | +1 | +1 | +1 |
| Khanal et al., 2023 [99] | 2022 | +1 | - | +1 | - | +1 | +1 |
| 2022 | +1 | - | +1 | +1 | +1 | +1 | |
| Khataniar et al., 2023 [98] | 2023 | +1 | +1 | +1 | +1 | +1 | +1 |
| Kodisinghe et al., 2011 [67] | 2011 | +1 | - | +1 | +1 | - | - |
| Krithika et al., 2018 [77] | 2017 | +1 | +1 | - | - | - | - |
| Kumar Das et al., 2024 [117] | 2023 | +1 | +1 | +1 | +1 | +1 | +1 |
| Kumar et al., 2016 [68] | 2016 | +1 | +1 | +1 | +1 | +1 | +1 |
| Kumar et al., 2017 [70] | 2017 | +1 | +1 | +1 | +1 | +1 | +1 |
| Kumar et al., 2018 [69] | 2018 | +1 | +1 | +1 | +1 | +1 | +1 |
| Lee et al., 2013 [66] | 2013 | +1 | +1 | +1 | +1 | +1 | +1 |
| Lee et al., 2021 [120] | 2021 | +1 | - | +1 | - | +1 | +1 |
| Lu et al., 2020 [85] | 2019 | +1 | +1 | +1 | +1 | +1 | +1 |
| Mishra et al., 2019 [75] | 2019 | +1 | +1 | +1 | +1 | +1 | +1 |
| Naik et al., 2021 [106] | 2021 | +1 | +1 | +1 | +1 | +1 | +1 |
| Nakazaki et al., 2024 [109] | 2023 | +1 | +1 | +1 | +1 | +1 | +1 |
| Nawal et al., 2018 [72] | 2016 | +1 | +1 | +1 | +1 | +1 | +1 |
| Nguyen et al., 2023 [114] | 2023 | +1 | - | +1 | +1 | +1 | +1 |
| Nogueira et al., 2022 [91] | 2022 | +1 | +1 | +1 | +1 | +1 | +1 |
| Prabhu et al., 2025 [119] | 2025 | +1 | - | +1 | +1 | - | - |
| Rahman et al., 2020 [97] | 2021 | +1 | +1 | +1 | +1 | +1 | +1 |
| Rajesh et al., 2008 [34] | 2008 | +1 | - | +1 | +1 | - | - |
| Ramindla et al., 2025 [95] | 2025 | +1 | +1 | +1 | +1 | +1 | +1 |
| Ridho et al., 2000 [60] | 2000 | +1 | +1 | +1 | +1 | +1 | +1 |
| Rodriguez Gonzalez et al., 2025 [116] | 2025 | +1 | +1 | +1 | +1 | +1 | +1 |
| Saber et al., 2021 [102] | 2021 | +1 | +1 | +1 | +1 | +1 | +1 |
| Seetharam et al., 2010 [78] | 2010 | +1 | - | +1 | - | +1 | +1 |
| Sharma et al., 2018 [74] | 2018 | +1 | +1 | +1 | +1 | +1 | +1 |
| Simadibrata et al., 2012 [83] | 2012 | +1 | +1 | +1 | +1 | +1 | +1 |
| Singh Lakra et al., 2022 [96] | 2022 | +1 | +1 | +1 | +1 | +1 | - |
| Sudulagunta et al., 2016 [65] | 2016 | +1 | +1 | +1 | +1 | +1 | +1 |
| Teja Derbesula et al., 2016 [86] | 2016 | +1 | +1 | +1 | +1 | +1 | +1 |
| Thadchanamoorthy et al., 2022 [111] | 2022 | +1 | - | +1 | +1 | +1 | +1 |
| Ullah et al., 2025 [107] | 2024 | +1 | - | +1 | +1 | +1 | +1 |
| Vilela Assis et al., 2021 [112] | 2020 | +1 | +1 | +1 | +1 | +1 | +1 |
| Wijekoon et al., 2010 [61] | 2009 | +1 | +1 | +1 | +1 | +1 | +1 |
| Summary (N/70, %) | 68 (97.1%) | 46 (65.7%) | 69 (98.6%) | 62 (88.6%) | 63 (90.0%) | 59 (84.3%) |
| Variable | TOT. | N/TOT. (%) | Interstitial vs. Necrotizing Pancreatitis | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Interstitial (n./46, %) | Necrotizing (n./24, %) | p Value 1 | Death (n./8, %) | Remission (n./62, %) | p Value 1 | |||
| Gender | 70 | 0.829 | 1.000 | |||||
| Male | 44 (62.9%) | 28 (60.9%) | 16 (66.7%) | 5 (62.5%) | 39 (62.9%) | |||
| Female | 26 (37.1%) | 18 (39.1%) | 8 (33.3%) | 3 (37.5%) | 23 (37.1%) | |||
| Age (years, average ± S.D.) | 70 | 31.6 ± 14.5 | 30.2 ± 13.8 | 34.5 ± 15.5 | 0.262 | 34.1 ± 20.3 | 31.3 ± 13.7 | 0.714 |
| Comorbidities | ||||||||
| Any | 70 | 19 (27.1%) | 9 (19.6%) | 10 (41.7%) | 0.091 | 5 (62.5%) | 14 (22.6%) | 0.049 |
| Diabetes | 70 | 5 (7.1%) | 1 (2.2%) | 4 (16.7%) | 0.081 | 1 (12.5%) | 4 (6.5%) | 1.000 |
| Hypertension | 70 | 2 (2.9%) | 0 (-) | 2 (8.3%) | 0.218 | 1 (12.5%) | 1 (1.6%) | 0.540 |
| Obesity | 70 | 3 (4.3%) | 2 (4.3%) | 1 (4.2%) | 1.000 | 2 (25.0%) | 1 (1.6%) | 0.032 |
| Previous pancreatitis | 70 | 1 (1.4%) | 0 (-) | 1 (4.2%) | 0.739 | 0 (-) | 1 (1.6%) | 1.000 |
| Alcohol consumption | 70 | 2 (2.9%) | 2 (4.3%) | 0 (-) | 0.779 | 0 (-) | 2 (3.2%) | 1.000 |
| History of gallstones | 70 | 0 (-) | 0 (-) | 0 (-) | - | 0 (-) | 0 (-) | - |
| Diagnosis by means of | ||||||||
| RT-qPCR | 70 | 7 (10.0%) | 6 (13.0%) | 1 (4.2%) | 0.450 | 1 (12.5%) | 6 (9.7%) | 1.000 |
| NS1 antigen | 70 | 35 (50.0%) | 23 (50.0%) | 12 (50.0%) | 1.000 | 2 (25.0%) | 33 (53.2%) | 0.260 |
| IgM (ELISA) | 70 | 40 (57.1%) | 26 (56.5%) | 14 (58.3%) | 1.000 | 4 (50.0%) | 36 (58.1%) | 0.957 |
| IgG (ELISA) | 70 | 12 (17.1%) | 7 (15.2%) | 5 (20.8%) | 0.797 | 0 (-) | 12 (19.4%) | 0.385 |
| Day of admission | 66 | 5.7 ± 7.8 | 4.7 ± 3.8 | 7.9 ± 12.7 | 0.274 | 11.1 ± 19.9 | 5.0 ± 4.1 | 0.409 |
| Symptoms at admission | ||||||||
| Fever (Temp. ≥ 38.0 °C) | 70 | 46 (65.7%) | 31 (67.4%) | 15 (62.5%) | 0.886 | 5 (62.5%) | 41 (66.1%) | 1.000 |
| Epigastric pain | 70 | 59 (84.3%) | 39 (84.8%) | 20 (83.3%) | 1.000 | 7 (87.5%) | 52 (83.9%) | 1.000 |
| Nausea | 70 | 38 (54.3%) | 26 (56.5%) | 12 (50.0%) | 0.789 | 5 (62.5%) | 33 (53.2%) | 0.906 |
| Vomiting | 70 | 41 (58.6%) | 28 (60.9%) | 13 (54.2%) | 0.776 | 5 (62.5%) | 36 (58.1%) | 1.000 |
| Altered consciousness | 70 | 17 (24.3%) | 9 (19.6%) | 8 (33.3%) | 0.326 | 3 (37.5%) | 14 (22.6%) | 0.625 |
| Headache | 70 | 28 (40.0%) | 22 (47.8%) | 6 (25.0%) | 0.111 | 5 (62.5%) | 23 (37.1%) | 0.319 |
| Rash | 70 | 16 (22.9%) | 11 (23.9%) | 5 (20.8%) | 1.000 | 2 (25.0%) | 14 (22.6%) | 1.000 |
| Jaundice | 70 | 7 (10.0%) | 2 (4.3%) | 5 (20.8%) | 0.078 | 1 (12.5%) | 6 (9.7%) | 1.000 |
| Conjunctivitis | 70 | 5 (7.1%) | 3 (6.5%) | 2 (8.3%) | 1.000 | 0 (-) | 5 (8.1%) | 0.917 |
| Retro-orbital pain | 70 | 17 (24.3%) | 15 (32.6%) | 2 (8.3%) | 0.051 | 1 (12.5%) | 14 (25.8%) | 0.698 |
| Body aches | 70 | 34 (48.6%) | 26 (56.5%) | 8 (33.3%) | 0.112 | 5 (62.5%) | 29 (46.8%) | 0.644 |
| Capillary refill disorders | 70 | 9 (12.9%) | 9 (19.6%) | 0 (-) | 0.052 | 0 (-) | 9 (14.5%) | 0.553 |
| Minor bleedings | 70 | 11 (15.7%) | 5 (10.9%) | 6 (25.0%) | 0.232 | 2 (25.0%) | 9 (14.5%) | 0.802 |
| Shortness of breath | 70 | 21 (30.0%) | 12 (26.1%) | 9 (37.5%) | 0.475 | 3 (37.5%) | 18 (29.0%) | 0.935 |
| Peripheral edemas | 70 | 9 (12.9%) | 6 (13.0%) | 3 (12.5%) | 1.000 | 1 (12.5%) | 8 (12.9%) | 1.000 |
| Hypotension | 70 | 31 (44.3%) | 18 (39.1%) | 13 (54.2%) | 0.343 | 7 (87.5%) | 24 (38.7%) | 0.025 |
| Tachycardia | 70 | 35 (50.0%) | 22 (47.8%) | 13 (54.2%) | 0.801 | 5 (62.5%) | 30 (48.4%) | 0.707 |
| Primary vs. Secondary Dengue | ||||||||
| Secondary dengue 1 | 70 | 14 (20.0%) | 9 (19.6%) | 5 (20.8%) | 1.000 | 14 (22.6%) | 0 (-) | 0.302 |
| Gallbladder disorders | ||||||||
| Stones | 67 | 0 | 0 (-) | 0 (-)- | 0 (-) | 0 (-) | (-)- | (-) |
| Ultrasonography performed/reported | 67 | 46 (68.7%) | 33 (76.7%) | 13 (54.2%) | 0.102 | 5 (62.5%) | 41 (69.5%) | 1.000 |
| n./33 | n./15 | n./5 | n./43 | |||||
| Ultrasonographic signs of pancreatic disease | 48 | 29 (60.4%) | 19 (57.6%) | 10 (66.7%) | 0.781 | 4 (80.0%) | 25 (58.1%) | 0.643 |
| Ultrasonographic signs of gallbladder disease | 48 | 5 (10.4%) | 5 (15.2%) | 0 (-) | 0.342 | 1 (20.0%) | 4 (9.3%) | 1.000 |
| CECT performed during hospital stay | 67 | 45 (67.2%) | 26 (57.8%) | 19 (42.2%) | 0.196 | 5 (62.5%) | 40 (67.8%) | 1.000 |
| CTSI SCORE (0–10) | 45 | 4.6 ± 2.6 | 3.1 ± 1.8 | 6.8 ± 1.8 | <0.001 | 7.2 ± 3.3 | 4.3 ± 2.3 | 0.016 |
| Characteristics of Pancreatitis | 70 | - | 0.029 | |||||
| Interstitial | 46 (65.7%) | - | - | 2 (25.0%) | 44 (71.0%) | |||
| Necrotizing | 24 (34.3%) | - | - | 6 (75.0%) | 18 (29.0%) | |||
| Imaging data available | 70 | 67 (95.7%) | 45 (97.8%) | 22 (91.7%) | 0.558 | 8 (100%) | 59 (95.2%) | 1.000 |
| Imaging features | ||||||||
| Swollen pancreas | 70 | 62 (88.6%) | 40 (87.0%) | 22 (91.7%) | 0.848 | 6 (75.0%) | 56 (90.3%) | 0.489 |
| Intrinsic pancreatic abnormalities | 70 | 39 (55.7%) | 23 (50.0%) | 16 (66.7%) | 0.281 | 4 (50.0%) | 35 (56.5%) | 1.000 |
| Inflammatory changes in peripancreatic fat | 70 | 26 (37.1%) | 16 (34.8%) | 10 (41.7%) | 0.760 | 3 (37.5%) | 23 (37.1%) | 1.000 |
| Pancreatic/peripancreatic fluid | 70 | 22 (31.4%) | 12 (26.1%) | 10 (41.7%) | 0.288 | 5 (62.5%) | 17 (27.4%) | 0.108 |
| Peripancreatic fat necrosis | 70 | 11 (15.7%) | 4 (8.7%) | 7 (29.2%) | 0.059 | 3 (37.5%) | 8 (12.9%) | 0.200 |
| Hepatomegaly | 70 | 21 (30.0%) | 15 (32.6%) | 6 (25.0%) | 0.701 | 3 (37.5%) | 18 (29.0%) | 0.935 |
| Ascites | 70 | 40 (57.1%) | 25 (54.3%) | 15 (62.5%) | 0.698 | 6 (75.0%) | 34 (54.8%) | 0.481 |
| Pleural effusions | 70 | 27 (38.6%) | 18 (39.1%) | 9 (37.5%) | 1.000 | 0 (-) | 27 (43.5%) | 0.046 |
| Collection(s) | 70 | 29 (41.4%) | 11 (23.9%) | 18 (75.0%) | <0.001 | 7 (87.5%) | 22 (35.5%) | 0.015 |
| n./11 | n./18 | n./7 | n./22 | |||||
| Lesser sac | 29 | 3 (10.3%) | 0 (-) | 3 (16.7%) | 0.423 | 1 (14.3%) | 2 (9.1%) | 1.000 |
| Intrapancreatic | 29 | 8 (27.6%) | 2 (18.2%) | 6 (33.3%) | 0.647 | 0 (-) | 8 (36.4%) | 0.165 |
| Peripancreatic | 29 | 18 (62.1%) | 7 (63.6%) | 11 (61.1%) | 1.000 | 5 (71.4%) | 13 (59.1%) | 0.890 |
| Perihepatic | 29 | 3 (10.3%) | 1 (9.1%) | 2 (11.1%) | 1.000 | 1 (14.3%) | 2 (9.1%) | 1.000 |
| Epigastric | 29 | 4 (13.8%) | 0 (-) | 4 (22.2%) | 0.259 | 1 (14.3%) | 3 (13.6%) | 1.000 |
| Mesenteric | 29 | 1 (3.4%) | 0 (-) | 1 (5.6%) | 1.000 | 1 (14.3%) | 0 (-) | 0.539 |
| Paracolic | 29 | 1 (3.4%) | 1 (9.1%) | 0 (-) | 0.800 | 1 (14.3%) | 0 (-) | 0.539 |
| Splenic hilar region | 29 | 2 (6.9%) | 0 (-) | 2 (11.1%) | 0.696 | 0 (-) | 2 (9.1%) | 1.000 |
| Pericholecystic | 29 | 1 (3.4%) | 1 (9.1%) | 0 (-) | 0.800 | 1 (14.3%) | 0 (-) | 0.539 |
| Psoas | 29 | 2 (6.9%) | 0 (-) | 2 (11.1%) | 0.696 | 1 (14.3%) | 1 (4.5%) | 0.976 |
| Drainage of collections | 29 | 7 (24.1%) | 1 (9.1%) | 6 (33.3%) | 0.302 | 1 (14.3%) | 6 (27.3%) | 0.847 |
| Severity | 70 | <0.001 | 0.002 | |||||
| Mild | 36 (51.4%) | 33 (71.7%) | 3 (12.5%) | 0 (-) | 36 (58.1%) | |||
| Moderately severe | 15 (21.4%) | 9 (19.6%) | 6 (25.0%) | 2 (25.0%) | 13 (21.0%) | |||
| Severe | 19 (27.1%) | 4 (8.7%) | 15 (62.5%) | 6 (75.0%) | 13 (21.0%) | |||
| Signs of Multiorgan Failure | 70 | 17 (24.3%) | 6 (13.0%) | 11 (45.8%) | 0.002 | 6 (75.0%) | 11 (17.7%) | 0.002 |
| Respiratory failure | 70 | 12 (17.1%) | 4 (8.7%) | 8 (33.3%) | 0.024 | 6 (75.0%) | 6 (9.7%) | < 0.001 |
| Signs of acute kidney failure | 70 | 15 (21.4%) | 7 (15.2%) | 8 (33.3%) | 0.148 | 4 (50.0%) | 11 (17.7%) | 0.102 |
| Hospital stay | 68 | 12.2 ± 8.8 | 10.1 ± 5.0 | 16.6 ± 12.7 | 0.030 | 8.4 ± 9.5 | 12.7 ± 8.6 | 0.196 |
| Death | 70 | 8 (11.4%) | 2 (4.3%) | 6 (25.0%) | 0.029 | - | - | - |
| Laboratory Findings | TOTAL (No. Sampled Cases) | n./TOTAL (%) | Interstitial vs. Necrotizing Pancreatitis | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Interstitial (n./N, %) | Necrotizing (n./N, %) | p Value | Death (n./N) | Remission (n./N) | p Value | |||
| Glycemia | 23 (32.9%) | |||||||
| Increased | 14 (60.9%) | 8 (57.1%) | 6 (42.9%) | 0.572 | 1 (7.1%) | 13 (92.9%) | 0.292 | |
| Normal | 9 (39.1%) | 7 (77.8%) | 2 (22.2%) | 3 (33.3%) | 6 (66.7%) | |||
| Creatinine levels | 35 (50.0%) | |||||||
| Increased creatinine levels | 15 (42.9%) | 7 (46.7%) | 8 (53.3%) | 0.296 | 4 (26.7%) | 11 (73.3%) | 0.185 | |
| Normal | 20 (57.1%) | 14 (70.0%) | 6 (30.0%) | 1 (5.0%) | 19 (95.0%) | |||
| Bilirubin levels | 27 (38.6%) | |||||||
| Increased | 22 (81.5%) | 15 (68.2%) | 7 (31.8%) | 1.000 | 3 (13.6%) | 19 (86.4%) | 1.000 | |
| Normal | 5 (18.5%) | 4 (80.0%) | 1 (20.0%) | 1 (20.0%) | 4 (80.0%) | |||
| White Blood Cell Count | 53 (75.7%) | 0.007 | 0.909 | |||||
| Normal range | 21 (39.6%) | 18 (85.7%) | 3 (14.3%) | 2 (9.5%) | 19 (90.5%) | |||
| Leukopenia | 18 (34.0%) | 13 (72.2%) | 5 (27.8%) | 2 (11.1%) | 16 (88.9%) | |||
| Leukocytosis | 14 (26.4%) | 5 (13.9%) | 9 (52.9%) | 2 (14.3%) | 12 (85.7%) | |||
| Thrombocytes | 62 (88.6%) | |||||||
| Reduced | 52 (83.9%) | 34 (65.4%) | 18 (34.6%) | 0.592 | 6 (11.5%) | 46 (88.5%) | 1.000 | |
| Normal | 10 (16.1%) | 8 (80.0%) | 2 (20.0%) | 1 (10.0%) | 9 (90.0%) | |||
| Hemoglobin | 48 (68.6%) | 0.911 | 0.464 | |||||
| Normal range | 14 (28.9%) | 9 (64.3%) | 5 (35.7%) | 1 (7.1%) | 13 (92.9%) | |||
| Reduced concentration (anemia) | 27 (57.8%) | 19 (70.4%) | 8 (29.6%) | 4 (14.8%) | 23 (85.2%) | |||
| Increased concentration (hemoconcentration) | 7 (13.3%) | 5 (71.4%) | 2 (28.6%) | 0 (-) | 7 (100%) | |||
| Albumin | 14 (20.0%) | |||||||
| Reduced concentration | 9 (64.3%) | 5 (55.6%) | 4 (44.4%) | 0.252 | 2 (22.2%) | 7 (77.8%) | 0.733 | |
| Normal | 5 (35.7%) | 5 (100%) | 0 (-) | 0 (-) | 5 (100%) | |||
| Gamma-glutamyl transferase | 10 (14.3%) | |||||||
| Increased concentration | 9 (90.0%) | 6 (66.7%) | 3 (33.3%) | 1.000 | - | 9 (100%) | 1.000 | |
| Normal | 1 (10.0%) | 1 (100%) | 0 (-) | - | 1 (100%) | |||
| Alkaline phosphatase | 28 (40.0%) | |||||||
| Increased concentration | 16 (57.1%) | 8 (50.0%) | 8 (50.0%) | 0.342 | 2 (12.5%) | 14 (87.5%) | 0.596 | |
| Normal | 12 (42.9%) | 9 (75.0%) | 3 (25.0%) | 0 (-) | 12 (100%) | |||
| Aspartate transaminase | 45 (64.3%) | |||||||
| Increased concentration | 43 (95.6%) | 31 (72.1%) | 12 (27.9%) | 1.000 | 5 (11.6%) | 38 (88.4%) | 1.000 | |
| Normal | 2 (4.4%) | 1 (50.0%) | 1 (50.0%) | 0 (-) | 2 (100%) | |||
| Alanine aminotransferase | 51 (72.9%) | |||||||
| Increased concentration | 41 (80.4%) | 32 (78.0%) | 9 (22.0%) | 0.165 | 5 (12.2%) | 36 (87.8%) | 0.569 | |
| Normal | 10 (19.6%) | 5 (50.0%) | 5 (50.0%) | 0 (-) | 10 (100%) | |||
| Lipase | 55 (78.6%) | |||||||
| Increased concentration | 50 (90.9%) | 34 (68.0%) | 16 (32.0%) | 0.963 | 4 (8.0%) | 46 (92.0%) | 0.941 | |
| Normal | 5 (9.1%) | 4 (80.0%) | 1 (20.0%) | 1 (20.0%) | 4 (80.0%) | |||
| Amylase, increased concentration | 61 (87.1%) | |||||||
| Increased concentration | 56 (91.8%) | 39 (69.6%) | 17 (30.4%) | 1.000 | 5 (8.9%) | 51 (91.1%) | 1.000 | |
| Normal | 5 (8.2%) | 4 (80.0%) | 1 (20.0%) | 0 (-) | 5 (100%) | |||
| Variable | No. | Mean | Standard Deviation | p Value * | |
|---|---|---|---|---|---|
| Blood Glucose Levels (mmol/L) | Total | 23 | 7.71 | 4.28 | <0.001 |
| AIP | 15 | 6.39 | 2.12 | 0.087 | |
| ANP | 8 | 10.18 | 6.15 | ||
| HD | 19 | 8.08 | 4.57 | 0.188 | |
| Deaths | 4 | 5.94 | 1.99 | ||
| Creatinine (µmol/L) | Total | 34 | 162.94 | 154.81 | <0.001 |
| AIP | 21 | 137.78 | 140.12 | 0.138 | |
| ANP | 13 | 203.59 | 174.01 | ||
| HD | 29 | 134.31 | 121.40 | 0.050 | |
| Deaths | 5 | 329.02 | 232.65 | ||
| Bilirubin (µmol/L) | Total | 27 | 71.38 | 67.00 | <0.001 |
| AIP | 19 | 63.39 | 62.98 | 0.217 | |
| ANP | 8 | 90.35 | 79.92 | ||
| HD | 23 | 77.01 | 71.95 | 0.303 | |
| Deaths | 4 | 38.99 | 21.53 | ||
| White Blood Cell (WBC) Total Count at Admission (cells/µL) | Total | 53 | 8170.20 | 6977.80 | <0.001 |
| AIP | 36 | 6383.61 | 4616.86 | 0.039 | |
| ANP | 17 | 11,953.53 | 9445.86 | ||
| HD | 47 | 7896.17 | 5912.37 | 0.924 | |
| Deaths | 6 | 10,316.67 | 13,361.95 | ||
| WBC Count (lowest level documented, cells/µL) | Total | 20 | 4618.50 | 3500.00 | 0.084 |
| AIP | 16 | 4038.75 | 2497.93 | 0.099 | |
| ANP | 4 | 6937.50 | 2988.14 | ||
| HD | 18 | 4415.00 | 2683.403 | 0.263 | |
| Deaths | 2 | 6450.00 | 4171.930 | ||
| WBC Count (peak level, cells/µL) | Total | 21 | 11,361.90 | 5562.11 | 0.006 |
| AIP | 17 | 10,783.53 | 5960.97 | 0.172 | |
| ANP | 4 | 13,820.00 | 2604.80 | ||
| HD | 19 | 11,197.89 | 5796.175 | 0.610 | |
| Deaths | 2 | 12,920.00 | 2941.564 | ||
| Lymphocyte Count at Admission (cells/µL) | Total | 18 | 2357.22 | 1595.00 | <0.001 |
| AIP | 15 | 2454.60 | 3429.93 | 0.654 | |
| ANP | 3 | 1870.33 | 1199.05 | ||
| HD | 16 | 2439.19 | 3301.862 | 0.732 | |
| Deaths | 2 | 1701.50 | 1982.020 | ||
| Platelets (cells/µL) | Total | 62 | 62,758.58 | 65,968.10 | <0.001 |
| AIP | 42 | 69,157.14 | 66,445.16 | 0.092 | |
| ANP | 20 | 49,321.60 | 64,528.77 | ||
| HD | 55 | 65,182.40 | 68,727.84 | 0.601 | |
| Deaths | 7 | 43,714.29 | 35,419.93 | ||
| Haematocrit (%) | Total | 33 | 42.59 | 9.48 | 0.170 |
| AIP | 24 | 43.27 | 9.42 | 0.506 | |
| ANP | 9 | 40.76 | 9.95 | ||
| HD | 29 | 42.01 | 9.58 | 0.360 | |
| Deaths | 4 | 46.73 | 8.75 | ||
| Hemoglobin (g/L) | Total | 49 | 128.13 | 34.49 | 0.425 |
| AIP | 34 | 132.35 | 32.73 | 0.200 | |
| ANP | 15 | 118.57 | 37.55 | ||
| HD | 44 | 131.47 | 32.29 | 0.168 | |
| Deaths | 5 | 98.80 | 43.19 | ||
| Albumin (g/L) | Total | 14 | 31.79 | 6.96 | 0.426 |
| AIP | 10 | 33.30 | 7.54 | 0.198 | |
| ANP | 4 | 28.00 | 3.56 | ||
| HD | 12 | 32.25 | 7.44 | 0.659 | |
| Deaths | 2 | 29.00 | 1.41 | ||
| Gamma Glutamyl Transpeptidase (IU/dL) | Total | 10 | 355.20 | 316.98 | 0.330 |
| AIP | 7 | 227.71 | 197.74 | 0.184 | |
| ANP | 3 | 652.67 | 381.08 | ||
| HD | 10 | 355.20 | 316.98 | - | |
| Deaths | 0a | - | - | ||
| Alkaline Phosphatase (IU/dL) | Total | 28 | 216.11 | 224.25 | <0.001 |
| AIP | 17 | 206.47 | 276.65 | 0.082 | |
| ANP | 11 | 231.00 | 113.68 | ||
| HD | 26 | 217.62 | 232.821 | 0.429 | |
| Deaths | 2 | 196.50 | 43.134 | ||
| Aspartate Transaminase (AST, IU/dL) | Total | 45 | 336.26 | 427.41 | <0.001 |
| AIP | 32 | 345.61 | 335.35 | 0.082 | |
| ANP | 13 | 313.25 | 615.21 | ||
| HD | 40 | 317.018 | 426.77 | 0.118 | |
| Deaths | 5 | 490.200 | 447.57 | ||
| Alanine Transferase (ALT, IU/dL) | Total | 51 | 268.56 | 451.55 | <0.001 |
| AIP | 37 | 312.01 | 509.30 | 0.057 | |
| ANP | 14 | 153.70 | 215.25 | ||
| HD | 46 | 270.659 | 468.37 | 0.747 | |
| Deaths | 5 | 249.200 | 283.34 | ||
| Amylase (U/L) | Total | 60 | 802.25 | 675.92 | 0.008 |
| AIP | 43 | 609.42 | 514.32 | 0.001 | |
| ANP | 17 | 1300.59 | 791.55 | ||
| HD | 55 | 766.18 | 661.65 | 0.145 | |
| Deaths | 5 | 1235.00 | 759.52 | ||
| Lipase (U/L) | Total | 54 | 1224.86 | 1404.21 | <0.001 |
| AIP | 38 | 1041.62 | 1212.20 | 0.315 | |
| ANP | 16 | 1660.06 | 1747.97 | ||
| HD | 49 | 1259.05 | 1432.63 | 0.321 | |
| Deaths | 5 | 889.80 | 1158.73 |
| ANP vs. AIP | Death vs. Survival | |
|---|---|---|
| HR (95%CI) | HR (95%CI) | |
| Any comorbidity | 0.360 (0.081; 1.602) | 1.218 (0.175; 8.472) |
| Any sign of collection | 5.544 (1.335; 23.024) | 15.531 (1.102; 218.816) |
| Any sign of organ failure | 2.538 (0.820; 7.853) | 9.030 (1.472; 55.405) |
| Hypotension | - | 7.216 (0.664; 78.416) |
| WBC range | - | |
| Normal | Reference | - |
| Leukocytosis | 7.990 (1.865; 32.273) | - |
| Leukopenia | 1.638 (0.354; 7.581) | - |
| ANP vs. AIP | - | 0.820 (0.107; 6.283) |
| Outcome (death) | 1.418 (0.275; 7.313) | - |
References
- Pourzangiabadi, M.; Najafi, H.; Fallah, A.; Goudarzi, A.; Pouladi, I. Dengue Virus: Etiology, Epidemiology, Pathobiology, and Developments in Diagnosis and Control—A Comprehensive Review. Infect. Genet. Evol. 2025, 127, 105710. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Chia, P.Y.; Low, J.G.; Ooi, E.E. Dengue and Severe Dengue. Clin. Microbiol. Rev. 2025; e0024424, epub ahead of print. [Google Scholar] [CrossRef]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.R.; Harris, E. The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue Virus Life Cycle: Viral and Host Factors Modulating Infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, N.; Bishoyi, A.K. Dengue: Epidemiology, Diagnosis Methods, Treatment Options, and Prevention Strategies. Arch. Virol. 2025, 170, 48. [Google Scholar] [CrossRef]
- Barrows, N.J.; Anglero-Rodriguez, Y.; Kim, B.; Jamison, S.F.; Le Sommer, C.; McGee, C.E.; Pearson, J.L.; Dimopoulos, G.; Ascano, M.; Bradrick, S.S.; et al. Dual Roles for the ER Membrane Protein Complex in Flavivirus Infection: Viral Entry and Protein Biogenesis. Sci. Rep. 2019, 9, 9711. [Google Scholar] [CrossRef] [PubMed]
- Lescar, J.; Soh, S.; Lee, L.T.; Vasudevan, S.G.; Kang, C.; Lim, S.P. The Dengue Virus Replication Complex: From RNA Replication to Protein-Protein Interactions to Evasion of Innate Immunity. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1062, pp. 115–129. [Google Scholar]
- Garcia-Blanco, M.A.; Vasudevan, S.G.; Bradrick, S.S.; Nicchitta, C. Flavivirus RNA Transactions from Viral Entry to Genome Replication. Antivir. Res. 2016, 134, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Luvira, V.; Thawornkuno, C.; Lawpoolsri, S.; Thippornchai, N.; Duangdee, C.; Ngamprasertchai, T.; Leaungwutiwong, P. Diagnostic Performance of Dengue NS1 and Antibodies by Serum Concentration Technique. Trop. Med. Infect. Dis. 2023, 8, 117. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue Virus: Epidemiology, Biology, and Disease Aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of Fifth Serotype of Dengue Virus (Denv-5): A New Public Health Dilemma in Dengue Control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Pagani, G.; Zanchetta, N.; Galimberti, L.; Oreni, L.; Passerini, S.; Giacomelli, A.; Cordier, L.; Gismondo, M.R.; Rizzardini, G.; Galli, M.; et al. Imported Dengue Fever: A 16-Years Retrospective Analysis in Milan (Italy) and a Brief Review of the European Literature. Infez. Med. 2020, 28, 243–252. [Google Scholar]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- Grobusch, M.P.; Díaz-Menéndez, M.; de Gomensoro, E.B.; Mächler, C.; Milovanović, B. The Burden of Dengue Fever in Travellers: A Systematic Literature Review. New Microbes New Infect. 2025, 67, 101631. [Google Scholar] [CrossRef] [PubMed]
- Naderian, R.; Eslami, M.; Ahmad, S.; Paraandavaji, E.; Yaghmayee, S.; Soltanipur, M.; Naderian, R.; Pajand, O.; Tajdini, P.; Alizadeh, A.; et al. Efficacy, Immune Response, and Safety of Dengue Vaccines in Adolescents: A Systematic Review. Rev. Med. Virol. 2025, 35, e70035. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Peruzzi, S.; Balzarini, F.; Zaniboni, A.; Ranzieri, S. Dengue Fever in Italy: The “Eternal Return” of an Emerging Arboviral Disease. Trop. Med. Infect. Dis. 2022, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Niaré, D.; Debin, M.; Blanchon, T. Aedes Albopictus (Tiger Mosquito) and Arboviroses: What to Expect in the Coming Years? Rev. Med. Interne 2025, 46, 229–235. [Google Scholar] [CrossRef]
- Logiudice, J.; Alberti, M.; Ciccarone, A.; Rossi, B.; Tiecco, G.; De Francesco, M.A.; Quiros-Roldan, E. Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens 2025, 14, 63. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge Gaps, Unmet Needs, and Research Priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Quam, M.B.; Khan, K.; Sears, J.; Hu, W.; Rocklöv, J.; Wilder-Smith, A. Estimating Air Travel-Associated Importations of Dengue Virus into Italy. J. Travel. Med. 2015, 22, 186–193. [Google Scholar] [CrossRef]
- Pang, T.; Mak, T.K.; Gubler, D.J. Prevention and Control of Dengue—The Light at the End of the Tunnel. Lancet Infect. Dis. 2017, 17, e79–e87. [Google Scholar] [CrossRef]
- Haider, N.; Hasan, M.N.; Onyango, J.; Billah, M.; Khan, S.; Papakonstantinou, D.; Paudyal, P.; Asaduzzaman, M. Global Dengue Epidemic Worsens with Record 14 Million Cases and 9,000 Deaths Reported in 2024. Int. J. Infect. Dis. 2025, 158, 107940. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhao, T.Y.; Wang, C.C.; He, Y.; Lu, H.Z.; Zhang, H.T.; Wang, L.M.; Zhang, M.; Li, C.X.; Deng, S.Q. Assessing the Global Dengue Burden: Incidence, Mortality, and Disability Trends over Three Decades. PLoS Negl. Trop. Dis. 2025, 19, e0012932. [Google Scholar] [CrossRef]
- Jessie, K.; Fong, M.Y.; Devi, S.; Lam, S.K.; Wong, K.T. Localization of Dengue Virus in Naturally Infected Human Tissues, by Immunohistochemistry and In Situ Hybridization. J. Infect. Dis. 2004, 189, 1411–1418. [Google Scholar] [CrossRef]
- Matusali, G.; Colavita, F.; Carletti, F.; Lalle, E.; Bordi, L.; Vairo, F.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Performance of Rapid Tests in the Management of Dengue Fever Imported Cases in Lazio, Italy 2014–2019. Int. J. Infect. Dis. 2020, 99, 193–198. [Google Scholar] [CrossRef]
- Begum, F.; Das, S.; Mukherjee, D.; Mal, S.; Ray, U. Insight into the Tropism of Dengue Virus in Humans. Viruses 2019, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO); Special Programme for Research and Training in Tropical Diseases (TDR). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control Treatment, Prevention and Control Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Ajlan, B.A.; Alafif, M.M.; Alawi, M.M.; Akbar, N.A.; Aldigs, E.K.; Madani, T.A. Assessment of the New World Health Organization’s Dengue Classification for Predicting Severity of Illness and Level of Healthcare Required. PLoS Negl. Trop. Dis. 2019, 13, e0007144. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Jiang, M.; Pan, C.Q.; Li, J.; Xu, L.G. The Global, Regional, and National Burden of Acute Pancreatitis in 204 Countries and Territories, 1990–2019. BMC Gastroenterol. 2021, 21, 332. [Google Scholar] [CrossRef]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Simons-Linares, C.R.; Imam, Z.; Chahal, P. Viral-Attributed Acute Pancreatitis: A Systematic Review. Dig. Dis. Sci. 2021, 66, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, G.; Nair, A.S.; Narayanan, V.A.; Balakrishnan, V.N. Acute Pancreatitis in Viral Infections, with Possible Progression to Chronic Pancreatitis. Indian J. Gastroenterol. 2008, 27, 162–164. [Google Scholar] [PubMed]
- de Andrade Vieira Alves, F.; Oliveira, L.d.L.S.; Salomão, N.G.; Provance, D.W.; Basilio-De-Oliveira, C.A.; Basílio-DeOliveira, R.; Moragas, L.J.; de Carvalho, J.J.; Mohana-Borges, R.; Rabelo, K.; et al. Cytokines and Inflammatory Mediators: Markers Involved in Interstitial Damage to the Pancreas in Two Dengue Fever Cases Associated with Acute Pancreatitis. PLoS ONE 2022, 17, e0262785. [Google Scholar] [CrossRef]
- Imam, Z.; Simons-Linares, C.R.; Chahal, P. Infectious Causes of Acute Pancreatitis: A Systematic Review. Pancreatology 2020, 20, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Estofolete, C.F.; de Oliveira Mota, M.T.; Bernardes Terzian, A.C.; de Aguiar Milhim, B.H.G.; Ribeiro, M.R.; Nunes, D.V.; Mourão, M.P.; Rossi, S.L.; Nogueira, M.L.; Vasilakis, N. Unusual Clinical Manifestations of Dengue Disease—Real or Imagined? Acta Trop. 2019, 199, 105134. [Google Scholar] [CrossRef]
- Ortiz Morales, C.M.; Girela Baena, E.L.; Olalla Muñoz, J.R.; Parlorio de Andrés, E.; López Corbalán, J.A. Radiology of Acute Pancreatitis Today: The Atlanta Classification and the Current Role of Imaging in Its Diagnosis and Treatment. Radiol. (Engl. Ed.) 2019, 61, 453–466. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Windsor, J.A.; Horvath, K.D.; et al. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Foster, B.R.; Jensen, K.K.; Bakis, G.; Shaaban, A.M.; Coakley, F.V. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. Radiographics 2016, 36, 675–687. [Google Scholar] [CrossRef]
- Zhao, K.; Adam, S.Z.; Keswani, R.N.; Horowitz, J.M.; Miller, F.H. Acute Pancreatitis: Revised Atlanta Classification and the Role of Cross-Sectional Imaging. Am. J. Roentgenol. 2015, 205, W32–W41. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.A.B.G.; Costa, L.B.; Bessa, P.P.d.N.; de Alcântara, G.F.T.; de Oliveira, J.L.; Silva, T.D.N.; Morais, G.d.F.L.; Neto, L.V.P.; Cavalcanti, L.P.G. Dengue Mimickers: Which Clinical Conditions Can Resemble Dengue Fever? Rev. Soc. Bras. Med. Trop. 2024, 57, e002062024. [Google Scholar] [CrossRef]
- Robert, S.; Diagbouga, P.S.; Djibougou, A.D.; Guy, D.; Bagnall, R.; Ravel, F. Dengue Diagnosis and Impact on Clinical Management: A Literature Review. PLoS Negl. Trop. Dis. 2025, 19, e0013196. [Google Scholar] [CrossRef]
- Yung, C.F.; Lee, K.S.; Thein, T.L.; Tan, L.K.; Gan, V.C.; Wong, J.G.X.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Dengue Serotype-Specific Differences in Clinical Manifestation, Laboratory Parameters and Risk of Severe Disease in Adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Bianchi, F.P.; Frisicale, E.M.; Guicciardi, S.; Fiacchini, D.; Tafuri, S.; et al. (Re-)Emergence of Oropouche Virus (OROV) Infections: Systematic Review and Meta-Analysis of Observational Studies. Viruses 2024, 16, 1498. [Google Scholar] [CrossRef]
- Ismail, O.Z.; Bhayana, V. Lipase or Amylase for the Diagnosis of Acute Pancreatitis? Clin. Biochem. 2017, 50, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Beaussac, M.; Luciano, L.; Savini, H.; Deniel, C.; Javelle, E.; Coton, T.; Simon, F. Primary Infectious Acute Pancreatitis. Pancreas 2020, 49, e55–e57. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New Insights into Acute Pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef]
- Mittal, N.; Oza, V.M.; Muniraj, T.; Kothari, T.H. Diagnosis and Management of Acute Pancreatitis. Diagnostics 2025, 15, 258. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Yazici, C.; Evans Phillips, A.; Forsmark, C.E. Diagnosis and Management of Acute Pancreatitis. Gastroenterology 2024, 167, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A. Inflammation in Acute and Chronic Pancreatitis. Curr. Opin. Gastroenterol. 2015, 31, 395–399. [Google Scholar] [CrossRef]
- Østergaard, A.A.; Sydenham, T.V.; Nybo, M.; Andersen, Å.B. Cerebrospinal Fluid Pleocytosis Level as a Diagnostic Predictor? A Cross-Sectional Study. BMC Clin. Pathol. 2017, 17, 15. [Google Scholar] [CrossRef]
- Spanier, B.W.M.; Nio, Y.; Van Der Hulst, R.W.M.; Tuynman, H.A.R.E.; Dijkgraaf, M.G.W.; Bruno, M.J. Practice and Yield of Early CT Scan in Acute Pancreatitis: A Dutch Observational Multicenter Study. Pancreatology 2010, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski Andreucci, B.; de Toledo-Mendes, J.; Gonçalves Dias, N.; Araújo Santana Tavares, M.; Bekhor, D.; Oliveira Pacheco, E.; Torres, U.S.; Talans, A.; D’Ippolito, G. Abdominal Imaging Findings in Tropical Endemic Diseases: A Pictorial Review. Abdom. Radiol. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological Quality and Synthesis of Case Series and Case Reports. Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Jusuf, H.; Sudjana, P.; Djumhana, A.; Abdurachman, S.A. DHF with Complication of Acute Pancreatitis Related Hyperglycemia: A Case Report. Southeast Asian J. Trop. Med. Public Health 1998, 29, 367–369. [Google Scholar]
- Alvarez, M.E.; Juan, S.; Rico, P.; RAMiREZ-RONDA, C.H. Dengue and Hepatic Failure. Am. J. Med. 1985, 79, 670–674. [Google Scholar] [CrossRef]
- Ridho, S.; Hidayat, S.; Kolopaking, M.S. Acute pancreatitis as a complication of dengue fever. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2000, 1, 29–32. [Google Scholar]
- Wijekoon, C.N.; Wijekoon, P. Case report dengue hemorrhagic fever presenting with acute pancreatitis. Southeast Asian J. Trop. Med. Public Health 2010, 41, 864–866. [Google Scholar] [PubMed]
- Derycke, T.; Levy, P.; Genelle, B.; Ruszniewski, P.; Merzeau, C. Pancréatite Aiguë Secondarie à Une Dengue. Gastroenterol. Clin. Biol. 2005, 29, 85–86. [Google Scholar] [CrossRef]
- Chen, T.-C.; Perng, D.-S.; Tsai, J.-J.; Lu, P.-L.; Chen, T.-P. Dengue Hemorrhagic Fever Complicated with Acute Pancreatitis and Seizure. J. Formos. Med. Assoc. 2004, 103, 865–868. [Google Scholar]
- Gonzalez-Fontal, G.R.; Felipe Henao-Martinez, A. Dengue Hemorrhagic Fever Complicated by Pancreatitis. Braz. J. Infect. Dis. 2011, 15, 490–492. [Google Scholar] [CrossRef]
- Sudulagunta, S.R.; Sodalagunta, M.B.; Sepehrar, M.; Raja, S.K.B.; Nataraju, A.S.; Kumbhat, M.; Sathyanarayana, D.; Gummadi, S.; Burra, H.K. Dengue Shock Syndrome. Oxf. Med. Case Rep. 2016, 2016, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Tsai, H.-C.; Lee, S.S.-J.; Lin, C.-K.; Huang, J.-S.; Chen, Y.-S. Case report dengue hemorrhagic fever presenting with hemorrhagic pancreatitis and an intramural hematoma of the duodenal wall: A case report and review of the literature. J. Trop. Med. Public Health 2013, 44, 400–408. [Google Scholar]
- Kodisinghe, S.K.; Fernando, A.H.N. A Case of Dengue Fever Complicated by Acute Pancreatitis. Galle Med. J. 2011, 16, 43–44. [Google Scholar] [CrossRef]
- Kumar, K.J.; Chandrashekar, A.; Basavaraja, C.K.; Kumar, H.C.K. Acute Pancreatitis Complicating Dengue Hemorrhagic Fever. Rev. Soc. Bras. Med. Trop. 2016, 49, 656–659. [Google Scholar] [CrossRef]
- Kumar, P.; Thapa, B.R.; Himral, H.; Kapil, V. Acute Pancreatitis in Dengue Fever. Indian. J. Pediatr. 2018, 85, 318–319. [Google Scholar] [CrossRef]
- Kumar, S.; Lakhiwal, R.; Bhandiwad, C.; Chhimpa, A.; Gupta, A. Dengue Fever Presenting as Acute Pancreatitis. Int. J. Res. Med. Sci. 2017, 5, 5476. [Google Scholar] [CrossRef]
- Jain, V.; Gupta, O.P.; Rao, T.; Rao, S. Acute Pancreatitis Complicating Severe Dengue. J. Glob. Infect. Dis. 2014, 6, 76–78. [Google Scholar] [CrossRef]
- Nawal, C.; Meena, P.; Shyam Chejara, R.; Jain, S.; Marker, S.; Tuteja, V. Dengue Fever as a Rare Cause of Acute Pancreatitis. J. Assoc. Physicians India 2018, 66, 82–83. [Google Scholar]
- Agrawal, A.; Jain, N.; Gutch, M.; Shankar, A. Acute Pancreatitis and Acute Respiratory Distress Syndrome Complicating Dengue Haemorrhagic Fever. BMJ Case Rep. 2011, 2011, bcr1020114891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.J.; Sharma, V.; Uddin, A.S.; Sharma, R.K.; Gupta, R.; Rana, S.S. Acute Pancreatitis Due to Dengue: Report of an Uncommon Complication and Literature Review. Trop. Gastroenterol. 2018, 39, 218–222. [Google Scholar]
- Mishra, A.; Saini, R.; Kallani, M. Case Report Acute Pancreatitis Associated with Dengue Fever: An Interesting and Rare Complication of Dengue Virus. Indian. J. Case Rep. 2019, 5, 29–32. [Google Scholar] [CrossRef]
- Devi, J.; Farooque, R.; Memon, M.S. Letter: Dengue Fever Complicated by Acute Pancreatitis. J. Gastrointest. Infect. 2022, 12, 080–082. [Google Scholar] [CrossRef]
- Krithika, A.P.; Ramya, R. Acute Pancreatitis: A Late Complication of Dengue Fever. Int. J. Contemp. Pediatr. 2018, 5, 676–677. [Google Scholar] [CrossRef]
- Seetharam, P.; Rodrigues, G. Dengue Fever Presenting as Acute Pancreatitis. Eurasian J. Med. 2010, 42, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Anam, A.M.; Rabbani, R.; Shumy, F. Expanded Dengue Syndrome: Three Concomitant Uncommon Presentations in the Same Patient. Trop. Dr. 2017, 47, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Anam, A.M.; Rabbani, R.; Shumy, F.; Polash, M.M.I. Subsequent Pancreatitis and Haemothorax in a Patient of Expanded Dengue Syndrome. Trop. Dr. 2016, 46, 40–42. [Google Scholar] [CrossRef]
- Bashir Bhatti, A.; Ali, F. Severe dengue complicated by acute pancreatitis. Int. J. Curr. Res. 2015, 7, 16160–16163. Available online: https://www.journalcra.com/article/severe-dengue-complicated-acute-pancreatitis (accessed on 30 September 2025).
- Iqbal, N.; Viswanathan, S.; Remalayam, B.; Muthu, V.; George, T. Pancreatitis and Mods Due to Scrub Typhus and Dengue Co-Infection. Trop. Med. Health 2012, 40, 19–21. [Google Scholar] [CrossRef]
- Simadibrata, M. Acute Pancreatitis in Dengue Hemorrhagic Fever. Acta Med. Indones. 2012, 44, 57–61. [Google Scholar] [PubMed]
- Correa, R.; Ortega-Loubon, C.; Zapata, L.; Armién, B.; Culquichicón, C. Dengue with Hemorrhagic Manifestations and Acute Pancreatitis: Case Report and Review. Cureus 2019, 11, e4895. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Liu, N.; Yu, F. Acute Pancreatisis in Dengue Fever: A Case Report. Clin. Emerg. J. 2020, 21, 504–506. [Google Scholar] [CrossRef]
- Teja Durbesula, A.; Usham, G.; Meriga, R.; Karnati, R.; Graduate Student, P.; Professor, A. A Rare Combination in Dengue Fever: Acute Pancreatitis with Normal Enzyme Levels. Int. J. Med. Health Sci. J. Home 2016, 5, 57–60. [Google Scholar]
- Dalugama, C.; Gawarammana, I.B. Dengue Hemorrhagic Fever Complicated with Transient Diabetic Ketoacidosis: A Case Report. J. Med. Case Rep. 2017, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, A.C.; Ong, Y.; Najmuddin Ghazi, A.F.; Rasidi, A.M. Acute Pancreatitis with Para-Duodenal Hematoma in Patient with Dengue Fever. Brunei Int. Med. J. 2016, 12, 176–180. [Google Scholar]
- Cunha, M.d.P.; Duarte-Neto, A.N.; Pour, S.Z.; Hajjar, L.A.; Frassetto, F.P.; Dolhnikoff, M.; Saldiva, P.H.d.N.; Zanotto, P.M.d.A. Systemic Dengue Infection Associated with a New Dengue Virus Type 2 Introduction in Brazil—A Case Report. BMC Infect. Dis. 2021, 21, 311. [Google Scholar] [CrossRef]
- Karoli, R.; Fatima, J.; Singh, G.; Maini, S. Acute Pancreatitis: An Unusual Complication of Dengue Fever. J. Assoc. Physicians India 2012, 6, 64–65. [Google Scholar]
- Nogueira, T.d.O.; Frainer, D.A.; Pisacane, L.; Altenburg, T.; Lemos, R.; Haritsch, F. Pancreatite Aguda Pelo Vírus Da Dengue Com Necessidade de Intervenção Cirúrgica. Brasília Médica 2022, 59, 1–5. [Google Scholar] [CrossRef]
- Arora, N.; Vojjala, N.; Singh, H.; Mohindra, R.; Suri, V.; Singh, A.K.; Bhalla, A.; Sachan, A. Acute Pancreatitis in the Spectrum of Expanded Dengue Syndrome. J. Clin. Infect. Dis. Soc. 2023, 1, 265–267. [Google Scholar] [CrossRef]
- Dutta, S.; Khullar, N. Atypical Presentation of Dengue Fever. Int. J. Contemp. Pediatr. 2025, 12, 1006–1010. [Google Scholar] [CrossRef]
- Gomes, R.R. Expanded Dengue Syndrome Presenting as Acute Pancreatitis. Clin. Med. Rev. Rep. 2021, 3, 01–06. [Google Scholar] [CrossRef]
- Ramindla, S.; Quaiser, S.; Parvez, A.; Khan, S.U.; Khan, R. Rare Presentations of a Common Infection. Ann. Med. Sci. Res. 2025, 4, 27–34. [Google Scholar] [CrossRef]
- Singh Lakra, M.; Lahiya, S.; Meshram, R.J.; Taksande, A.; Damke, S. A Rare Case of Unusual Presentation of Acute Necrotising Pancreatitis as a Presenting Feature of Dengue Haemorrhagic Fever in a Child. J. Pediatr. Neonatal Care 2022, 12, 20–22. [Google Scholar] [CrossRef]
- Rahman, S.; Wazib, A.; Bahar, T.; Irteeja, S. Acute Pancreatitis Complicating Dengue Fever—A Case Report. Sri Lanka J. Med. 2020, 29, 39. [Google Scholar] [CrossRef]
- Khataniar, H.; Vellankal, S. Acute Hemorrhagic Pancreatitis as a Rare Complication of Dengue Fever. ACG Case Rep. J. 2023, 10, e01152. [Google Scholar] [CrossRef]
- Khanal, K.; Poudel, S.; Ghimire, A.; Regmi, A.; Bhattarai, R.; Pandey, A. Dengue Fever Presenting as Acute Pancreatitis: A Case Series. J. Adv. Intern. Med. 2023, 11, 60–63. [Google Scholar] [CrossRef]
- Acherjya, G.K.; Tarafder, K.; Sayeed, M.A.; Ghosh, G.K.; Hossain, M.J.; Hossain, S.; Ali, M.; Kabir, M.A.; Chakrabortty, R. Dengue Presenting as a Case of Acute Pancreatitis—A Rare Case Report. Clin. Case Rep. 2023, 11, e6926. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.F.; Martorell, S.; Fernandez, Y.D.; Fourzans, G.; Galzenati, F.; Piccolo Ramos, E. Pancreatitis y dengue. Rev. Hosp. Durand 2024, 1, 48–51. [Google Scholar]
- Saber, S.; Alam, R.F.; Tarek, M.; Hossain, M.M. Dengue Fever Presenting with Acute Pancreatitis: A Rare Complication of Dengue Virus. Br. J. Med. Health Sci. (BJMHS) 2021, 3, 1182–1184. [Google Scholar]
- Biswas, U.; León-Ruiz, M.; Ghosh, R.; Joarder, U.; Islam, K.M.; Bheeman, R.; Benito-León, J. An Intriguing Case of Expanded Dengue Syndrome with Co-Existing Encephalitis, Pancreatitis, and Hepatitis: The Classic Thalamic “Double-Doughnut” Sign Revisited. Neurohospitalist 2024, 14, 316–321. [Google Scholar] [CrossRef]
- Islam, J.; Mondal, K.; Ghosh, S.K.; Datta, A.K.; Ghosh, S. A Rare Presentation of Dengue Fever: Bilateral Psoas Muscle Hematoma, Intrahepatic Cholestatic Hepatitis, Pancreatitis and Pancytopenia. Oxf. Med. Case Rep. 2023, 2023, omad115. [Google Scholar] [CrossRef]
- Kashyap, A.; Dhamala, M.; Dahal, A.; Obaidullah; Ghimire, S. Dengue Fever Presenting with Acute Pancreatitis: A Case Report. SAGE Open Med. Case Rep. 2024, 12, 1–3. [Google Scholar] [CrossRef]
- Naik, S.; Mahajan, S.; Talwar, D.; Jagtap, G. Acute Pancreatitis Complicating a Case of Dengue Fever: Double Trouble. Cureus 2021, 13, e19523. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, H.; Rehman, M.Z.; Fatima, N.; Ali, M.H. A Rare Confluence: Concurrent Dengue and Enteric Fever Presenting with Acute Pancreatitis and Pleural Effusion. J. Clin. Images Med. Case Rep. 2024, 5, 3401. [Google Scholar] [CrossRef]
- Alnuaimi, M.K.; Alsubai, J.A.; Alnuaimi, A. Pancreatitis as a Rare Complication of Dengue Fever: A Case Report and Review of the Literature. Cureus 2025, 17, e82725. [Google Scholar] [CrossRef]
- Nakazaki, J.C.F.; Cotera-Ramón, A.I.; Medina, R.G. Case Report: Acute Pancreatitis in a Patient with Dengue Fever. Iberoam. J. Med. 2024, 6, 98–102. [Google Scholar] [CrossRef]
- Flor, M.A.; Andrade, J.V.; Bucaram, J.A. Acute Pancreatitis Secondary to Dengue Fever: An Uncommon Presentation of a Common Endemic Illness. Case Rep. Infect. Dis. 2022, 2022, 9540705. [Google Scholar] [CrossRef]
- Thadchanamoorthy, V.; Dayasiri, K. Expanded Dengue Syndrome Presenting with Acute Liver Failure, Acute Kidney Injury, Pancreatic Involvement, Coagulopathy, and Multiple Intracranial Hemorrhages in a Young Child: A Case Report. J. Med. Case Rep. 2022, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Nathália Vilela Assis, M.; Lourdes Esteves Mesquita, C.; Bento Alves Esber Kanaan, B.; Mansur, G.; Clara Faustino Linhares, M.; Antônio Santos Anjo, V.; Naves de Resende, P. Abdome agudo e dengue, uma apresentação atípica: Relato de caso acute abdome and dengue, an atypical presentation: Case report. J. Cienc. Biomed. E Saude 2021, 6, 64–68. [Google Scholar]
- Arredondo-Nontol, M.; Arredondo-Nontol, R.; Fernández-Guzmán, D.; Ccami-Bernal, F.; Arredondo-Reto, M.N.; Cabrera-Hipólito, S.E.; Ugas-Charcape, C.F. Dengue as a Rare Cause of Acute Pancreatitis in Obese Child. Rev. Mex. Pediatr. 2022, 89, 254–258. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Nguyen, H.Q. A Rare Case of Acute Pancreatitis as Dengue Complication. Case Rep. Infect. Dis. 2023, 2023, 2619785. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Wasim, M.A.; Kazi, A.N.; Akber, H.; Sheikh, M.; Patel, M.J. A Curious Case of Expanded Dengue Syndrome. Trop. Dr. 2024, 54, 179–181. [Google Scholar] [CrossRef]
- Rodriguez Gonzalez, S.A.; Banegas, L.; Cantillano Quintero, E.M.; Domínguez-Rojas, J. Severe Dengue with Hyperinflammatory State and Associated Acute Pancreatitis. Case Rep. Infect. Dis. 2025, 2025, 8029446. [Google Scholar] [CrossRef]
- Kumar Das, K.; Basu, R. Dengue Pancreatitis with Ketoacidosis—A Rare Manifestation in Endemicity. Oxf. Med. Case Rep. 2024, 2024, omae148. [Google Scholar] [CrossRef]
- Jahan, Y.; Rahman, A. Management of Dengue Hemorrhagic Fever in a Secondary Level Hospital in Bangladesh: A Case Report. IDCases 2020, 21, e00880. [Google Scholar] [CrossRef]
- Prabhu, P.; K.C, G.; Eswarappa, M.; M.S, G.; M, R.; Yousuff, M.; V, H.; Sreeram, S.; S.N, S. WCN25-2960 rare case of dengue virus triggered graft versus host disease in a renal transplant recipient. Kidney Int. Rep. 2025, 10, S522. [Google Scholar] [CrossRef]
- Lee, S.L.; Yann Ng, C.; Sidhu, J. Infected Pancreatic Pseudocyst Following Severe Dengue Infection. Med. J. Malays. 2021, 76, 927–929. [Google Scholar]
- Basile, G.; Vacante, M.; Corsaro, A.; Evola, F.R.; Maugeri, G.; Barchitta, M.; Biondi, A.; Musumeci, G.; D’Agata, V.; Evola, G. Treatment of Acute Pancreatitis. Minerva Surg. 2025, 80, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zeng, Y.; Ai, L.; Wang, G.; Fu, Y. Clinical Predictors and Prevalence of Enteral Nutrition Intolerance in Acute Pancreatitis: An Updated Systematic Review and Meta-Analysis. Nutrients 2025, 17, 910. [Google Scholar] [CrossRef] [PubMed]
- Marchi, G.; Vianello, A.; Crisafulli, E.; Maroccia, A.; Crinò, S.F.; Pecori, S.; Zamboni, G.A.; Mazzaferri, F.; Tacconelli, E.; Girelli, D. Cytomegalovirus-Induced Gastrointestinal Bleeding and Pancreatitis Complicating Severe COVID-19 Pneumonia: A Paradigmatic Case. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020060. [Google Scholar] [CrossRef]
- Jayarajah, U.; Lahiru, M.; de Zoysa, I.; Seneviratne, S.L. Review Article Dengue Infections and the Surgical Patient. Am. J. Trop. Med. Hyg. 2021, 104, 52–59. [Google Scholar] [CrossRef]
- Mohan, K.; Malaiyan, J.; Nasimuddin, S.; Devasir, R.; Meenakshi-Sundaram, P.; Selvaraj, S.; Krishnasamy, B.; Gnanadesikan, S.; Karthikeyan, M.; Kandasamy, M.; et al. Clinical Profile and Atypical Manifestation of Dengue Fever Cases between 2011 and 2018 in Chennai, India. J. Fam. Med. Prim. Care 2020, 9, 1119. [Google Scholar] [CrossRef]
- Dinkar, A.; Singh, J. Dengue Infection in North India: An Experience of a Tertiary Care Center from 2012 to 2017. Tzu Chi Med. J. 2020, 32, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.I.; Chi, C.Y.; Wang, Y.P.; Chien, Y.W. Risks of Acute Cholecystitis, Acute Pancreatitis, and Acute Appendicitis in Patients with Dengue Fever: A Population-Based Cohort Study in Taiwan. Infect. Dis. Ther. 2023, 12, 1677–1693. [Google Scholar] [CrossRef]
- Ravi Shankar, B.; Bharani, I.; Viswanath Reddy, D.; Madhu Sudhan, E.; Reddy Vamsi Krishna, B.; Vijay Kumar, B.; Jhansi Rani, K. Acute Pancreatitis Complicating Dengue Fever—A Retrospective Study. JOP 2023, 24, 119–124. Available online: https://www.primescholars.com/articles/acute-pancreatitis-complicating-dengue-fever--a-retrospective-observational-study.pdf (accessed on 30 September 2025).
- Ricco, M.; Peruzzi, S.; Balzarini, F. Epidemiology of West Nile Virus Infections in Humans, Italy, 2012–2020: A Summary of Available Evidences. Trop. Med. Infect. Dis. 2021, 6, 61. [Google Scholar] [CrossRef]
- Ghweil, A.A.; Osman, H.A.; Khodeary, A.; Okasha, A.; Hassan, M.H. Relative Frequency of Acute Pancreatitis from Dengue Outbreaks as a Late Complication, in Egypt. Virusdisease 2019, 30, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Sunder, A.; Pathak, S. Clinicolaboratory Profile of Expanded Dengue Syndrome—Our Experience in a Teaching Hospital. J. Fam. Med. Prim. Care 2019, 8, 1022. [Google Scholar] [CrossRef]
- Majumdar, R.; Jana, C.K.; Ghosh, S.; Biswas, U. Clinical Spectrum of Dengue Fever in a Tertiary Care Centre with Particular Reference to Atypical Presentation in the 2012 Outbreak in Kolkata. J. Indian Med. Assoc. 2012, 110, 904–906. [Google Scholar]
- Ramos-De La Medina, A.; Remes-Troche, J.M.; González-Medina, M.F.; Anitúa-Valdovinos, M.d.M.; Cerón, T.; Zamudio, C.; Díaz-Vega, A. Síntomas Abdominales y Gastrointestinales Del Dengue. Análisis de Una Cohorte de 8.559 Pacientes. Gastroenterol. Hepatol. 2011, 34, 243–247. [Google Scholar] [CrossRef]
- Póvoa, T.F.; Oliveira, E.R.A.; Basílio-de-Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.A.; Salomão, N.G.; Mota, E.M.; Paes, M.V. Peripheral Organs of Dengue Fatal Cases Present Strong Pro-Inflammatory Response with Participation of IFN-Gamma-, TNF-Alphaand RANTES-Producing Cells. PLoS ONE 2016, 11, e0168973. [Google Scholar] [CrossRef]
- Rosser, A. Necrotizing Pancreatitis. Surgery 2021, 39, 730–735. [Google Scholar]
- Jayasundara, B.; Perera, L.; de Silva, A. Dengue Fever May Mislead the Surgeons When It Presents as an Acute Abdomen. Asian Pac. J. Trop. Med. 2017, 10, 15–19. [Google Scholar] [CrossRef]
- Setiawan, M.W.; Samsi, T.K.; Wulur, H.; Sugianto, D.; Pool, T.N. Epigastric Pain and Sonographic Assessment of the Pancreas in Dengue Hemorrhagic Fever. J. Clin. Ultrasound 1998, 26, 257–259. [Google Scholar] [CrossRef]
- Muniraj, T.; Dang, S.; Pitchumoni, C.S. Pancreatitis or not?—Elevated Lipase and Amylase in ICU Patients. J. Crit. Care 2015, 30, 1370–1375. [Google Scholar] [CrossRef]
- Lee, I.-K.; Khor, B.-S.; Kee, K.-M.; Yang, K.D.; Liu, J.-W. Hyperlipasemia/Pancreatitis in Adults with Dengue Hemorrhagic Fever. Pancreas 2007, 35, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Breslin, P.A.S. Salivary Amylase: Digestion and Metabolic Syndrome. Curr. Diabetes Rep. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Junjappa, R.; Handigund, M.; Kim, H.R.; Chae, H.J. The Imprint of Salivary Secretion in Autoimmune Disorders and Related Pathological Conditions. Autoimmun. Rev. 2018, 17, 376–390. [Google Scholar] [CrossRef]
- Techapornroong, M.; Kitjarak, R.; Chetanachan, M.; Nakaviroj, S. Dengue Hemorrhagic Fever with Acute Liver Failure, A Case Report with Total Plasma Exchange Therapy Case Report. J. Prapokklao Hosp. Clin. Med. Educ. Cent. 2016, 33, 230–235. [Google Scholar]
- Rana, S.S.; Chaudhary, V.; Sharma, R.; Sharma, V.; Chhabra, P.; Bhasin, D.K. Comparison of Abdominal Ultrasound, Endoscopic Ultrasound and Magnetic Resonance Imaging in Detection of Necrotic Debris in Walled-off Pancreatic Necrosis. Gastroenterol. Rep. 2015, 4, 50–53. [Google Scholar] [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; D’Onofrio, M.; Pozzi Mucelli, R. Ultrasonography of the Pancreas. Radiol. Clin. N. Am. 2012, 50, 395–406. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Barbi, E.; Dietrich, C.F.; Kitano, M.; Numata, K.; Sofuni, A.; Principe, F.; Gallotti, A.; Zamboni, G.A.; Mucelli, R.P. Pancreatic Multicenter Ultrasound Study (PAMUS). Eur. J. Radiol. 2012, 81, 630–638. [Google Scholar] [CrossRef]
- Burrowes, D.P.; Choi, H.H.; Rodgers, S.K.; Fetzer, D.T.; Kamaya, A. Utility of Ultrasound in Acute Pancreatitis. Abdom. Radiol. 2020, 45, 1253–1264. [Google Scholar] [CrossRef]
- Brizi, M.G.; Perillo, F.; Cannone, F.; Tuzza, L.; Manfredi, R. The Role of Imaging in Acute Pancreatitis. Radiol. Medica 2021, 126, 1017–1029. [Google Scholar] [CrossRef]
- Sayre, J.W.; Toklu, H.Z.; Ye, F.; Mazza, J.; Yale, S. Case Reports, Case Series—From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education. Cureus 2017, 9, e1546. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahman, F.K.; binti Wan Puteh, S.E.; bin Zainuddin, M.A. E-Dengue System Insights: Exploring the Factors Influencing Dengue-Related Deaths in an Urbanized State in a Low-Middle Income Country (LMIC). BMC Public Health 2024, 24, 3055. [Google Scholar] [CrossRef]
- Srisawat, N.; Thisyakorn, U.; Ismail, Z.; Rafiq, K.; Gubler, D.J. World Dengue Day: A Call for Action. PLoS Negl. Trop. Dis. 2022, 16, e0010586. [Google Scholar] [CrossRef] [PubMed]
- De Pretis, N.; Capuano, F.; Amodio, A.; Pellicciari, M.; Casetti, L.; Manfredi, R.; Zamboni, G.; Capelli, P.; Negrelli, R.; Campagnola, P.; et al. Clinical and Morphological Features of Paraduodenal Pancreatitis: An Italian Experience with 120 Patients. Pancreas 2017, 46, 489–495. [Google Scholar] [CrossRef]
- Pillay, K.; Keddie, S.H.; Fitchett, E.; Akinde, C.; Bärenbold, O.; Bradley, J.; Falconer, J.; Keogh, R.H.; Lim, Z.N.; Nezafat Maldonado, B.; et al. Evaluating the Performance of Common Reference Laboratory Tests for Acute Dengue Diagnosis: A Systematic Review and Meta-Analysis of RT-PCR, NS1 ELISA, and IgM ELISA. Lancet Microbe 2025, 6, 101088. [Google Scholar] [CrossRef]
- Rathore, A.P.; Farouk, F.S.; St. John, A.L. Risk Factors and Biomarkers of Severe Dengue. Curr. Opin. Virol. 2020, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sudhish Nimmagadda, S.; Mahabala, C.; Boloor, A.; Manibettu Raghuram, P.; Akshatha Nayak, U. Atypical Manifestations of Dengue Fever—Where Do We Stand Today? J. Clin. Diagn. Res. 2014, 8, 71–73. [Google Scholar] [CrossRef]
- Horstick, O.; Runge-Ranziger, S. WHO 2009 guidelines and case classification: An update and short report. Southeast Asian J. Trop. Med. Public Health 2017, 48, 68–74. [Google Scholar]
| Database | Search Strategy | Entries |
|---|---|---|
| PubMed | (“Dengue”[Mesh] OR “Severe Dengue”[Mesh] OR “Dengue Virus”[Mesh]) AND (“Pancreatitis”[Mesh] OR “pancreatitis” OR “acute pancreatitis” OR “necrotizing pancreatitis” OR “interstitial pancreatitis” OR “hemorrhagic pancreatitis”) | 32 |
| EMBASE | (‘dengue’/exp OR ‘dengue’ OR ‘dengue virus’) AND (‘acute pancreatitis’ OR ‘acute hemorrhagic pancreatitis’ OR ‘acute interstitial pancreatitis’) | 77 |
| Scopus | ((acute) OR (interstitial) OR (necrotizing)) AND (Pancreatitis) AND ((Dengue) OR (Severe Dengue) OR (Dengue Virus)) | 96 |
| MedRxiv | 2 | |
| BioRxiv | 7 |
| Reference | Year | Country | Age (years) | Gender | Day 1 | Additional Diagnoses | Dengue Diagnosis | Severity | Class. | CT Score (0–10) | Outcome | Quality 2 (0–6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acherjya et al., 2023 [100] | 2022 | Bangladesh | 55 | Male | 3 | - | NS1 | M | Int. | 4 | Survived | 6 |
| Agrawal et al., 2011 [73] | 2011 | India | 38 | Male | 10 | - | IgM | S | Nec. | 6 | Survived | 6 |
| Ahmed et al., 2024 [115] | 2024 | Pakistan | 20 | Male | 1 | - | NS1 | S | N.A. | N.A. | Survived | 4 |
| Alnuaimi et al., 2025 [108] | 2024 | United Arab Emirates | 44 | Female | 5 | - | PCR | M | Int. | 2 | Survived | 6 |
| Alvarez et al., 1985 [59] | 1981 | United States | 51 | Male | 1 | HA | MS | Nec. | - | Survived | 3 | |
| Anam et al., 2016 [80] | 2013 | Bangladesh | 20 | Male | 8 | - | IgM, IgG | S | Int. | 2 | Survived | 5 |
| Anam et al., 2017 [79] | 2015 | Bangladesh | 44 | Male | 1 | - | NS1 | M | Int. | N.A. | Survived | 6 |
| Arora et al., 2023 [92] | 2023 | India | 47 | Male | 3 | - | NS1, IgM | MS | Int. | 4 | Survived | 4 |
| 2023 | India | 63 | Female | 5 | DM, HPT, OC | IgM | MS | Nec. | 6 | Survived | 5 | |
| 2023 | India | 45 | Male | 6 | HPT | IgM | S | Nec. | 8 | Death | 5 | |
| Arredondo-Nontol et al., 2022 [113] | 2022 | Peru | 13 | Male | 3 | OBS | NS1 | MS | Int. | 4 | Survived | 6 |
| Bashir Bhatti et al., 2015 [81] | 2015 | India | 16 | Female | 3 | - | NS1 | M | Int. | 1 | Survived | 6 |
| Beaussac et al., 2015 [49] | 2020 | France (European) | 18 | Female | 7 | - | IgM | M | Int. | 0 | Survived | 3 |
| Biswas et al., 2024 [103] | 2024 | India | 35 | Female | 5 | ENC, HEP | IgM | S | Int. | 4 | Survived | 6 |
| Che Jusoh et al., 2016 [88] | 2015 | Brunei | 22 | Male | 3 | - | PCR, NS1 | MS | Int. | 4 | Survived | 5 |
| Chen et al., 2004 [63] | 2004 | Taiwan | 66 | Female | DM, seizures | IgM, IgG | MS | Nec. | 6 | Survived | 3 | |
| Correa et al., 2019 [84] | 2018 | Panama | 37 | Female | 5 | - | PCR, IgM | M | Int. | - | Survived | 6 |
| Dalugama et al., 2017 [87] | 2016 | Sri Lanka | 26 | Male | 3 | - | NS1, IgM, IgG | M | Nec. | - | Survived | 5 |
| de Andrade et al., 2022 [35] | 2002 | Brazil | 63 | Male | 1 | DM | IgM | MS | Nec. | 6 | Death | 6 |
| 2002 | Brazil | 21 | Female | 8 | OBS | IgM | S | Nec. | - | Death | 4 | |
| Derycke et al., 2005 [62] | 2005 | France (Nouvelle Caledonie) | 29 | Male | 1 | - | PCR, IgM, IgG | MS | Int. | 2 | Survived | 6 |
| Devi et al., 2022 [76] | 2019 | Pakistan | 35 | Male | 3 | - | IgM | M | Int. | - | Survived | 4 |
| dos Passos Cunha et al., 2021 [89] | 2019 | Brazil | 58 | Male | 3 | TB, cancer (thymoma), pleuropneumonectomy | PCR | S | Int. | 2 | Death | 6 |
| Duarte et al., 2024 [101] | 2023 | Argentina | 28 | Male | 14 | Previous removal of gallbladder | NS1 | M | Int. | - | Survived | 4 |
| Dutta et al., 2025 [93] | 2025 | India | 4 | Male | 1 | NS1 | S | Nec. | 6 | Survived | 6 | |
| Flor et al., 2022 [110] | 2021 | Ecuador | 26 | Male | 5 | Alcohol (abuse) | IgM, IgG | M | Int. | - | Survived | 6 |
| Gomes et al., 2021 [94] | 2021 | Bangladesh | 35 | Female | 4 | - | NS1 | M | Int. | - | Survived | 6 |
| Gonzalez-Fontal et al., 2011 [64] | 2009 | Colombia | 27 | Male | 4 | ERSD, DM | IgM | M | Int. | 4 | Survived | 6 |
| Iqbal et al., 2012 [82] | 2012 | India | 40 | Female | 4 | Scrub typhus | IgM | M | Int. | 4 | Survived | 6 |
| Islam et al., 2023 [104] | 2023 | India | 25 | Male | 4 | - | NS1 | M | Nec. | - | Survived | 6 |
| Jahan et al., 2020 [118] | 2020 | Bangladesh | 42 | Male | 3 | - | IgM | M | Int. | - | Survived | 6 |
| Jain et al., 2014 [71] | 2014 | India | 27 | Male | 4 | Sickle cell | NS1 | S | Nec. | N.A. | Death | 6 |
| Jusuf et al., 1998 [58] | 1997 | Indonesia | 25 | Female | 5 | - | IgM, IgG | S | Nec. | 8 | Survived | 4 |
| Karoli et al., 2012 [90] | 2012 | India | 35 | Female | - | NS1 | MS | Nec. | - | Survived | 3 | |
| Kashyap et al., 2024 [105] | 2024 | Nepal | 24 | Female | 4 | - | IgM | M | Int. | 2 | Survived | 6 |
| Khanal et al., 2023 [99] | 2022 | Nepal | 25 | Male | - | NS1 | M | Int. | - | Survived | 5 | |
| 2022 | Nepal | 51 | Male | - | NS1 | M | Nec. | 6 | Survived | 4 | ||
| Khataniar et al., 2023 [98] | 2023 | India | 28 | Female | 2 | - | IgM, IgG | S | Nec. | 8 | Survived | 6 |
| Kodisinghe et al., 2011 [67] | 2011 | Sri Lanka | 35 | Male | 4 | - | IgM | M | Int. | - | Survived | 3 |
| Krithika et al., 2018 [77] | 2017 | India | 11 | Male | 7 | - | IgM, IgG | M | Int. | 4 | Survived | 2 |
| Kumar Das et al., 2024 [117] | 2023 | India | 26 | Male | 3 | - | NS1 | MS | Int. | 6 | Survived | 6 |
| Kumar et al., 2016 [68] | 2016 | India | 10 | Female | 1 | AIHA | NS1, IgM | MS | Int. | - | Survived | 6 |
| Kumar et al., 2017 [70] | 2017 | India | 32 | Female | 4 | - | IgM | M | Int. | - | Survived | 6 |
| Kumar et al., 2018 [69] | 2018 | India | 8 | Female | 3 | - | NS1, IgG | M | Int. | 2 | Survived | 6 |
| Lee et al., 2013 [66] | 2013 | Taiwan | 47 | Male | 6 | HBV | IgM, IgG | S | Nec. | 8 | Survived | 6 |
| Lee et al., 2021 [120] | 2021 | Malaysia | 31 | Female | 10 | psoas muscle hematoma, cholestatic HEP | NS1 | MS | Nec. | 6 | Survived | 4 |
| Lu et al., 2020 [85] | 2019 | China | 33 | Female | 5 | - | PCR, NS1, IgM | M | Int. | 2 | Survived | 6 |
| Mishra et al., 2019 [75] | 2019 | India | 17 | Female | 5 | - | IgM | S | Nec. | 10 | Death | 6 |
| Naik et al., 2021 [106] | 2021 | India | 21 | Male | 3 | - | NS1 | M | Int. | 2 | Survived | 6 |
| Nakazaki et al., 2024 [109] | 2023 | Peru | 18 | Female | 5 | - | NS1 | M | Int. | 4 | Survived | 6 |
| Nawal et al., 2018 [72] | 2016 | India | 25 | Male | 3 | - | NS1 | M | Int. | 4 | Survived | 6 |
| Nguyen et al., 2023 [114] | 2023 | Vietnam | 31 | Male | 2 | - | NS1 | M | Int. | 8 | Survived | 5 |
| Nogueira et al., 2022 [91] | 2022 | Brazil | 41 | Male | 2 | Previous pancreatitis (EBV) | NS1, IgM | S | Nec. | 8 | Survived | 6 |
| Prabhu et al., 2025 [119] | 2025 | India | 34 | Male | 60 | Kidney transplant, CRD | N.P. | S | Nec. | 10 | Death | 3 |
| Rahman et al., 2020 [97] | 2021 | Bangladesh | 32 | Female | 2 | - | NS1 | M | Int. | - | Survived | 6 |
| Rajesh et al., 2008 [34] | 2008 | India | 16 | Female | 21 | Cassava | IgM | M | Int. | 2 | Survived | 3 |
| Ramindla et al., 2025 [95] | 2025 | India | 17 | Male | 10 | - | IgM | M | Int. | 6 | Survived | 6 |
| Ridho et al., 2000 [60] | 2000 | Indonesia | 28 | Male | 2 | - | IgM | M | Int. | - | Survived | 6 |
| Rodriguez Gonzalez et al., 2025 [116] | 2025 | Peru | 17 | Female | 4 | - | NS1, IgM, IgG | M | Int. | 2 | Survived | 6 |
| Saber et al., 2021 [102] | 2021 | Bangladesh | 47 | Male | 4 | - | NS1 | MS | Int. | - | Survived | 6 |
| Seetharam et al., 2010 [78] | 2010 | India | 56 | Male | 1 | - | IgM | M | Int. | 2 | Survived | 4 |
| Sharma et al., 2018 [74] | 2018 | India | 32 | Male | 4 | - | NS1 | S | Nec. | 6 | Survived | 6 |
| Simadibrata et al., 2012 [83] | 2012 | Indonesia | 59 | Male | 4 | - | IgM, IgG | M | Int. | 4 | Survived | 6 |
| Singh Lakra et al., 2022 [96] | 2022 | India | 15 | Male | 5 | - | NS1, IgM | S | Nec. | 8 | Survived | 5 |
| Sudulagunta et al., 2016 [65] | 2016 | India | 30 | Male | 6 | DM | PCR, NS1, IgM | S | Nec. | 6 | Survived | 6 |
| Teja Derbesula et al., 2016 [86] | 2016 | India | 36 | Male | 20 | - | NS1 | M | Nec. | 6 | Survived | 6 |
| Thadchanamoorthy et al., 2022 [111] | 2022 | Sri Lanka | 6 | Female | 5 | - | NS1 | MS | Int. | - | Death | 5 |
| Ullah et al., 2025 [107] | 2024 | Pakistan | 25 | Male | 14 | - | NS1 | M | Int. | - | Survived | 5 |
| Vilela Assis et al., 2021 [112] | 2020 | Brazil | 24 | Female | 2 | - | IgM | M | Int. | 0 | Survived | 6 |
| Wijekoon et al., 2010 [61] | 2009 | Sri Lanka | 47 | Male | 7 | Alcohol (occasional), surgery, asthma | IgM, IgG | MS | Int. | - | Survived | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Cascio, A.; Pipitò, L.; Bottazzoli, M.; Manzoni, P.; Brandolisio, L.R.; Nobili, C.; Giuri, P.G. Dengue and Acute Pancreatitis: A Systematic Review. Trop. Med. Infect. Dis. 2025, 10, 330. https://doi.org/10.3390/tropicalmed10120330
Riccò M, Cascio A, Pipitò L, Bottazzoli M, Manzoni P, Brandolisio LR, Nobili C, Giuri PG. Dengue and Acute Pancreatitis: A Systematic Review. Tropical Medicine and Infectious Disease. 2025; 10(12):330. https://doi.org/10.3390/tropicalmed10120330
Chicago/Turabian StyleRiccò, Matteo, Antonio Cascio, Luca Pipitò, Marco Bottazzoli, Paolo Manzoni, Lilian Romina Brandolisio, Cecilia Nobili, and Pasquale Gianluca Giuri. 2025. "Dengue and Acute Pancreatitis: A Systematic Review" Tropical Medicine and Infectious Disease 10, no. 12: 330. https://doi.org/10.3390/tropicalmed10120330
APA StyleRiccò, M., Cascio, A., Pipitò, L., Bottazzoli, M., Manzoni, P., Brandolisio, L. R., Nobili, C., & Giuri, P. G. (2025). Dengue and Acute Pancreatitis: A Systematic Review. Tropical Medicine and Infectious Disease, 10(12), 330. https://doi.org/10.3390/tropicalmed10120330