Review of the Canadian Nontuberculous Mycobacterial Disease Landscape—Challenges and Opportunities
Abstract
1. Introduction
2. Historical Context and Epidemiology of NTM Disease in Canada
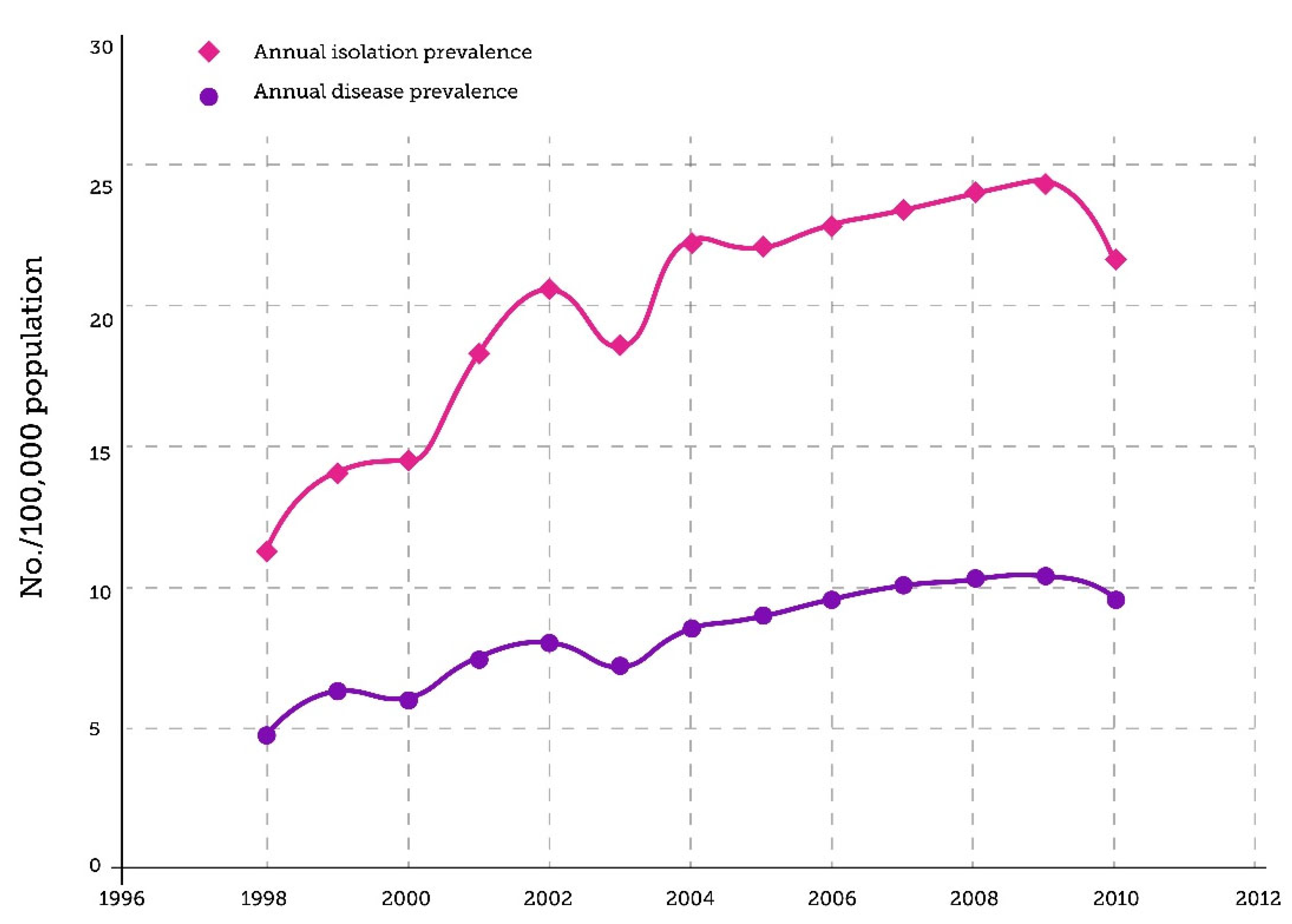
2.1. NTM Pulmonary Disease
2.2. Extrapulmonary NTM Disease
3. Evolution of NTM Therapies
4. Role of Non-Medication Treatment in the Management of NTM Disease
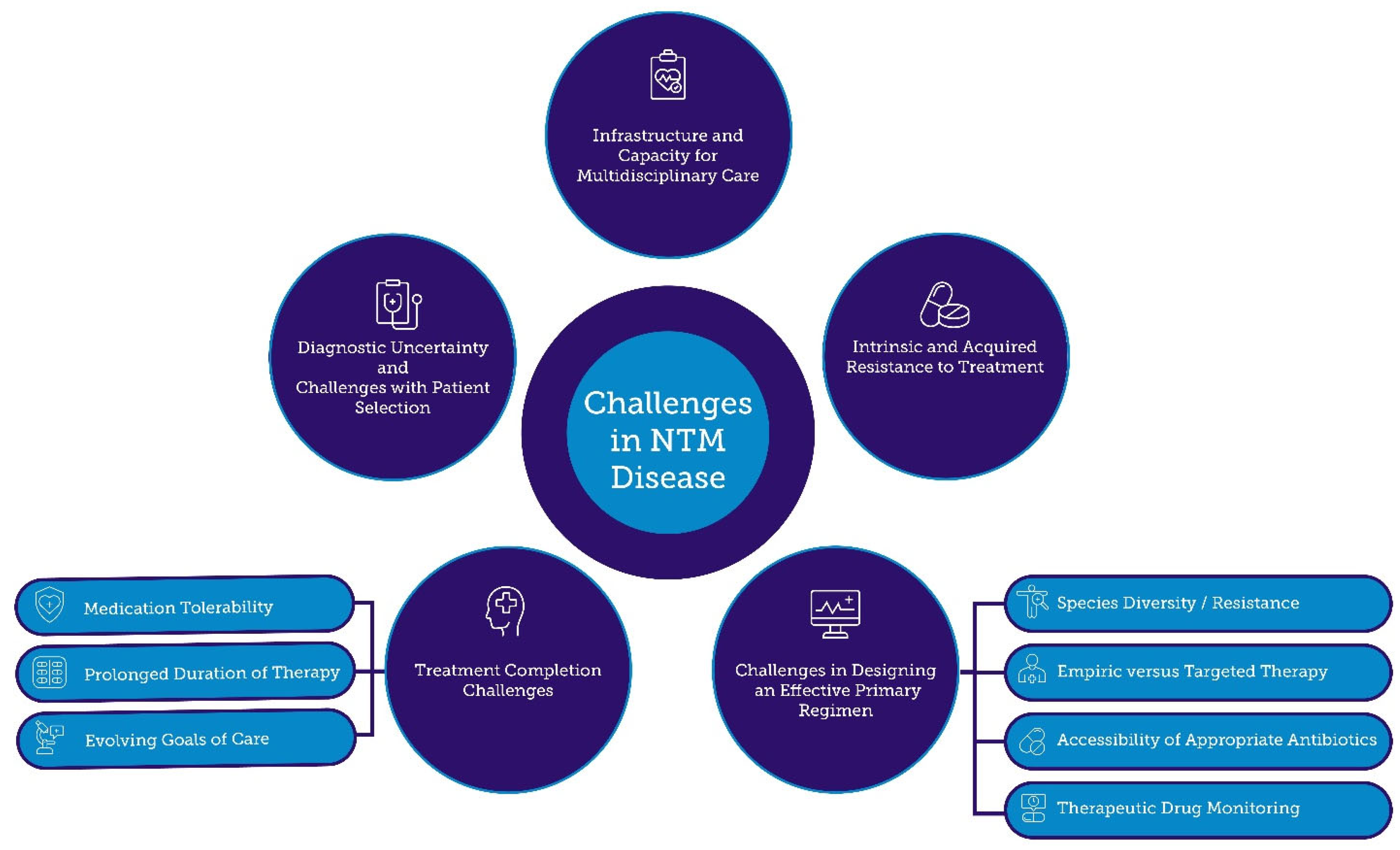
5. Challenges in NTM Treatment
5.1. Challenges with Patient Selection
5.2. Resistance to Antibiotic Treatment
5.2.1. Macrolide Resistance
5.2.2. Aminoglycoside Resistance
5.2.3. Rifamycin Resistance
5.2.4. Fluoroquinolone Resistance
5.2.5. Clofazimine Resistance
5.3. Challenges in Designing an Effective Regimen
5.3.1. Species Diversity and Limited Evidence
5.3.2. Empiric Versus Targeted Therapy
5.3.3. Accessibility of Appropriate Antibiotics
5.3.4. Therapeutic Drug Monitoring: Utility and Limitations in NTM Care
5.4. Treatment Completion Challenges
5.5. Infrastructure and Capacity for Multidisciplinary Care
6. Opportunities—Emerging Therapies and Precision Approaches in NTM-PD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marras, T.K.; Mendelson, D.; Marchand-Austin, A.; May, K.; Jamieson, F.B. Pulmonary Nontuberculous Mycobacterial Disease, Ontario, Canada, 1998–2010. Emerg. Infect. Dis. 2013, 19, 1889–1891. [Google Scholar] [CrossRef]
- Brode, S.K.; Marchand-Austin, A.; Jamieson, F.B.; Marras, T.K. Pulmonary versus Nonpulmonary Nontuberculous Mycobacteria, Ontario, Canada. Emerg. Infect. Dis. 2017, 23, 1898–1901. Available online: http://wwwnc.cdc.gov/eid/article/23/11/17-0959_article.htm (accessed on 2 August 2025). [CrossRef] [PubMed]
- Dahl, V.N.; Mølhave, M.; Fløe, A.; Van Ingen, J.; Schön, T.; Lillebaek, T.; Andersen, A.B.; Wejse, C. Global Trends of Pulmonary Infections with Nontuberculous Mycobacteria: A Systematic Review. Int. J. Infect. Dis. 2022, 125, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Cowman, S.; Van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-Tuberculous Mycobacterial Pulmonary Disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- Pennington, K.M.; Vu, A.; Challener, D.; Rivera, C.G.; Shweta, F.N.U.; Zeuli, J.D.; Temesgen, Z. Approach to the Diagnosis and Treatment of Non-Tuberculous Mycobacterial Disease. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 24, 100244. [Google Scholar] [CrossRef]
- Malhotra, A.M.; Arias, M.; Backx, M.; Gadsby, J.; Goodman, A.; Gourlay, Y.; Milburn, H.; Moncayo-Nieto, O.L.; Shimmin, D.; Dedicoat, M.; et al. Extrapulmonary Nontuberculous Mycobacterial Infections: A Guide for the General Physician. Clin. Med. 2024, 24, 100016. [Google Scholar] [CrossRef]
- Conyers, L.E.; Saunders, B.M. Treatment for Non-Tuberculous Mycobacteria: Challenges and Prospects. Front. Microbiol. 2024, 15, 1394220. [Google Scholar] [CrossRef]
- Andréjak, C.; Thomsen, V.Ø.; Johansen, I.S.; Riis, A.; Benfield, T.L.; Duhaut, P.; Sørensen, H.T.; Lescure, F.-X.; Thomsen, R.W. Nontuberculous Pulmonary Mycobacteriosis in Denmark: Incidence and Prognostic Factors. Am. J. Respir. Crit. Care Med. 2010, 181, 514–521. [Google Scholar] [CrossRef]
- Lai, C.-C.; Tan, C.-K.; Chou, C.-H.; Hsu, H.-L.; Liao, C.-H.; Huang, Y.-T.; Yang, P.-C.; Luh, K.-T.; Hsueh, P.-R. Increasing Incidence of Nontuberculous Mycobacteria, Taiwan, 2000–2008. Emerg. Infect. Dis. 2010, 16, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Kruijshaar, M.E.; Ormerod, L.P.; Drobniewski, F.; Abubakar, I. Increasing Reports of Non-Tuberculous Mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health 2010, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, C.-H.; Lee, S.-M.; Yang, S.-C.; Yoo, C.-G.; Kim, Y.W.; Han, S.K.; Shim, Y.-S.; Yim, J.-J. Rapid Increase of Non-Tuberculous Mycobacterial Lung Diseases at a Tertiary Referral Hospital in South Korea. Int. J. Tuberc. Lung Dis. 2010, 14, 1069–1071. [Google Scholar] [PubMed]
- Taiwo, B.; Glassroth, J. Nontuberculous Mycobacterial Lung Diseases. Infect. Dis. Clin. N. Am. 2010, 24, 769–789. [Google Scholar] [CrossRef]
- Donohue, M.J.; Wymer, L. Increasing Prevalence Rate of Nontuberculous Mycobacteria Infections in Five States, 2008–2013. Ann. ATS 2016, 13, 2143–2150. [Google Scholar] [CrossRef]
- Diel, R.; Jacob, J.; Lampenius, N.; Loebinger, M.; Nienhaus, A.; Rabe, K.F.; Ringshausen, F.C. Burden of Non-Tuberculous Mycobacterial Pulmonary Disease in Germany. Eur. Respir. J. 2017, 49, 1602109. [Google Scholar] [CrossRef]
- Thomson, R.; Donnan, E.; Konstantinos, A. Notification of Nontuberculous Mycobacteria: An Australian Perspective. Ann. ATS 2017, 14, 318–323. [Google Scholar] [CrossRef]
- Queensland Health. 2024. Notifiable Conditions Annual Reporting. Queensland Government Website. Available online: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/surveillance/reports/notifiable/annual (accessed on 20 December 2024).
- Marshall, J.E.; Mercaldo, R.A.; Lipner, E.M.; Prevots, D.R. Nontuberculous Mycobacteria Testing and Culture Positivity in the United States. BMC Infect. Dis. 2024, 24, 288. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Xue, Y.; Huo, F.; Jia, J.; Dong, L.; Zhao, L.; Jiang, G.; Huang, H. Doubled Nontuberculous Mycobacteria Isolation as a Consequence of Changes in the Diagnosis Algorithm. Infect. Drug Resist. 2022, 15, 3347–3355. [Google Scholar] [CrossRef]
- Schiff, H.F.; Jones, S.; Achaiah, A.; Pereira, A.; Stait, G.; Green, B. Clinical Relevance of Non-Tuberculous Mycobacteria Isolated from Respiratory Specimens: Seven Year Experience in a UK Hospital. Sci. Rep. 2019, 9, 1730. [Google Scholar] [CrossRef]
- Prevots, D.R.; Marras, T.K. Epidemiology of Human Pulmonary Infection with Nontuberculous Mycobacteria: A Review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Al Houqani, M.; Jamieson, F.; Chedore, P.; Mehta, M.; May, K.; Marras, T.K. Isolation Prevalence of Pulmonary Nontuberculous Mycobacteria in Ontario in 2007. Can. Respir. J. 2011, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.R. Environmental Sources and Transmission of Nontuberculous Mycobacteria. Clin. Chest Med. 2023, 44, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; De Giacomi, F.; Bodini, B.D.; Stainer, A.; Fumagalli, A.; Bini, F.; Luppi, F.; Aliberti, S. Nontuberculous Mycobacterial Pulmonary Disease: An Integrated Approach beyond Antibiotics. ERJ Open Res. 2021, 7, 00574–02020. [Google Scholar] [CrossRef]
- Honda, J.R.; Bernhard, J.N.; Chan, E.D. Natural Disasters and Nontuberculous Mycobacteria: A Recipe for Increased Disease? Chest 2015, 147, 304–308. [Google Scholar] [CrossRef]
- Thornton, C.S.; Mellett, M.; Jarand, J.; Barss, L.; Field, S.K.; Fisher, D.A. The Respiratory Microbiome and Nontuberculous Mycobacteria: An Emerging Concern in Human Health. Eur. Respir. Rev. 2021, 30, 200299. [Google Scholar] [CrossRef]
- Brode, S.K.; Daley, C.L.; Marras, T.K. The Epidemiologic Relationship between Tuberculosis and Non-Tuberculous Mycobacterial Disease: A Systematic Review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef]
- Marras, T.K.; Nelson, P.; Peci, A.; Richard-Greenblatt, M.; Brode, S.; Sullivan, A.; Jamieson, F.B.; Kus, J.V. Pulmonary Nontuberculous Mycobacteria, Ontario, Canada, 2020. Emerg. Infect. Dis. 2023, 29, 1415–1419. [Google Scholar] [CrossRef]
- Marras, T.K.; Daley, C.L. Epidemiology of Human Pulmonary Infection with Mycobacteria Nontuberculous. Clin. Chest Med. 2002, 23, 553–567. [Google Scholar] [CrossRef]
- Elwood, R.K.; Opazo Saez, A.M.; Lentini, V.; Shadmani, R. Incidence of Pulmonary Disease Caused by Mycobacteria Other than Tuberculosis in British Columbia. Can. Respir. J. 2002, 9, 319–323. [Google Scholar] [CrossRef]
- Marras, T.K.; Mehta, M.; Chedore, P.; May, K.; Houqani, M.A.; Jamieson, F. Nontuberculous Mycobacterial Lung Infections in Ontario, Canada: Clinical and Microbiological Characteristics. Lung 2010, 188, 289–299. [Google Scholar] [CrossRef]
- Khan, K.; Wang, J.; Marras, T.K. Nontuberculous Mycobacterial Sensitization in the United States: National Trends over Three Decades. Am. J. Respir. Crit. Care Med. 2007, 176, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.T.; Eisemann, E.; Parrish, N. A Brief Update on Mycobacterial Taxonomy, 2020 to 2022. J. Clin. Microbiol. 2023, 61, e00331-22. [Google Scholar] [CrossRef] [PubMed]
- Public Health Ontario. Enhanced Epidemiological Summary: Nontuberculous Mycobacteria Disease in Ontario, January 1, 2020 to December 31, 2020. Published October 2022. Available online: https://www.publichealthontario.ca/-/media/Documents/N/2022/nontuberculous-mycobacteria-disease-ontario-2020.pdf?rev=83fc8c24d7b64fadb17352a53b5df0c4&sc_lang=en#:~:text=Among%20individuals%20tested%20for%20NTM,was%2019.0%20per%20100%2C000%20population (accessed on 14 November 2024).
- Hernández-Garduño, E.; Rodrigues, M.; Elwood, R.K. The Incidence of Pulmonary Non-Tuberculous Mycobacteria in British Columbia, Canada. Int. J. Tuberc. Lung Dis. 2009, 13, 1086–1093. [Google Scholar]
- Hoefsloot, W.; Van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The Geographic Diversity of Nontuberculous Mycobacteria Isolated from Pulmonary Samples: An NTM-NET Collaborative Study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef]
- Yu, X.; Liu, P.; Liu, G.; Zhao, L.; Hu, Y.; Wei, G.; Luo, J.; Huang, H. The Prevalence of Non-Tuberculous Mycobacterial Infections in Mainland China: Systematic Review and Meta-Analysis. J. Infect. 2016, 73, 558–567. [Google Scholar] [CrossRef]
- Zaheen, A.; Hirama, T.; Mehrabi, M.; Brode, S.K.; Marras, T.K. Clinical Outcomes in Mycobacterium xenopi versus Mycobacterium avium Complex Pulmonary Disease: A Retrospective Matched Cohort Study. Respir. Med. 2020, 167, 105967. [Google Scholar] [CrossRef]
- Thangavelu, K.; Krishnakumariamma, K.; Pallam, G.; Dharm Prakash, D.; Chandrashekar, L.; Kalaiarasan, E.; Das, S.; Muthuraj, M.; Joseph, N.M. Prevalence and Speciation of Non-Tuberculous Mycobacteria among Pulmonary and Extrapulmonary Tuberculosis Suspects in South India. J. Infect. Public. Health 2021, 14, 320–323. [Google Scholar] [CrossRef]
- Chapter 11: Nontuberculous Mycobacteria. In Canadian Tuberculosis Standards, 7th ed.; Canadian Lung Association: Ottawa, ON, Canada; Public Health Agency of Canada: Ottawa, ON, Canada; Tuberculosis Prevention and Control: Ottawa, ON, Canada, 2014; Available online: https://www.phac-aspc.gc.ca/tbpc-latb/pubs/tb-canada-7/assets/pdf/tb-standards-tb-normes-ch11-eng.pdf (accessed on 10 February 2025).
- Bents, S.J.; Mercaldo, R.A.; Powell, C.; Henkle, E.; Marras, T.K.; Prevots, D.R. Nontuberculous Mycobacterial Pulmonary Disease (NTM PD) Incidence Trends in the United States, 2010–2019. BMC Infect. Dis. 2024, 24, 1094. [Google Scholar] [CrossRef]
- Wi, Y.M. Treatment of Extrapulmonary Nontuberculous Mycobacterial Diseases. Infect. Chemother. 2019, 51, 245. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.A.; Løkke, A.; Fløe, A.; Ibsen, R.; Johansen, I.S.; Hilberg, O. Nationwide Increasing Incidence of Nontuberculous Mycobacterial Diseases Among Adults in Denmark. Chest 2024, 166, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, A.B.; Drage, L.A.; Wengenack, N.L.; Wilson, J.W.; Lohse, C.M. Increased Incidence of Cutaneous Nontuberculous Mycobacterial Infection, 1980 to 2009: A Population-Based Study. Mayo Clin. Proc. 2013, 88, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.-T.-V.; Daniel, S.J.; Quach, C. Nontuberculous Mycobacteria in Children: A Changing Pattern. J. Otolaryngol. 2005, 34 (Suppl. S1), S40–S44. [Google Scholar]
- Sander, M.A.; Isaac-Renton, J.L.; Tyrrell, G.J. Cutaneous Nontuberculous Mycobacterial Infections in Alberta, Canada: An Epidemiologic Study and Review. J. Cutan. Med. Surg. 2018, 22, 479–483. [Google Scholar] [CrossRef]
- Ren, H.; Xiao, Y.; Tang, B.; Shi, Y.; Zeng, Z.; Qiu, X.; Ding, Y.; Xiao, R. The Price of Beauty: A Literature Review on Non-Tuberculous Mycobacteria Infection After Cosmetic Procedures. Aesthetic Surg. J. 2024, 44, NP574–NP584. [Google Scholar] [CrossRef]
- McNamara, K.X.; Perz, J.F.; Perkins, K.M. Association of Healthcare and Aesthetic Procedures with Infections Caused by Nontuberculous Mycobacteria, France, 2012–2020. Emerg. Infect. Dis. 2022, 28, 1303–1304. [Google Scholar] [CrossRef]
- Kham-ngam, I.; Chetchotisakd, P.; Ananta, P.; Chaimanee, P.; Sadee, P.; Reechaipichitkul, W.; Faksri, K. Epidemiology of and Risk Factors for Extrapulmonary Nontuberculous Mycobacterial Infections in Northeast Thailand. PeerJ 2018, 6, e5479. [Google Scholar] [CrossRef]
- Schreiber, P.W.; Kohl, T.A.; Kuster, S.P.; Niemann, S.; Sax, H. The Global Outbreak of Mycobacterium chimaera Infections in Cardiac Surgery—A Systematic Review of Whole-Genome Sequencing Studies and Joint Analysis. Clin. Microbiol. Infect. 2021, 27, 1613–1620. [Google Scholar] [CrossRef]
- Van Ingen, J.; Kohl, T.A.; Kranzer, K.; Hasse, B.; Keller, P.M.; Katarzyna Szafrańska, A.; Hillemann, D.; Chand, M.; Schreiber, P.W.; Sommerstein, R.; et al. Global Outbreak of Severe Mycobacterium chimaera Disease after Cardiac Surgery: A Molecular Epidemiological Study. Lancet Infect. Dis. 2017, 17, 1033–1041. [Google Scholar] [CrossRef]
- O’Neil, C.R.; Taylor, G.; Smith, S.; Joffe, A.M.; Antonation, K.; Shafran, S.; Kunimoto, D. Mycobacterium chimaera Infection After Aortic Valve Replacement Presenting With Aortic Dissection and Pseudoaneurysm. Open Forum Infect. Dis. 2018, 5, ofy018. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, N.; Kohl, T.A.; Diricks, M.; Mas-Peiro, S.; Holubec, T.; Kessel, J.; Graf, C.; Koch, B.; Herrmann, E.; Vehreschild, M.J.G.T.; et al. Clinical Characteristics and Outcome of Mycobacterium chimaera Infections after Cardiac Surgery: Systematic Review and Meta-Analysis of 180 Heater-Cooler Unit-Associated Cases. Clin. Microbiol. Infect. 2023, 29, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Rössle, M.; Hoffmann, M.; Deggim, V.; Kuster, S.; Zimmermann, D.R.; Bloemberg, G.; Hombach, M.; Hasse, B. Prosthetic Valve Endocarditis and Bloodstream Infection Due to Mycobacterium chimaera. J. Clin. Microbiol. 2013, 51, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, T.; Taylor, G.; Johnston, L.; Amaratunga, K.; Muller, M.; Coady, A.; Defalco, K.; Dunn, K.; Johnstone, J.; Smith, S.; et al. Mycobacterium chimaera Infections in Post-Operative Patients Exposed to Heater-Cooler Devices: An Overview. Can. Commun. Dis. Rep. 2017, 43, 107–113. [Google Scholar] [CrossRef]
- Hamad, R.; Noly, P.-E.; Perrault, L.P.; Pellerin, M.; Demers, P. Mycobacterium chimaera Infection After Cardiac Surgery: First Canadian Outbreak. Ann. Thorac. Surg. 2017, 104, e43–e45. [Google Scholar] [CrossRef]
- Kasperbauer, S.H.; Daley, C.L. Mycobacterium chimaera Infections Related to the Heater–Cooler Unit Outbreak: A Guide to Diagnosis and Management. Clin. Infect. Dis. 2019, 68, 1244–1250. [Google Scholar] [CrossRef]
- Wallace, R.J.; O’Brien, R.; Glassroth, J.; Raleigh, J.; Dutt, A. Diagnosis and Treatment of Disease Caused by Nontuberculous Mycobacteria. Am. Rev. Respir. Dis. 1990, 142, 940–953. [Google Scholar] [CrossRef]
- Wallace, R.; Cook, J.; Glassroth, J.; Griffith, D.; Olivier, K.; Gordin, F. Diagnosis and Treatment of Disease Caused by Nontuberculous Mycobacteria. Am. J. Resp. Crit. Care Med. 1997, 156 Pt 2, s1–s25. [Google Scholar] [CrossRef]
- Shafran, S.D.; Singer, J.; Zarowny, D.P.; Phillips, P.; Salit, I.; Walmsley, S.L.; Fong, I.W.; Gill, M.J.; Rachlis, A.R.; Lalonde, R.G.; et al. A Comparison of Two Regimens for the Treatment of Mycobacterium avium Complex Bacteremia in AIDS: Rifabutin, Ethambutol, and Clarithromycin versus Rifampin, Ethambutol, Clofazimine, and Ciprofloxacin. N. Engl. J. Med. 1996, 335, 377–384. [Google Scholar] [CrossRef]
- Koh, W.-J.; Kwon, O.J.; Lee, K.S. Diagnosis and Treatment of Nontuberculous Mycobacterial Pulmonary Diseases: A Korean Perspective. J. Korean Med. Sci. 2005, 20, 913–925. [Google Scholar] [CrossRef]
- Jarand, J.; Davis, J.P.; Cowie, R.L.; Field, S.K.; Fisher, D.A. Long-Term Follow-up of Mycobacterium avium Complex Lung Disease in Patients Treated with Regimens Including Clofazimine and/or Rifampin. Chest 2016, 149, 1285–1293. [Google Scholar] [CrossRef]
- National Institutes of Health, HIV Medicine Association, and Infectious Diseases Society of America. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection (accessed on 1 November 2025).
- Nguyen, V.-D.; Duong, H.; Lee, M.-C.; Chen, J.-H.; Huang, W.-C.; Chen, H.-E.; Lin, J.-C.; Wang, J.-Y.; Lee, C.-H. Two-Drug versus Three-Drug Regimens for Treating Mycobacterium avium Complex Infection: A Systematic Review and Meta-Analysis. J. Infect. Public. Health 2025, 18, 102711. [Google Scholar] [CrossRef]
- Im, Y.; Choe, J.; Kim, D.H.; Kim, S.-Y.; Jhun, B.W. Treatment Outcomes of Mycobacterium avium Complex Pulmonary Disease with a 2-Drug Daily Regimen Using Macrolide and Ethambutol. Open Forum Infect. Dis. 2025, 12, ofaf292. [Google Scholar] [CrossRef]
- Lange, C.; Böttger, E.C.; Cambau, E.; Griffith, D.E.; Guglielmetti, L.; Van Ingen, J.; Knight, S.L.; Marras, T.K.; Olivier, K.N.; Santin, M.; et al. Consensus Management Recommendations for Less Common Non-Tuberculous Mycobacterial Pulmonary Diseases. Lancet Infect. Dis. 2022, 22, e178–e190. [Google Scholar] [CrossRef]
- Kim, H.-J. Nonpharmacological Treatment for Nontuberculous Mycobacterial Pulmonary Disease. Tuberc. Respir. Dis. 2024, 87, 451–457. [Google Scholar] [CrossRef]
- Youssefnia, A.; Pierre, A.; Hoder, J.M.; MacDonald, M.; Shaffer, M.J.B.; Friedman, J.; Mehler, P.S.; Bontempo, A.; da Silva, F.C.N.; Chan, E.D. Ancillary Treatment of Patients with Lung Disease Due to Non-Tuberculous Mycobacteria: A Narrative Review. J. Thorac. Dis. 2022, 14, 3575–3597. [Google Scholar] [CrossRef]
- Murray, M.P.; Pentland, J.L.; Hill, A.T. A Randomised Crossover Trial of Chest Physiotherapy in Non-Cystic Fibrosis Bronchiectasis. Eur. Respir. J. 2009, 34, 1086–1092. [Google Scholar] [CrossRef]
- McShane, P.J. Investigation and Management of Bronchiectasis in Nontuberculous Mycobacterial Pulmonary Disease. Clin. Chest Med. 2023, 44, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, A.; Segal, L.; Samuels, J.; Feintuch, J.; Feintuch, J.; Alter, K.; Moffson, D.; Scott, A.; Addrizzo-Harris, D.; Liu, M.; et al. Effects of Chest Physical Therapy in Patients with Non-Tuberculous Mycobacteria. Int. J. Respir. Pulm. Med. 2017, 4, 065. [Google Scholar] [CrossRef] [PubMed]
- Huiberts, A.; Zweijpfenning, S.M.H.; Pennings, L.J.; Boeree, M.J.; van Ingen, J.; Magis-Escurra, C.; Hoefsloot, W. Outcomes of Hypertonic Saline Inhalation as a Treatment Modality in Nontuberculous Mycobacterial Pulmonary Disease. Eur. Respir. J. 2019, 54, 1802143. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.M.; Jhun, B.W.; Baek, S.-Y.; Kim, S.; Jeon, K.; Ko, R.-E.; Shin, S.H.; Lee, H.; Kwon, O.J.; Huh, H.J.; et al. Long-Term Natural History of Non-Cavitary Nodular Bronchiectatic Nontuberculous Mycobacterial Pulmonary Disease. Respir. Med. 2019, 151, 1–7. [Google Scholar] [CrossRef]
- van Ingen, J.; Verhagen, A.F.T.M.; Dekhuijzen, P.N.R.; van Soolingen, D.; Magis-Escurra, C.; Boeree, M.J.; de Lange, W.C.M. Surgical Treatment of Non-Tuberculous Mycobacterial Lung Disease: Strike in Time. Int. J. Tuberc. Lung Dis. 2010, 14, 99–105. [Google Scholar]
- Shiraishi, Y.; Nakajima, Y.; Takasuna, K.; Hanaoka, T.; Katsuragi, N.; Konno, H. Surgery for Mycobacterium avium Complex Lung Disease in the Clarithromycin Era. Eur. J. Cardio-Thorac. Surg. 2002, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Nakajima, Y.; Katsuragi, N.; Kurai, M.; Takahashi, N. Pneumonectomy for Nontuberculous Mycobacterial Infections. Ann. Thorac. Surg. 2004, 78, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y. Surgical Treatment of Nontuberculous Mycobacterial Lung Disease. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.A.; Pomerantz, M.; Bishop, A.; Weyant, M.J.; Mitchell, J.D. Lady Windermere Revisited: Treatment with Thoracoscopic Lobectomy/Segmentectomy for Right Middle Lobe and Lingular Bronchiectasis Associated with Non-Tuberculous Mycobacterial Disease. Eur. J. Cardio-Thorac. Surg. 2011, S1010794010011152. [Google Scholar] [CrossRef]
- Kang, H.K.; Park, H.Y.; Kim, D.; Jeong, B.-H.; Jeon, K.; Cho, J.H.; Kim, H.K.; Choi, Y.S.; Kim, J.; Koh, W.-J. Treatment Outcomes of Adjuvant Resectional Surgery for Nontuberculous Mycobacterial Lung Disease. BMC Infect. Dis. 2015, 15, 76. [Google Scholar] [CrossRef]
- Taylor, L.J.; Mitchell, J.D. Surgical Resection in Nontuberculous Mycobacterial Pulmonary Disease. Clin. Chest Med. 2023, 44, 861–868. [Google Scholar] [CrossRef]
- Holt, M.; Kasperbauer, S. Management of Extrapulmonary Nontuberculous Mycobacterial Infections. Semin. Respir. Crit. Care Med. 2018, 39, 399–410. [Google Scholar] [CrossRef]
- Bi, S.; Hu, F.-S.; Yu, H.-Y.; Xu, K.-J.; Zheng, B.-W.; Ji, Z.-K.; Li, J.-J.; Deng, M.; Hu, H.-Y.; Sheng, J.-F. Nontuberculous Mycobacterial Osteomyelitis. Infect. Dis. 2015, 47, 673–685. [Google Scholar] [CrossRef]
- Ortega-Portas, C.; Auñon, A.; Esteban, J. Clinical Treatment of Mycobacterial Prosthetic Joint Infections. Expert. Rev. Anti Infect. Ther. 2025, 23, 829–841. [Google Scholar] [CrossRef]
- Jabbour, S.F.; Malek, A.E.; Kechichian, E.G.; Tomb, R.R.; Nasr, M.W. Nontuberculous Mycobacterial Infections After Cosmetic Procedures: A Systematic Review and Management Algorithm. Dermatol. Surg. 2020, 46, 116–121. [Google Scholar] [CrossRef]
- Kadota, N.; Ishikawa, K.; Kubono, Y.; Konishi, K.; Fujimaru, T.; Ito, Y.; Nagahama, M.; Taki, F.; Kawai, F.; Mori, N.; et al. Systematic Literature Review of the Diagnosis, Prognosis, and Treatment of Peritoneal Dialysis-Related Infection Caused by Nontuberculous Mycobacteria. BMC Nephrol. 2024, 25, 432. [Google Scholar] [CrossRef] [PubMed]
- El Helou, G.; Viola, G.M.; Hachem, R.; Han, X.Y.; Raad, I.I. Rapidly Growing Mycobacterial Bloodstream Infections. Lancet Infect. Dis. 2013, 13, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Veve, M.P.; Kenney, R.M.; Aljundi, A.M.; Dierker, M.S.; Athans, V.; Shallal, A.B.; Patel, N. Multicenter, Retrospective Cohort Study of Antimycobacterial Treatment-Related Harms among Patients with Non-Tuberculosis Mycobacterium Infections in the United States. Antimicrob. Agents Chemother. 2025, 69, e01596-24. [Google Scholar] [CrossRef] [PubMed]
- Sawka, A.; Burke, A. Medications and Monitoring in Treatment of Nontuberculous Mycobacterial Pulmonary Disease. Clin. Chest Med. 2023, 44, 815–828. [Google Scholar] [CrossRef]
- Furuuchi, K.; Morimoto, K.; Kurashima, A.; Fujiwara, K.; Nakamoto, K.; Tanaka, Y.; Tachibana, H.; Yoshimori, K.; Sasaki, Y.; Ohta, K. Treatment Duration and Disease Recurrence Following the Successful Treatment of Patients with Mycobacterium avium Complex Lung Disease. Chest 2020, 157, 1442–1445. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, S.; Hong, Y.; Lee, S.-D.; Kim, W.S.; Kim, D.S.; Shim, T.S.; Jo, K.-W. Risk Factors for Recurrence after Successful Treatment of Mycobacterium avium Complex Lung Disease. Antimicrob. Agents Chemother. 2015, 59, 2972–2977. [Google Scholar] [CrossRef]
- Koh, W.-J.; Moon, S.M.; Kim, S.-Y.; Woo, M.-A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.-S.; et al. Outcomes of Mycobacterium avium Complex Lung Disease Based on Clinical Phenotype. Eur. Respir. J. 2017, 50, 1602503. [Google Scholar] [CrossRef]
- Kwon, B.S.; Shim, T.S.; Jo, K.-W. The Second Recurrence of Mycobacterium avium Complex Lung Disease after Successful Treatment for First Recurrence. Eur. Respir. J. 2019, 53, 1801038. [Google Scholar] [CrossRef]
- Santin, M.; Dorca, J.; Alcaide, F.; Gonzalez, L.; Casas, S.; Lopez, M.; Guerra, M.R. Long-Term Relapses after 12-Month Treatment for Mycobacterium kansasii Lung Disease. Eur. Respir. J. 2009, 33, 148–152. [Google Scholar] [CrossRef]
- Raats, D.; Brode, S.K.; Mehrabi, M.; Marras, T.K. Increasing and More Commonly Refractory Mycobacterium avium Pulmonary Disease, Toronto, Ontario, Canada. Emerg. Infect. Dis. 2022, 28, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Trudel, C.; Onotera, M.; Gibson, D.; Ahmad Khan, F.; Behr, M.A.; Menzies, D. A Retrospective Review of Non-Tuberculous Mycobacterium Treatment in Montreal. Can. J. Respir. Crit. Care Sleep Med. 2025, 9, 152–159. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Kamolratanakul, S.; Rotcheewaphan, S.; Leelahavanichkul, A.; Schultz, M.J. Recent Advances in Immunopathogenesis and Clinical Practice: Mastering the Challenge-Managing of Non-Tuberculous Mycobacteria. Front. Immunol. 2025, 16, 1554544. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Woods, G.L. Antimycobacterial Susceptibility Testing of Nontuberculous Mycobacteria. J. Clin. Microbiol. 2019, 57, e00834-19. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Boeree, M.J.; Van Soolingen, D.; Mouton, J.W. Resistance Mechanisms and Drug Susceptibility Testing of Nontuberculous Mycobacteria. Drug Resist. Updates 2012, 15, 149–161. [Google Scholar] [CrossRef]
- Kobashi, Y.; Yoshida, K.; Miyashita, N.; Niki, Y.; Oka, M. Relationship between Clinical Efficacy of Treatment of Pulmonary Mycobacterium avium Complex Disease and Drug-Sensitivity Testing of Mycobacterium avium Complex Isolates. J. Infect. Chemother. 2006, 12, 195–202. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Langsjoen, B.; Zhang, Y.; Pan, X.; Girard, W.; Nelson, K.; Caccitolo, J.; Alvarez, J.; Shepherd, S.; et al. Clinical and Molecular Analysis of Macrolide Resistance in Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 2006, 174, 928–934. [Google Scholar] [CrossRef]
- Moon, S.M.; Park, H.Y.; Kim, S.-Y.; Jhun, B.W.; Lee, H.; Jeon, K.; Kim, D.H.; Huh, H.J.; Ki, C.-S.; Lee, N.Y.; et al. Clinical Characteristics, Treatment Outcomes, and Resistance Mutations Associated with Macrolide-Resistant Mycobacterium avium Complex Lung Disease. Antimicrob. Agents Chemother. 2016, 60, 6758–6765. [Google Scholar] [CrossRef]
- Morimoto, K.; Namkoong, H.; Hasegawa, N.; Nakagawa, T.; Morino, E.; Shiraishi, Y.; Ogawa, K.; Izumi, K.; Takasaki, J.; Yoshiyama, T.; et al. Macrolide-Resistant Mycobacterium avium Complex Lung Disease: Analysis of 102 Consecutive Cases. Ann. ATS 2016, 13, 1904–1911. [Google Scholar] [CrossRef]
- Koh, W.-J.; Jeon, K.; Lee, N.Y.; Kim, B.-J.; Kook, Y.-H.; Lee, S.-H.; Park, Y.K.; Kim, C.K.; Shin, S.J.; Huitt, G.A.; et al. Clinical Significance of Differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 2011, 183, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Kwon, O.J.; Lee, N.Y.; Kim, B.-J.; Kook, Y.-H.; Lee, S.-H.; Park, Y.K.; Kim, C.K.; Koh, W.-J. Antibiotic Treatment of Mycobacterium abscessus Lung Disease: A Retrospective Analysis of 65 Patients. Am. J. Respir. Crit. Care Med. 2009, 180, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Iakhiaeva, E.; Griffith, D.E.; Woods, G.L.; Stout, J.E.; Wolfe, C.R.; Turenne, C.Y.; Wallace, R.J. In Vitro Activity of Amikacin against Isolates of Mycobacterium avium Complex with Proposed MIC Breakpoints and Finding of a 16S rRNA Gene Mutation in Treated Isolates. J. Clin. Microbiol. 2013, 51, 3389–3394. [Google Scholar] [CrossRef]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar] [CrossRef]
- Tsiolakkis, G.; Liontos, A.; Filippas-Ntekouan, S.; Matzaras, R.; Theodorou, E.; Vardas, M.; Vairaktari, G.; Nikopoulou, A.; Christaki, E. Mycobacterium marinum: A Case-Based Narrative Review of Diagnosis and Management. Microorganisms 2023, 11, 1799. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Parrish, N.; Luethke, R.; Dionne, K.; Carroll, K.; Riedel, S. Case of Mycobacterium marinum Infection with Unusual Patterns of Susceptibility to Commonly Used Antibiotics. J. Clin. Microbiol. 2011, 49, 2056–2058. [Google Scholar] [CrossRef]
- Huh, H.J.; Kim, S.-Y.; Shim, H.J.; Kim, D.H.; Yoo, I.Y.; Kang, O.-K.; Ki, C.-S.; Shin, S.Y.; Jhun, B.W.; Shin, S.J.; et al. GenoType NTM-DR Performance Evaluation for Identification of Mycobacterium avium Complex and Mycobacterium abscessus and Determination of Clarithromycin and Amikacin Resistance. J. Clin. Microbiol. 2019, 57, e00516-19. [Google Scholar] [CrossRef]
- Mougari, F.; Loiseau, J.; Veziris, N.; Bernard, C.; Bercot, B.; Sougakoff, W.; Jarlier, V.; Raskine, L.; Cambau, E. Evaluation of the New GenoType NTM-DR Kit for the Molecular Detection of Antimicrobial Resistance in Non-Tuberculous Mycobacteria. J. Antimicrob. Chemother. 2017, 72, 1669–1677. [Google Scholar] [CrossRef]
- Falkinham, J.O. Challenges of NTM Drug Development. Front. Microbiol. 2018, 9, 1613. [Google Scholar] [CrossRef]
- Dubé, M.P.; Sattler, F.R.; Torriani, F.J.; See, D.; Havlir, D.V.; Kemper, C.A.; Dezfuli, M.G.; Bozzette, S.A.; Bartok, A.E.; Leedom, J.M.; et al. A Randomized Evaluation of Ethambutol for Prevention of Relapse and Drug Resistance during Treatment of Mycobacterium avium Complex Bacteremia with Clarithromycin-Based Combination Therapy. J. Infect. Dis. 1997, 176, 1225–1232. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Nash, K.A.; Petrofsky, M.; Young, L.S.; Inderlied, C.B. Effect of Ethambutol on Emergence of Clarithromycin-Resistant Mycobacterium avium Complex in the Beige Mouse Model. J. Infect. Dis. 1996, 174, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, E.H.; Jung, I.; Park, G.; Kang, Y.A. Clinical Characteristics and Treatment Outcomes of Patients with Macrolide-Resistant Mycobacterium avium Complex Pulmonary Disease: A Systematic Review and Meta-Analysis. Respir. Res. 2019, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.-L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society Consensus Recommendations for the Management of Non-Tuberculous Mycobacteria in Individuals with Cystic Fibrosis. Thorax 2016, 71 (Suppl. S1), i1–i22. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society Guidelines for the Management of Adult Bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Renvoisé, A.; Brossier, F.; Galati, E.; Veziris, N.; Sougakoff, W.; Aubry, A.; Robert, J.; Cambau, E.; Jarlier, V.; Bernard, C. Assessing Primary and Secondary Resistance to Clarithromycin and Amikacin in Infections Due to Mycobacterium avium Complex Antimicrob. Agents Chemother. 2015, 59, 7153–7155. [Google Scholar] [CrossRef]
- Tuberculosis Physician Manual Section 12: Pulmonary Non-Tuberculous Mycobacteria (NTM). British Columbia Centre for Disease Control, Provincial Health Services Authority: Vancouver, BC, Canada, 2022. Available online: https://www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/Guidelines%20and%20Manuals/TB/TB%20physician%20manual_section%2012.pdf (accessed on 20 April 2025).
- Ren, W.; Tan, Y.; Ma, Z.; Shang, Y.; Li, S.; Zhang, X.; Wang, W.; Yao, C.; Yuan, J.; Li, L.; et al. In Vitro Susceptibility of Nontuberculous Mycobacteria in China. BMC Infect. Dis. 2024, 24, 118. [Google Scholar] [CrossRef]
- Hershko, Y.; Adler, A. Antimicrobial Susceptibility Distributions of Clinical Isolates of Nontuberculous Mycobacteria in Israel. Microb. Drug Resist. 2023, 29, 302–308. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, J.; Zou, W.; Li, Q.; Qian, X.; Zhu, Z. Antimicrobial Susceptibility Profiles of Mycobacterium abscessus Complex Isolates from Respiratory Specimens in Shanghai, China. J. Glob. Antimicrob. Resist. 2021, 25, 72–76. [Google Scholar] [CrossRef]
- Portell-Buj, E.; Bonet-Rossinyol, Q.; López-Gavín, A.; Roman, A.; Fernández-Pittol, M.; Tudó, G.; Gonzalez-Martin, J. Comparison of Two-Drug Combinations, Amikacin/Tigecycline/Imipenem and Amikacin/Tigecycline/Clarithromycin against Mycobacteroides abscessus subsp. abscessus Using the in Vitro Time-Kill Assay. J. Antibiot. 2021, 74, 285–290. [Google Scholar] [CrossRef]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; Pfyffer, G.E.; Ridderhof, J.C.; Siddiqi, S.H.; Wallace, R.J.; et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes, 2nd ed.; CLSI Publication/Clinical and Laboratory Standards Institute, No. 31.5; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544374/ (accessed on 14 November 2025).
- Kim, S.-Y.; Kim, D.H.; Moon, S.M.; Song, J.Y.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Koh, W.-J.; Jhun, B.W. Association between 16S rRNA Gene Mutations and Susceptibility to Amikacin in Mycobacterium avium Complex and Mycobacterium abscessus Clinical Isolates. Sci. Rep. 2021, 11, 6108. [Google Scholar] [CrossRef]
- Ka Lip, C.; Go, J.; Binte Abu Bakar, N.A.; Octavia, S.; Pin Lin, R.T.; Teo, J.W.P. Whole-Genome Phylogenetic Analysis of Mycobacterium avium Complex from Clinical Respiratory Samples. Microbiol. Spectr. 2025, 13, e01600-24. [Google Scholar] [CrossRef]
- González Martínez, A.; Aguilera, M.; Tarriño, M.; Alberola, A.; Reguera, J.A.; Sampedro, A.; Navarro, J.M.; Rodríguez Granger, J. Susceptibility Patterns in Clinical Isolates of Mycobacterium avium Complex from a Hospital in Southern Spain. Microorganisms 2024, 12, 2613. [Google Scholar] [CrossRef]
- Sirichoat, A.; Kaewprasert, O.; Hinwan, Y.; Faksri, K. Phenotypic Drug-Susceptibility Profiles and Genetic Analysis Based on Whole-Genome Sequencing of Mycobacterium avium Complex Isolates in Thailand. PLoS ONE 2023, 18, e0294677. [Google Scholar] [CrossRef]
- Minuk, L.M.; Brode, S.K.; Mehrabi, M.; Sharma, M.K.; Stobart, M.; Soualhine, H.; Marras, T.K. Phenotypic Amikacin Resistance May Not Indicate Poor Response to Amikacin in Mycobacterium avium Complex Pulmonary Disease. Antimicrob. Agents Chemother. 2024, e00084-24. [Google Scholar] [CrossRef] [PubMed]
- Hunkins, J.-J.; de-Moura, V.-C.-N.; Eddy, J.-J.; Daley, C.-L.; Khare, R. In Vitro Susceptibility Patterns for Rapidly Growing Nontuberculous Mycobacteria in the United States. Diagn. Microbiol. Infect. Dis. 2023, 105, 115882. [Google Scholar] [CrossRef] [PubMed]
- Broda, A.; Jebbari, H.; Beaton, K.; Mitchell, S.; Drobniewski, F. Comparative Drug Resistance of Mycobacterium Abscessus and M. Chelonae Isolates from Patients with and without Cystic Fibrosis in the United Kingdom. J. Clin. Microbiol. 2013, 51, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Spaink, H.P.; Forn-Cuní, G. Drug Resistance in Nontuberculous Mycobacteria: Mechanisms and Models. Biology 2021, 10, 96. [Google Scholar] [CrossRef]
- Huang, H.-L.; Lu, P.-L.; Lee, C.-H.; Chong, I.-W. Treatment of Pulmonary Disease Caused by Mycobacterium kansasii. J. Formos. Med. Assoc. 2020, 119, S51–S57. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Liu, H.; Yang, J.; Wang, W.; Wang, B.; Li, M.; Yu, F. Clinical and Microbiological Characteristics of Mycobacterium kansasii Pulmonary Infections in China. Microbiol. Spectr. 2022, 10, e01475-21. [Google Scholar] [CrossRef]
- Andalibi, F.; Bostanghadiri, N.; Amirmozafari, N.; Irajian, G.; Mirkalantari, S. Efficacy and Treatment Outcome of Infected Patients with Pulmonary Mycobacterium kansasii: A Systematic Review. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 36, 100463. [Google Scholar] [CrossRef]
- Moon, S.M.; Kim, S.-Y.; Kim, D.H.; Huh, H.J.; Lee, N.Y.; Jhun, B.W. Relationship between Resistance to Ethambutol and Rifampin and Clinical Outcomes in Mycobacterium avium Complex Pulmonary Disease. Antimicrob. Agents Chemother. 2022, 66, e02027-21. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.-Y.; Huh, H.J.; Lee, N.Y.; Koh, W.-J.; Jhun, B.W. In Vitro Activity of Rifamycin Derivatives against Nontuberculous Mycobacteria, Including Macrolide- and Amikacin-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2021b, 65, e02611-20. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, B.; Nasiri, M.J.; Adkinson, B.C.; Azimi, T.; Khalili, F.; Goudarzi, M.; Dadashi, M.; Murthi, M.; Mirsaeidi, M. Comparison of Rifabutin-Based Versus Rifampin-Based Regimens for the Treatment of Mycobacterium avium Complex: A Meta-Analysis Study. Front. Pharmacol. 2021, 12, 693369. [Google Scholar] [CrossRef] [PubMed]
- Thapa, J.; Chizimu, J.Y.; Kitamura, S.; Akapelwa, M.L.; Suwanthada, P.; Miura, N.; Toyting, J.; Nishimura, T.; Hasegawa, N.; Nishiuchi, Y.; et al. Characterization of DNA Gyrase Activity and Elucidation of the Impact of Amino Acid Substitution in GyrA on Fluoroquinolone Resistance in Mycobacterium avium. Microbiol. Spectr. 2023, 11, e05088-22. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Hong, G.; Kim, S.-Y.; Jeong, B.-H.; Park, H.Y.; Jeon, K.; Kwon, O.J.; Lee, S.-H.; Kim, C.K.; Shin, S.J. Treatment of Refractory Mycobacterium avium Complex Lung Disease with a Moxifloxacin-Containing Regimen. Antimicrob. Agents Chemother. 2013, 57, 2281–2285. [Google Scholar] [CrossRef]
- Liu, C.-F.; Song, Y.-M.; He, W.-C.; Liu, D.-X.; He, P.; Bao, J.-J.; Wang, X.-Y.; Li, Y.-M.; Zhao, Y.-L. Nontuberculous Mycobacteria in China: Incidence and Antimicrobial Resistance Spectrum from a Nationwide Survey. Infect. Dis. Poverty 2021, 10, 59. [Google Scholar] [CrossRef]
- Hajikhani, B.; Nasiri, M.J.; Hosseini, S.S.; Khalili, F.; Karimi-Yazdi, M.; Hematian, A.; Nojookambari, N.Y.; Goudarzi, M.; Dadashi, M.; Mirsaeidi, M. Clofazimine Susceptibility Testing of Mycobacterium avium Complex and Mycobacterium abscessus: A Meta-Analysis Study. J. Glob. Antimicrob. Resist. 2021, 26, 188–193. [Google Scholar] [CrossRef]
- Kwak, N.; Whang, J.; Yang, J.S.; Kim, T.S.; Kim, S.A.; Yim, J.-J. Minimal Inhibitory Concentration of Clofazimine Among Clinical Isolates of Nontuberculous Mycobacteria and Its Impact on Treatment Outcome. Chest 2021, 159, 517–523. [Google Scholar] [CrossRef]
- Omar, S.; Whitfield, M.G.; Nolan, M.B.; Ngom, J.T.; Ismail, N.; Warren, R.M.; Klopper, M. Bedaquiline for Treatment of Non-Tuberculous Mycobacteria (NTM): A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2024, 79, 211–240. [Google Scholar] [CrossRef]
- Fröberg, G.; Maurer, F.P.; Chryssanthou, E.; Fernström, L.; Benmansour, H.; Boarbi, S.; Mengshoel, A.T.; Keller, P.M.; Viveiros, M.; Machado, D.; et al. Towards Clinical Breakpoints for Non-Tuberculous Mycobacteria—Determination of Epidemiological Cut off Values for the Mycobacterium avium Complex and Mycobacterium abscessus Using Broth Microdilution. Clin. Microbiol. Infect. 2023, 29, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Pawa, J. Exploring Specialized Care for Non-Tuberculous Mycobacterial (NTM) Patients in British Columbia (BC), 2018. British Columbia Centre for Disease Control, Provincial Health Services Authority: Vancouver, BC, Canada, 2018; Available online: https://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/TB/TB_NTM%20report.pdf (accessed on 9 March 2025).
- Shulha, J.A.; Escalante, P.; Wilson, J.W. Pharmacotherapy Approaches in Nontuberculous Mycobacteria Infections. Mayo Clin. Proc. 2019, 94, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-V.H.; Haas, M.K.; Kasperbauer, S.H.; Calado Nogueira De Moura, V.; Eddy, J.J.; Mitchell, J.D.; Khare, R.; Griffith, D.E.; Chan, E.D.; Daley, C.L. Nontuberculous Mycobacterial Pulmonary Disease: Patients, Principles, and Prospects. Clin. Infect. Dis. 2024, 79, e27–e47. [Google Scholar] [CrossRef]
- Peloquin, C.A.; Berning, S.E.; Nitta, A.T.; Simone, P.M.; Goble, M.; Huitt, G.A.; Iseman, M.D.; Cook, J.L.; Curran-Everett, D. Aminoglycoside Toxicity: Daily versus Thrice-Weekly Dosing for Treatment of Mycobacterial Diseases. Clin. Infect. Dis. 2004, 38, 1538–1544. [Google Scholar] [CrossRef]
- Ferro, B.E.; Srivastava, S.; Deshpande, D.; Sherman, C.M.; Pasipanodya, J.G.; Van Soolingen, D.; Mouton, J.W.; Van Ingen, J.; Gumbo, T. Amikacin Pharmacokinetics/Pharmacodynamics in a Novel Hollow-Fiber Mycobacterium abscessus Disease Model. Antimicrob. Agents Chemother. 2016, 60, 1242–1248. [Google Scholar] [CrossRef]
- Van Ingen, J.; Aliberti, S.; Andrejak, C.; Chalmers, J.D.; Codecasa, L.R.; Daley, C.L.; Hasegawa, N.; Griffith, D.E.; Hoefsloot, W.; Huitt, G.; et al. Management of Drug Toxicity in Mycobacterium avium Complex Pulmonary Disease: An Expert Panel Survey. Clin. Infect. Dis. 2021, 73, e256–e259. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Jeon, K.; Park, H.Y.; Kim, S.-Y.; Lee, K.S.; Huh, H.J.; Ki, C.-S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Intermittent Antibiotic Therapy for Nodular Bronchiectatic Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 96–103. [Google Scholar] [CrossRef]
- Kamii, Y.; Nagai, H.; Kawashima, M.; Matsuki, M.; Nagoshi, S.; Sato, A.; Kohno, S.; Ohgiya, M.; Ohta, K. Adverse Reactions Associated with Long-Term Drug Administration in Mycobacterium avium Complex Lung Disease. Int. J. Tuberc. Lung Dis. 2018, 22, 1505–1510. [Google Scholar] [CrossRef]
- Leber, A.; Marras, T.K. The Cost of Medical Management of Pulmonary Nontuberculous Mycobacterial Disease in Ontario, Canada. Eur. Respir. J. 2011, 37, 1158–1165. [Google Scholar] [CrossRef]
- Goring, S.M.; Wilson, J.B.; Risebrough, N.R.; Gallagher, J.; Carroll, S.; Heap, K.J.; Obradovic, M.; Loebinger, M.R.; Diel, R. The Cost of Mycobacterium Avium Complex Lung Disease in Canada, France, Germany, and the United Kingdom: A Nationally Representative Observational Study. BMC Health Serv. Res. 2018, 18, 700. [Google Scholar] [CrossRef]
- Ramsay, L.C.; Shing, E.; Wang, J.; Marras, T.K.; Kwong, J.C.; Brode, S.K.; Jamieson, F.B.; Sander, B. Costs Associated with Nontuberculous Mycobacteria Infection, Ontario, Canada, 2001–2012. Emerg. Infect. Dis. 2020, 26, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Ali, J. A Multidisciplinary Approach to the Management of Nontuberculous Mycobacterial Lung Disease: A Clinical Perspective. Expert. Rev. Respir. Med. 2021, 15, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.S.; Stoker, N.G.; Potter, J.L.; Claassen, H.; Leslie, A.; Tweed, C.D.; Chiang, C.-Y.; Conradie, F.; Esmail, H.; Lange, C.; et al. Bedaquiline: What Might the Future Hold? Lancet Microbe 2024, 5, 100909. [Google Scholar] [CrossRef] [PubMed]
- Kurbatfinski, N.; Hill, P.J.; Tobin, N.; Kramer, C.N.; Wickham, J.; Goodman, S.D.; Hall-Stoodley, L.; Bakaletz, L.O. Disruption of Nontuberculous Mycobacteria Biofilms Induces a Highly Vulnerable to Antibiotic Killing Phenotype. Biofilm 2023, 6, 100166. [Google Scholar] [CrossRef]
- Muñoz-Egea, M.-C.; Akir, A.; Esteban, J. Mycobacterium Biofilms. Biofilm 2023, 5, 100107. [Google Scholar] [CrossRef]
- Kaur, P.; Krishnamurthy, R.V.; Shandil, R.K.; Mohan, R.; Narayanan, S. A Novel Inhibitor against the Biofilms of Non-Tuberculous Mycobacteria. Pathogens 2023, 13, 40. [Google Scholar] [CrossRef]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef]
- Cano-Fernández, M.; Esteban, J. New Antibiofilm Strategies for the Management of Nontuberculous Mycobacteria Diseases. Expert. Opin. Pharmacother. 2024, 25, 2035–2046. [Google Scholar] [CrossRef]
- Van Der Laan, R.; Snabilié, A.; Obradovic, M. Meeting the Challenges of NTM-PD from the Perspective of the Organism and the Disease Process: Innovations in Drug Development and Delivery. Respir. Res. 2022, 23, 376. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Sirbu, A.; Manley, A.; Das, A.; Chitra, S.; Deck, D.H.; Serio, A.W.; Anastasiou, D.; Villano, S. A Phase 2, Double-Blind, Randomized, Placebo-Controlled, Multi-Center Study to Evaluate the Efficacy, Safety, and Tolerability of Oral Omadacycline in Adults With Nontuberculous Mycobacterial Pulmonary Disease (NTM PD) Caused by Mycobacterium abscessus Complex (MABc). Am. J. Respir. Crit. Care Med. 2025, 211, A3168. [Google Scholar] [CrossRef]
- Thomson, R.M.; Loebinger, M.R.; Burke, A.J.; Morgan, L.C.; Waterer, G.W.; Ganslandt, C. OPTIMA: An Open-Label, Noncomparative Pilot Trial of Inhaled Molgramostim in Pulmonary Nontuberculous Mycobacterial Infection. Ann. ATS 2024, 21, 568–576. [Google Scholar] [CrossRef]
- Mejia-Chew, C.; Spec, A.; Walton, A.H.; Ulezko Antonova, A.; Dram, A.; Bhalla, S.; Colonna, M.; Morre, M.; Hotchkiss, R. Recombinant Interleukin-7 Treatment of Refractory Mycobacterium avium Complex Lung Disease (IMPULSE-7): A Pilot Phase II, Single-Center, Randomized, Clinical Trial. Ther. Adv. Infect. 2025, 12, 20499361251339300. [Google Scholar] [CrossRef]
- Daley, C.L.; Chalmers, J.D.; Flume, P.A.; Griffith, D.E.; Hasegawa, N.; Morimoto, K.; Winthrop, K.L.; Sheu, C.-C.; Hassan, M.; Nevoret, M.-L.; et al. Microbiologic Outcomes From a Randomized, Double-Blind Trial of Amikacin Liposome Inhalation Suspension in Adults With Newly Diagnosed or Recurrent Mycobacterium avium Complex Lung Disease: The ARISE Study. In A16. NTM with a Side of Bronchiectasis; American Thoracic Society: New York, NY, USA, 2024; p. A1033. [Google Scholar] [CrossRef]
- Flume, P.A.; Garcia, B.A.; Wilson, D.; Steed, L.; Dorman, S.E.; Winthrop, K. Inhaled Nitric Oxide for Adults with Pulmonary Non-Tuberculous Mycobacterial Infection. Respir. Med. 2023, 206, 107069. [Google Scholar] [CrossRef] [PubMed]
- Stemkens, R.; Lemson, A.; Koele, S.E.; Svensson, E.M.; Te Brake, L.H.M.; Van Crevel, R.; Boeree, M.J.; Hoefsloot, W.; Van Ingen, J.; Aarnoutse, R.E. A Loading Dose of Clofazimine to Rapidly Achieve Steady-State-like Concentrations in Patients with Nontuberculous Mycobacterial Disease. J. Antimicrob. Chemother. 2024, 79, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qi, X.; Zhang, W.; Wang, H.; Fu, L.; Wang, B.; Chen, X.; Chen, X.; Lu, Y. Efficacy of PBTZ169 and Pretomanid against Mycobacterium avium, Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum in BALB/c Mice Models. Front. Cell. Infect. Microbiol. 2023, 13, 1115530. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Mikušová, K. Development of Macozinone for TB Treatment: An Update. Appl. Sci. 2020, 10, 2269. [Google Scholar] [CrossRef]
- Rimal, B.; Howe, R.A.; Panthi, C.M.; Wang, W.; Lamichhane, G. Oral Oxaborole MRX-5 Exhibits Efficacy against Pulmonary Mycobacterium abscessus in Mouse. Antimicrob. Agents Chemother. 2024, 68, e01351-24. [Google Scholar] [CrossRef]
- MicuRx Newsletter. MicuRx’s Self-Developed MRX-5 for NTM Infection Clinical Trial Application Approved. 2025. Available online: https://www.micurx.com/2161.html (accessed on 20 May 2025).
- Mediaas, S.D.; Haug, M.; Louet, C.; Wahl, S.G.F.; Gidon, A.; Flo, T.H. Metformin Improves Mycobacterium avium Infection by Strengthening Macrophage Antimicrobial Functions. Front. Immunol. 2024, 15, 1463224. [Google Scholar] [CrossRef]
- Mercaldo, R.; Fennelly, K.P.; Honda, S.; Frankland, T.B.; Daida, Y.G.; Prevots, R. Metformin Use Reduces the Risk of Nontuberculous Mycobacteria Infection: Results From a Population-Based Cohort in Hawaii, USA. In C68. NTM: Challenging Science and Cases; American Thoracic Society: New York, NY, USA, 2024; p. A6302. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, W.; Xu, D.; Guo, F.; Yang, M.; Zhu, Y.; Shen, L.; Chen, S.; Tang, D.; Li, L.; et al. Discovery and Preclinical Profile of Sudapyridine (WX-081), a Novel Anti-Tuberculosis Agent. Bioorganic Med. Chem. Lett. 2022, 71, 128824. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, H.; Qi, X.; Zhang, W.; Wang, B.; Fu, L.; Chen, X.; Chen, X.; Lu, Y. Sudapyridine (WX-081) Antibacterial Activity against Mycobacterium avium, Mycobacterium abscessus, and Mycobacterium chelonae In Vitro and In Vivo. mSphere 2024, 9, e00518-23. [Google Scholar] [CrossRef]
- Fan, J.; Tan, Z.; He, S.; Li, A.; Jia, Y.; Li, J.; Zhang, Z.; Li, B.; Chu, H. TBAJ-587, a Novel Diarylquinoline, Is Active against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2024, 68, e00945-24. [Google Scholar] [CrossRef]
- Longo, B.M.; Trunfio, M.; Calcagno, A. Dual β-Lactams for the Treatment of Mycobacterium abscessus: A Review of the Evidence and a Call to Act against an Antibiotic Nightmare. J. Antimicrob. Chemother. 2024, 79, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo Torres, M.; Van Ingen, J. Dual β-Lactam Therapy to Improve Treatment Outcome in Mycobacterium abscessus Disease. Clin. Microbiol. Infect. 2024, 30, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, L.; Tan, F.; Zhang, Y.; Fan, J.; Wang, X.; Zhang, Z.; Li, B.; Chu, H. A Novel Oxazolidinone, Contezolid (MRX-I), Expresses Anti-Mycobacterium Abscessus Activity In Vitro. Antimicrob. Agents Chemother. 2021, 65, e00889-21. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Nie, W.; Liu, L.; Su, L.; You, Y.; Geng, R.; Chu, N. Antibacterial Activity of the Novel Oxazolidinone Contezolid (MRX-I) against Mycobacterium abscessus. Front. Cell. Infect. Microbiol. 2023, 13, 1225341. [Google Scholar] [CrossRef]
- Ruth, M.M.; Koeken, V.A.C.M.; Pennings, L.J.; Svensson, E.M.; Wertheim, H.F.L.; Hoefsloot, W.; Van Ingen, J. Is There a Role for Tedizolid in the Treatment of Non-Tuberculous Mycobacterial Disease? J. Antimicrob. Chemother. 2020, 75, 609–617. [Google Scholar] [CrossRef]
- Tang, Y.W.; Cheng, B.; Yeoh, S.F.; Lin, R.T.P.; Teo, J.W.P. Tedizolid Activity Against Clinical Mycobacterium abscessus Complex Isolates—An in Vitro Characterization Study. Front. Microbiol. 2018, 9, 2095. [Google Scholar] [CrossRef]
- Poon, Y.K.; La Hoz, R.M.; Hynan, L.S.; Sanders, J.; Monogue, M.L. Tedizolid vs. Linezolid for the Treatment of Nontuberculous Mycobacteria Infections in Solid Organ Transplant Recipients. Open Forum Infect. Dis. 2021, 8, ofab093. [Google Scholar] [CrossRef]
- Koomanan, N.; Yvonne Zhou, P.; Andrea Kwa, L.H.; Chung, S.J. P-799. Eravacycline in the Management of Nontuberculous Mycobacteria (NTM) Infections: A Single-Centre Experience. Open Forum Infect. Dis. 2025, 12 (Suppl. S1), ofae631.991. [Google Scholar] [CrossRef]
| Intervention | Comparator | Population | Trial Name | Status |
|---|---|---|---|---|
| Bedaquiline, clofazimine, linezolid + 2 or 3 drugs | N/A | Severe NTM-PD (defined based on CT) with macrolide-resistant or non-responsive MAC & MAB | NCT05494957 - Phase 4, Open label, Single group assignment | Not yet recruiting |
| 2-drug regimen (macrolide + rifampin OR ethambutol) | 3-drug regimen (macrolide + rifampin AND ethambutol) | Non-cavitary MAC-PD (n = 474) | NCT03672630 - Phase 2/3 | In progress, estimated completion date October 2025 |
| RHB-204 (fixed dose combination tablet: clarithromycin, rifabutin, and clofazimine | Placebo | Nodular-bronchiectatic MAC | NCT04616924 (CleaR-MAC), - Phase 3, randomized, double-blind, placebo-controlled | Terminated due to slow enrollment |
| Class | Intervention vs. Comparator | Proposed MOA | Population | Study ID/Phase | Core Findings |
|---|---|---|---|---|---|
| Aminobenzimidazole | SPR720 (fobrepodacin) vs. Placebo | Oral pro-drug → SPR719; GyrB ATPase inhibitor | MAC-PD, treatment naïve or off therapy at least 3 months (n = 25) | NCT04553406 - Phase 2 (suspended December 2024) | Interim analysis (n = 16) did not show sufficient separation from placebo. Safety concern with 3 cases of grade 3 hepatotoxicity |
| Aminomethylcycline (3rd generation Tetracycline) | Omadacycline vs. Placebo | Protein synthesis inhibition | Non-cavitary MAB-PD not on therapy (n = 66) | NCT04922554 - Phase 2, double-blind, randomized, parallel-group, placebo-controlled | 34% of patients had improvement in at least half of their symptoms vs. 20% in placebo group (p value > 0.05). Omadacycline improved secondary efficacy outcomes (hypothesis generating) for symptom-based global impression endpoints [167]. |
| Benzoxaborole | Epetraborole (EBO-301) + GBT vs. Placebo + GBT | Protein synthesis inhibition through inhibition of bacterial leucyl-tRNA synthetase | Refractory MAC-PD (n = 117) | NCT05327803 - Phase 2/3, double-blind, randomized, parallel-group, placebo-controlled (Terminated August 2024) | Truncated Phase 3 study (n = 97) misses primary endpoint; results unable to confirm clinical efficacy observed in Phase 2 study. |
| Biologics | Inhaled Molgramostim in addition to GBT or no treatment vs. (no comparator) | Unclear, likely stimulation of macrophage proliferation and function | Refractory NTM-PD (n = 32) | NCT03421743 (OPTIMA) Phase 2, prospective, open-label | 8 patients (25%) achieved culture conversion which was sustained only in 4 of them. Main safety finding was eosinophilia [168]. |
| Biologics | Recombinant Interleukin-7 (CYT107) vs. (no comparator) | Proposed to be immune activation | Refractory MAC-PD (n = 8) | NCT04154826 (IMPULSE-7) Phase 2, prospective, single-blinded | early termination due to futility. After treatment for 4 weeks, there was no culture conversion at 6 months and no change in symptoms [169]. |
| Inhaled liposomal aminoglycoside | ALIS (liposomal amikacin) in addition to AZI + EMB for 6 months vs. Empty liposomal control in addition to AZI + EMB for 6 months | protein synthesis inhibition | New diagnosis non-cavitary MAC-PD or recurrent MAC-PD not receiving therapy | NCT04677543 (ARISE) - Phase 3, Randomized double blind, placebo-controlled | Addition of ALIS to standard therapy for 6 months led to higher culture conversion rates by Month 6 (80.6% vs. 63.9%) and Month 7 (78.8% vs. 47.1%) vs. comparator. (p = 0.001). No resistance to amikacin or macrolide developed; recurrence was significantly lower with ALIS (12.8% vs. 50%) and occurred in the absence of resistant emergence [170]. |
| Inhaled liposomal aminoglycoside | ALIS (liposomal amikacin) in addition to AZI + EMB for 12 months vs. Empty liposomal control in addition to AZI + EMB for 12 months | protein synthesis inhibition | New diagnosis non-cavitary MAC-PD or recurrent MAC-PD not receiving therapy | NCT04677569 (ENCORE) - Phase 3, Randomized double blind, placebo-controlled | Study in progress. This is the confirmatory study for ARISE to evaluate safety/efficacy at month 13 [1 month off treatment] as well as % with durable culture conversion at month 15 [3 months off treatment]. |
| Metal-based compounds | Intravenous Gallium nitrate in addition to GBT or no treatment vs. (no comparator) | Iron substitution and interruption of bacterial metabolism | Refractory NTM-PD in CF patients (n = 40) | NCT04294043 (ABATE), - Phase 1, safety study, prospective | Study in progress |
| Nitric oxide | Intermittent inhaled nitric oxide vs. (no comparator) | Oxidative and nitrosative stress induction | NTM-PD in CF and non-CF (n = 10) | NCT03748992 - Phase 2, single group assignment, | 4 out of 10 patients achieved negative sputum culture within 3 weeks but were positive again within 3 months post therapy) [171]. |
| Oxazolidinone | Delpazolid in addition to GBT vs. (no comparator) | Protein synthesis inhibition | Refractory MAB-PD (n = 20) | NCT06004037 - Phase 2, prospective | Study in progress |
| Personalized bacteriophages | Phage cocktails in addition to GBT vs. (no comparator) | Lytic phages lyse Mab cell wall | Refractory MAB in CF patients (n = 10) | NCT06262282 (POSTSTAMP) Prospective, single group assignment | Enrolling phase |
| Riminophenazine | Clofazimine loading dose in addition to GBT vs. (no comparator) | Cell replication disruption and ROS generation through binding Mycobacterial DNA | Non-CF extrapulmonary or pulmonary NTM disease and weight > 45 kg (n = 12) | NCT05294146 (C-LOAD) - Phase 2, PK study, open label, single group assignment | 4-week loading dose regimen of 300 mg once daily reduced the time to target clofazimine concentrations by ~1.5 months and was well tolerated with GI symptoms as main side effect [172]. |
| Riminophenazine | Inhaled clofazimine in addition to GBT vs. Placebo in addition to GBT | Cell replication disruption and ROS generation through binding Mycobacterial DNA | MAC-PD currently on GBT | NCT06418711 (ICoN-1) - Phase 3, randomized, double-blind, placebo-controlled | Study in progress |
| Tetracycline | Minocycline in addition to rifampin and other GBT vs. (no comparator) | Protein synthesis inhibition through 30S ribosomal subunit | Non-CF MAC-PD (planned n = 15) | NCT05861258 (Mino-PK) - Phase 2, PK study, single group assignment | Recruiting |
| Class | Compound Name | Proposed MOA | Notable Studies | Notable Findings |
|---|---|---|---|---|
| Benzothiazinone | Macozinone (PBTZ169, MCZ) | Cell wall synthesis inhibition | M. avium, MAB, and M. fortuitum clinical strains|MAB, M. chelonae, and M. fortuitum mouse models | Despite high MICs in vitro, it was bactericidal against M. abscessus & M. chelonae and bacteriostatic against M. avium & M. fortuitum in the lung and spleen of murine model Also, under development for TB, completed phase 1 safety trials [173,174]. |
| Benzoxaborole | MRX-5 (oral prodrug for MRX-6038) | Protein synthesis inhibition through inhibition of bacterial leucyl-tRNA synthetase | MAB in mouse model|Healthy human subjects for PK study | Phase 1 clinical trial in Australia showed no SEs (unpublished data). Undergoing phase 2 in China; FDA Orphan Drug Designation granted in December 2024 [175,176]. |
| Biguanide (host-directed) | Metformin | AMPK activation → autophagy & ROS ↑ Enhance macrophage activity | M. avium mouse model Cohort of patients in a large population-based study | Mouse lung bacterial load ↓; macrophage killing ↑ ↓ hazard of incident NTM infection by 62.4% in a cohort of pt with MAC disease also on metformin [177,178]. |
| Diarylquinoline | Sudapyridine (WX-081) | BDQ analogue (ATP-synthase blockade), ↓ QTc prolonging based on studies in tuberculosis [179]. | Clinical isolate M. avium and MAB|Intracellular MAB in macrophages|Immunocompromised mouse model infected with M. avium, MAB or M. chelonae [undergoing phase 2 for MDR-TB] | Bactericidal in vitro in all three strains Intracellular antimicrobial activity comparable to BDQ [180]. |
| Diarylquinoline | TBAJ-876 (3,5-dialkoxypyridine analogue of BDQ) | BDQ analogue, ↓ lipophilicity | MAB reference strains|MAB clinical isolates|Intracellular MAB in macrophages|immunocompromised mouse model | Bactericidal activity in vitro Intracellular antimicrobial activity comparable to BDQ Clinical efficacy in mouse model comparable to BDQ Compatible with other NTM meds [181]. |
| Dual beta-lactam +/− beta-lactamase inhibitor | Different combinations based on in vitro synergy testing mostly carbapenems + cephalosporins (e.g., ceftaroline + imipenem or ceftazidime + imipenem) +/− relebactam or avibactam (e.g., Imipenem/cilastatin + relebactam + amoxicillin) | Disruption of cell envelope through inhibition of both D, D-transpeptidases and L, D-transpeptidases Beta-lactamase inhibitors increase activity of beta-lactam | MAB complex In vitro|mouse models|case studies in treatment refractory MAB-PD | Synergistic effect against MAB in vitro and in vivo (although very limited data). Patient cases suggest a higher risk of elevated liver enzymes. No current clinical trial [182,183]. |
| Oxazolidinone | Contezolid (MRX-I) | inhibits bacterial protein synthesis, ↓ hematologic and neurologic toxicity compared to linezolid | MAB complex In vitro strains|zebrafish Compassionate use in MAB SSTI unable to tolerate linezolid | Inhibited MAB growth and prolonged zebrafish survival. Successful treatment of MAB SSTI with no adverse events reported after 6 months [184,185]. |
| Oxazolidinone | tedizolid | inhibits bacterial protein synthesis | Invitro laboratory and clinical isolates|single-centre retrospective cohort in solid organ transplant recipients (n = 15 tedizolid, 9 linezolid) | Efficacy reported against MAC with a lower MIC compared to linezolid in in vitro research. Synergy with ethambutol reported [186,187]. Retrospective data in solid organ transplant recipients showed similar efficacy and safety compared to linezolid over 7 weeks of therapy [188]. |
| Tetracycline | Eravacycline (intravenous) | Protein synthesis inhibition | retrospective, single-centre cohort of 16 patients | Despite claims of clinical improvement, nearly half discontinued ERV due to adverse events and only 50% completed OPAT. Results are limited by short treatment duration and unclear what clinical improvement entailed as primary efficacy outcome [189]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahid, S.; Yan, M.; Turvey, S.L. Review of the Canadian Nontuberculous Mycobacterial Disease Landscape—Challenges and Opportunities. Trop. Med. Infect. Dis. 2025, 10, 328. https://doi.org/10.3390/tropicalmed10120328
Vahid S, Yan M, Turvey SL. Review of the Canadian Nontuberculous Mycobacterial Disease Landscape—Challenges and Opportunities. Tropical Medicine and Infectious Disease. 2025; 10(12):328. https://doi.org/10.3390/tropicalmed10120328
Chicago/Turabian StyleVahid, Sepideh, Marie Yan, and Shannon Lee Turvey. 2025. "Review of the Canadian Nontuberculous Mycobacterial Disease Landscape—Challenges and Opportunities" Tropical Medicine and Infectious Disease 10, no. 12: 328. https://doi.org/10.3390/tropicalmed10120328
APA StyleVahid, S., Yan, M., & Turvey, S. L. (2025). Review of the Canadian Nontuberculous Mycobacterial Disease Landscape—Challenges and Opportunities. Tropical Medicine and Infectious Disease, 10(12), 328. https://doi.org/10.3390/tropicalmed10120328






