Non-Coding RNAs as Emerging Biomarkers in Leishmaniasis and Chagas Disease
Abstract
1. Introduction
2. Clinical Diagnosis of Leishmaniasis and Trypanosomiasis
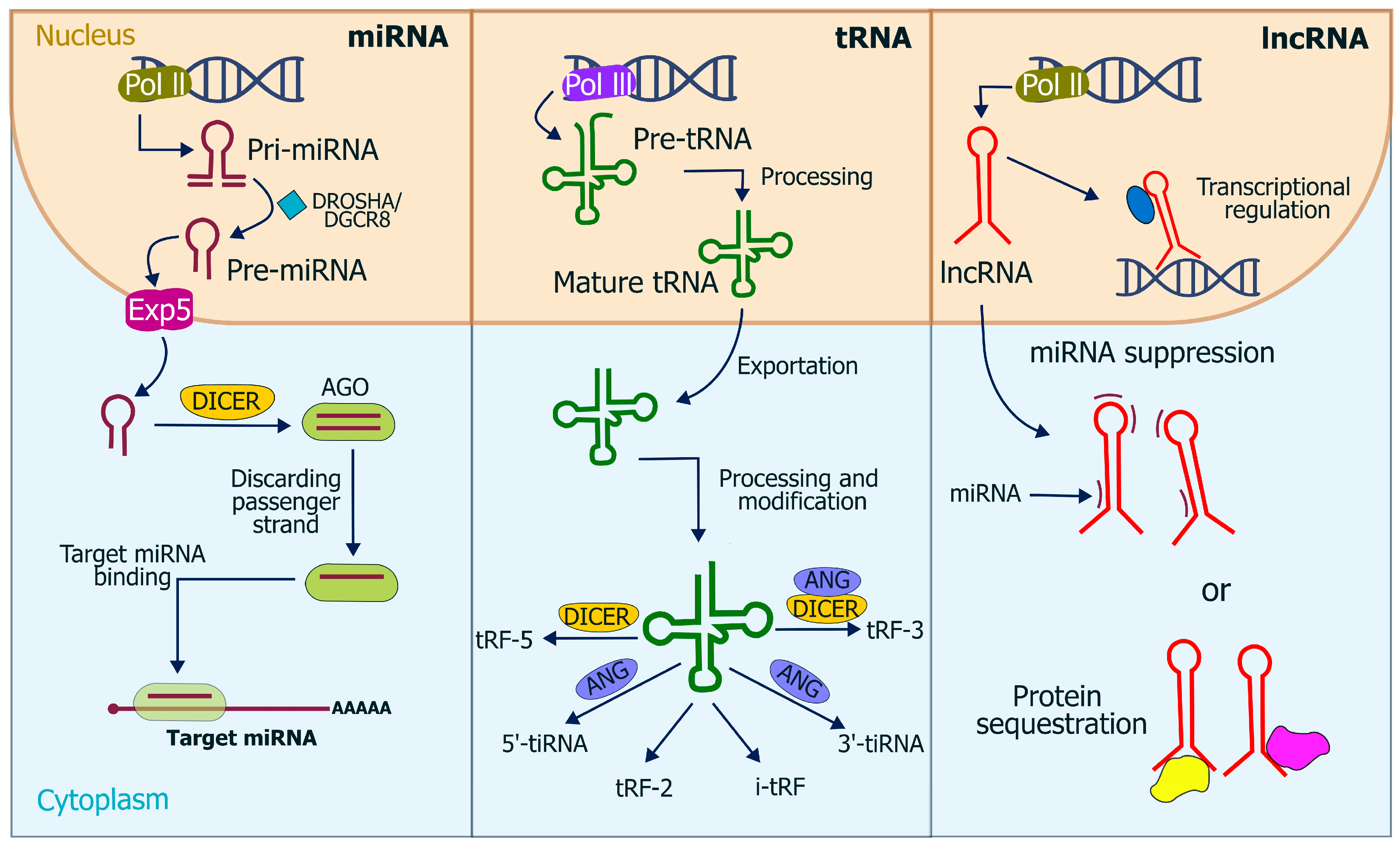
3. Characteristics of miRNA, piRNA, and lncRNA
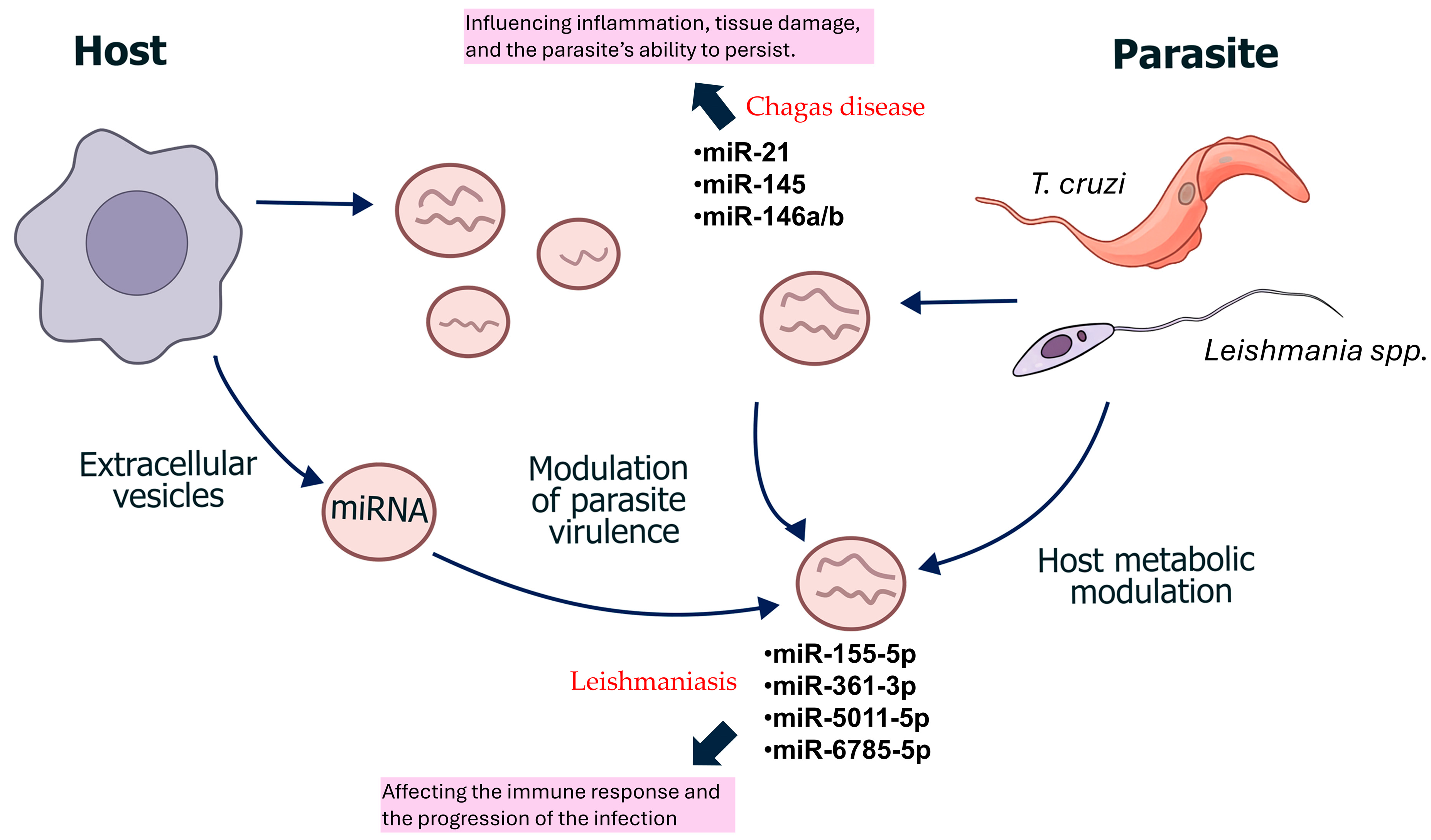
4. ncRNA in Kinetoplastids
5. ncRNA as Diagnostic Biomarkers in Leishmaniasis
6. ncRNAs as Emerging Biomarkers in the Diagnosis of Chagas Cardiomyopathy
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T. cruzi | Trypanosoma cruzi |
| miRNAs | microRNAs |
| ncRNAs | non-coding RNAs |
| lncRNAs | long non-coding RNAs |
| PAHO | Pan American Health Organization |
| piRNAs | PIWI-interacting RNAs |
| RNAome | repertoire of ncRNAs |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| siRNAs | short interfering RNAs |
| VL | visceral leishmaniasis |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 23 January 2024).
- Cruz-Reyes, A.; Pickering-López, J.M. Chagas disease in Mexico: An analysis of geographical distribution during the past 76 years—A review. Mem. Inst. Oswaldo Cruz 2006, 101, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, S.; Dondi, A.; Totaro, C.; Biagi, C.; Melchionda, F.; Zama, D.; Pierantoni, L.; Gennari, M.; Campagna, C.; Prete, A.; et al. Visceral Leishmaniasis: Epidemiology, Diagnosis, and Treatment Regimens in Different Geographical Areas with a Focus on Pediatrics. Microorganisms 2022, 10, 1887. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Castrejón, Ó.; Rivas-Sánchez, B. Apuntes para la historia de la enfermedad de Chagas en México. Bol. Med. Hosp. Infant. Mex. 2008, 65, 57–79. [Google Scholar]
- Reithinger, R.; Dujardin, J.C. Molecular diagnosis of leishmaniasis: Current status and future applications. J. Clin. Microbiol. 2007, 45, 21–25. [Google Scholar] [CrossRef]
- Lopez-Albizu, C.; Rivero, R.; Ballering, G.; Freilij, H.; Santini, M.S.; Bisio, M.M.C. Laboratory diagnosis of Trypanosoma cruzi infection: A narrative review. Front. Parasitol. 2023, 2, 1138375. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Clinical Testing and Diagnosis for Chagas Disease. Available online: https://www.cdc.gov/chagas/es/hcp/diagnosis-testing/pruebas-y-diagnostico-clinicos-para-la-enfermedad-de-chagas.html#:~:text=Use%20dos%20o%20m%C3%A1s%20pruebas,de%20anticuerpos%20inmunofluorescentes%20(IFA) (accessed on 3 January 2024).
- Pan American Health Organization (PAHO). Chagas Disease. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 3 January 2024).
- Bautista-Lopez, N.; Ndao, M. Usefulness of polymerase chain reaction tests in Chagas disease studies. Front. Parasitol. 2024, 3, 1292143. [Google Scholar] [CrossRef]
- Benatar, A.F.; Danesi, E.; Besuschio, S.A.; Bortolotti, S.; Cafferata, M.L.; Ramirez, J.C.; Albizu, C.L.; Scollo, K.; Baleani, M.; Lara, L.; et al. Prospective multicenter evaluation of real time PCR Kit prototype for early diagnosis of congenital Chagas disease. EBioMedicine 2021, 69, 103450. [Google Scholar] [CrossRef]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical Syndromes and Treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Diagnosis and Testing—Chagas Disease. Available online: https://www.cdc.gov/chagas/hcp/diagnosis-testing/index.html (accessed on 23 January 2024).
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.J.; Chiodini, P.L. Diagnosis and Clinical Management of Chagas Disease: An Increasing Challenge in Non-Endemic Areas. Res. Rep. Trop. Med. 2022, 13, 25–40. [Google Scholar] [CrossRef]
- Forsyth, C.J.; Manne-Goehler, J.; Bern, C.; Whitman, J.; Hochberg, N.S.; Edwards, M.; Marcus, R.; Beatty, N.L.; Castro-Sesquen, Y.E.; Coyle, C.; et al. Recommendations for Screening and Diagnosis of Chagas Disease in the United States. J. Infect. Dis. 2022, 225, 1601–1610. [Google Scholar] [CrossRef]
- Schijman, A.G.; Alonso-Padilla, J.; Britto, C.; Herrera Bernal, C.P. Retrospect, advances and challenges in Chagas disease diagnosis: A comprehensive review. Lancet Reg. Health Am. 2024, 36, 100821. [Google Scholar] [CrossRef] [PubMed]
- Pippadpally, S.; Venkatesh, T. Deciphering piRNA Biogenesis through Cytoplasmic Granules, Mitochondria and Exosomes. Arch. Biochem. Biophys. 2020, 695, 108597. [Google Scholar] [CrossRef]
- Russell, S.J.; Zhao, C.; Biondic, S.; Menezes, K.; Hagemann-Jensen, M.; Librach, C.L.; Petropoulos, S. An Atlas of Small Non-Coding RNAs in Human Preimplantation Development. Nat. Commun. 2024, 15, 8634. [Google Scholar] [CrossRef]
- Traber, G.M.; Yu, A.M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Deerberg, A.; Willkomm, S.; Restle, T. Minimal Mechanistic Model of siRNA-Dependent Target RNA Slicing by Recombinant Human Argonaute 2 Protein. Proc. Natl. Acad. Sci. USA 2013, 110, 17850–17855. [Google Scholar] [CrossRef]
- Atayde, V.D.; Shi, H.; Franklin, J.B.; Carriero, N.; Notton, T.; Lye, L.F.; Owens, K.; Beverley, S.M.; Tschudi, C.; Ullu, E. The Structure and Repertoire of Small Interfering RNAs in Leishmania (Viannia) braziliensis Reveal Diversification in the Trypanosomatid RNAi Pathway. Mol. Microbiol. 2013, 87, 580–593. [Google Scholar] [CrossRef]
- DaRocha, W.D.; Otsu, K.; Teixeira, S.M.; Donelson, J.E. Tests of Cytoplasmic RNA Interference (RNAi) and Construction of a Tetracycline-Inducible T7 Promoter System in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2004, 133, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most Mammalian mRNAs Are Conserved Targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, Action, and Degradation of RNA-Induced Silencing Complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la Différence: Biogenesis and Evolution of microRNAs in Plants and Animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef]
- Hynes, C.; Kakumani, P.K. Regulatory Role of RNA-Binding Proteins in microRNA Biogenesis. Front. Mol. Biosci. 2024, 11, 1374843. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Stoffel, M. MicroRNAs: A New Class of Regulatory Genes Affecting Metabolism. Cell Metab. 2006, 4, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.P.; Lau, N.C. Piwi Proteins and piRNAs Step onto the Systems Biology Stage. Adv. Exp. Med. Biol. 2014, 825, 159–197. [Google Scholar] [CrossRef]
- Théron, E.; Dennis, C.; Brasset, E.; Vaury, C. Distinct Features of the piRNA Pathway in Somatic and Germ Cells: From piRNA Cluster Transcription to piRNA Processing and Amplification. Mob. DNA 2014, 5, 28. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, P.J. Mammalian piRNAs: Biogenesis, Function, and Mysteries. Spermatogenesis 2014, 4, e27889. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Lu, Q.; Wei, J.; Yang, H.; Gu, M. Detection of Stably Expressed piRNAs in Human Blood. Int. J. Clin. Exp. Med. 2015, 8, 13353–13358. [Google Scholar] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Lima, F.M.; Ruiz, J.C.; Almeida, I.C.; da Silveira, J.F. Characterization of the Small RNA Content of Trypanosoma cruzi Extracellular Vesicles. Mol. Biochem. Parasitol. 2014, 193, 71–74. [Google Scholar] [CrossRef]
- Fort, R.S.; Chavez, S.; Trinidad Barnech, J.M.; Oliveira-Rizzo, C.; Smircich, P.; Sotelo-Silveira, J.R.; Duhagon, M.A. Current Status of Regulatory Non-Coding RNAs Research in the Tritryp. Noncoding RNA 2022, 8, 54. [Google Scholar] [CrossRef]
- Lye, L.F.; Dobson, D.E.; Beverley, S.M.; Tung, M.C. RNA Interference in Protozoan Parasites and Its Application. J. Microbiol. Immunol. Infect. 2025, 58, 281–287. [Google Scholar] [CrossRef]
- Poloni, J.F.; Oliveira, F.H.S.; Feltes, B.C. Localization Is the Key to Action: Regulatory Peculiarities of lncRNAs. Front. Genet. 2024, 15, 1478352. [Google Scholar] [CrossRef]
- Franzén, O.; Arner, E.; Ferella, M.; Nilsson, D.; Respuela, P.; Carninci, P.; Hayashizaki, Y.; Aslund, L.; Andersson, B.; Daub, C.O. The Short Non-Coding Transcriptome of the Protozoan Parasite Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2011, 5, e1283. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Gonçalves, A.N.A.; Floeter-Winter, L.M.; Nakaya, H.I.; Muxel, S.M. Comparative Transcriptomic Analysis of Long Noncoding RNAs in Leishmania-Infected Human FGs. Front. Genet. 2023, 13, 1051568. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular Vesicles Shed by Trypanosoma cruzi Are Linked to Small RNA Pathways, Life Cycle Regulation, and Susceptibility to Infection of Mammalian Cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Peng, R.; Santos, H.J.; Nozaki, T. Transfer RNA-Derived Small RNAs in the Pathogenesis of Parasitic Protozoa. Genes 2022, 13, 286. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Sanguinetti, J.; Cabrera-Cabrera, F.; Franzén, O.; Cayota, A. A Particular Set of Small Non-Coding RNAs Is Bound to the Distinctive Argonaute Protein of Trypanosoma cruzi: Insights from RNA-Interference Deficient Organisms. Gene 2014, 538, 379–384. [Google Scholar] [CrossRef]
- Ullu, E.; Tschudi, C.; Chakraborty, T. RNA Interference in Protozoan Parasites. Cell Microbiol. 2004, 6, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Marini, M.M.; da Silveira, J.F. Non-Coding RNAs in Host-Pathogen Interactions: Subversion of Mammalian Cell Functions by Protozoan Parasites. Front. Microbiol. 2017, 8, 474. [Google Scholar] [CrossRef]
- Martinez-Hernandez, J.E.; Aliaga-Tobar, V.; González-Rosales, C.; Monte-Neto, R.; Martin, A.J.M.; Maracaja-Coutinho, V. Comparative and Systems Analyses of Leishmania spp. Non-Coding RNAs through Developmental Stages. PLoS Negl. Trop. Dis. 2025, 19, e0013108. [Google Scholar] [CrossRef]
- Paul, S.; Ruiz-Manriquez, L.M.; Serrano-Cano, F.I.; Estrada-Meza, C.; Solorio-Diaz, K.A.; Srivastava, A. Human microRNAs in Host-Parasite Interaction: A Review. 3 Biotech 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Lago, T.S.; Silva, J.A.; Lago, E.L.; Carvalho, E.M.; Zanette, D.L.; Castellucci, L.C. The miRNA 361-3p, a Regulator of GZMB and TNF, Is Associated with Therapeutic Failure and Longer Time Healing of Cutaneous Leishmaniasis Caused by L. (Viannia) braziliensis. Front. Immunol. 2018, 9, 2621. [Google Scholar] [CrossRef]
- Masoudsinaki, T.; Hadifar, S.; Sarvnaz, H.; Farahmand, M.; Masoudzadeh, N.; Goyonlo, V.M.; Kerachian, M.; Salim, R.E.; Barhoumi, M.; Gargari, S.L.M.; et al. Altered miRNA Expression in the Lesions of Cutaneous Leishmaniasis Caused by L. major and L. tropica with Insights into Apoptosis Regulation. Sci. Rep. 2025, 15, 20680. [Google Scholar] [CrossRef]
- Varikuti, S.; Verma, C.; Natarajan, G.; Oghumu, S.; Satoskar, A.R. MicroRNA155 Plays a Critical Role in the Pathogenesis of Cutaneous Leishmania major Infection by Promoting a Th2 Response and Attenuating Dendritic Cell Activity. Am. J. Pathol. 2021, 191, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Akand, S.K.; Rahman, A.; Masood, M.; Tabrez, S.; Jawed, J.J.; Ahmed, M.Z.; Akhter, Y.; Haque, M.M.; Rub, A. Leishmania donovani alters the host sphingolipid biosynthetic pathway regulatory microRNA hsa-miR-15a-5p for its survival. Microbial pathogenesis. 2025, 208, 108019. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Zhang, Y.; Zhou, X. RNA-Seq Analysis of Differentially Expressed lncRNAs from Leishmaniasis Patients Compared to Uninfected Humans. Acta Trop. 2023, 238, 106738. [Google Scholar] [CrossRef]
- Rojas-Pirela, M.; Andrade-Alviárez, D.; Medina, L.; Castillo, C.; Liempi, A.; Guerrero-Muñoz, J.; Ortega, Y.; Maya, J.D.; Rojas, V.; Quiñones, W.; et al. MicroRNAs: Master Regulators in Host-Parasitic Protist Interactions. Open Biol. 2022, 12, 210395. [Google Scholar] [CrossRef]
- Villar, S.R.; Herreros-Cabello, A.; Callejas-Hernández, F.; Maza, M.C.; Del Moral-Salmoral, J.; Gómez-Montes, M.; Rodríguez-Angulo, H.O.; Carrillo, I.; Górgolas, M.; Bosch-Nicolau, P.; et al. Discovery of Circulating miRNAs as Biomarkers of Chronic Chagas Heart Disease via a Small RNA-Seq Approach. Sci. Rep. 2024, 14, 1187, Erratum in: Sci. Rep. 2024, 4, 18514. [Google Scholar] [CrossRef]
- Ribeiro, H.G.; Galdino, O.A.; de Souza, K.S.C.; Rosa Neta, A.P.; Lin-Wang, H.T.; Cunha-Neto, E.; Rezende, A.A.; Silbiger, V.N. Unraveling the Role of miRNAs as Biomarkers in Chagas Cardiomyopathy: Insights into Molecular Pathophysiology. PLoS Negl. Trop. Dis. 2024, 18, e0011865. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Macêdo, C.T.; Cavalcante, B.R.R.; Alcântara, A.C.; Silva, D.N.; Bezerra, M.D.R.; Caria, A.C.I.; Tavora, F.R.F.; Neto, J.D.S.; Noya-Rabelo, M.M.; et al. Circulating miRNAs as Potential Biomarkers Associated with Cardiac Remodeling and Fibrosis in Chagas Disease Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 4064. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, L.; Mariani, J.; Martínez, M.J.; Santalla, M.; Vensentini, N.; Kyle, D.A.; de Abreu, M.; Tajer, C.; Lacunza, E.; Ferrero, P. Circulating microRNAs as Biomarkers of Chagas Cardiomyopathy. Front. Cardiovasc. Med. 2023, 10, 1250029. [Google Scholar] [CrossRef] [PubMed]
- Rayford, K.J.; Cooley, A.; Strode, A.W.; Osi, I.; Arun, A.; Lima, M.F.; Misra, S.; Pratap, S.; Nde, P.N. Trypanosoma cruzi Dysregulates Expression Profile of piRNAs in Primary Human Cardiac Fibroblasts during Early Infection Phase. Front. Cell. Infect. Microbiol. 2023, 13, 1083379. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Cornet-Gomez, A.; O’Valle, F.; Garrido, J.M.; Serrano, F.R.; Nieto, A.I.; Osuna, A. Cardiac alterations induced by Trypanosoma cruzi extracellular vesicles and immune complexes. PLoS Negl. Trop. Dis. 2025, 19, e0013273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristic | Cutaneous Leishmaniasis | Mucocutaneous Leishmaniasis | Visceral Leishmaniasis |
|---|---|---|---|
| Causative agents | L. major, L. tropica (Old World); L. mexicana, L. amazonensis, L. guyanensis, L. panamensis, L. braziliensis (Americas) | Mainly L. braziliensis | L. donovani, L. infantum (Old World); L. infantum/L. chagasi (Americas); occasional cases caused by viscerotropic strains of L. tropica |
| Endemic regions | Old World: Africa, Middle East, Asia; New World: Central and South America | Primarily South America: Brazil, Peru, Bolivia; also seen in Colombia, Ecuador, Paraguay, and Venezuela | Old World: India, Pakistan, China, Africa, Mediterranean region; New World: mainly Brazil |
| Pathogenesis | Localized skin infection with an inflammatory reaction at the inoculation site | Spread or metastasis of the parasite from cutaneous lesions to nasopharyngeal and/or oropharyngeal mucosal tissues | Systemic dissemination to organs of the reticuloendothelial system (liver, spleen, bone marrow) |
| Clinical manifestations | Single or multiple; typically on exposed areas (face, arms, legs) | Progressive destruction of mucous membranes and soft tissues: nose, mouth, pharynx, eyelids, can cause severe disfigurement and respiratory/nutritional difficulties | Prolonged fever, weight loss, hepatosplenomegaly, anemia, pancytopenia, immunosuppression; HIV coinfection increases severity |
| Clinical course | Usually self-limiting, but may progress to mucocutaneous disease (especially with L. panamensis and L. braziliensis) | Chronic evolution can develop months or years after initial cutaneous lesions; high risk of complications | Potentially fatal if untreated; immunosuppression increases the risk of opportunistic infections. |
| Diagnosis | Visualization of amastigotes (Microscopic evaluation), culture, PCR (conventional and real-time); biopsy in diffuse cases | Parasitological and molecular diagnosis (DNA sequencing analysis; also cellulose acetate electrophoresis); evaluation of mucosal involvement | Combination of clinical findings, serology (ELISA, DAT), parasite visualization (smear, bone marrow aspirate), PCR (conventional and real-time) |
| Treatment | Local therapy in mild cases; systemic therapy in extensive disease or mucocutaneous risk | Prolonged systemic treatment; limited response, risk of recurrence | Requires immediate systemic therapy; management is more complex in HIV-coinfected patients |
| Prognosis | Good in most cases, especially with early treatment | Guarded; high morbidity and disfigurement; mortality mainly due to secondary infections and malnutrition | Severe if untreated; with appropriate therapy, mortality decreases significantly |
| Diagnostic Aspect | Acute Phase | Chronic Phase * |
|---|---|---|
| Morphology |
|
|
| Serology (Antibody Detection) |
|
|
| Molecular Testing |
|
|
| Feature | tRNA-Derived Small RNAs in T. cruzi | tRNA-Derived Small RNAs in Leishmania | lncRNAs |
|---|---|---|---|
| Size | 31–40 nt | 20–40 nt | >200 nt |
| Precursor | Mature tRNAs cleaved at anticodon loop (tRNA halves) or at D loop, T loop, anticodon loop, or 3′ leader (tRFs) | Mature tRNAs | Primary transcripts from RNA Pol I, II, or III |
| Processing enzymes | Dicer is absent in T. cruzi | Most Leishmania lack RNAi machinery | Standard RNA processing machinery |
| Associated proteins | TcPIWI-tryp (trypanosomatid-specific PIWI-like protein) | AGO/PIWI homolog (present in RNAi-deficient species) | Spliceosome, transcriptional regulators, RNA stability proteins |
| RNAi machinery | Absent (no canonical Dicer or Argonaute) | Absent in most species; retained only in L. braziliensis (Viannia subgenus) | N/A |
| Functional notes | TcPIWI-tryp binds small RNAs derived from structural RNAs; T. cruzi lost RNAi during evolution. | L. major, L. donovani, L. infantum lack RNAi; miRNA-like elements computationally predicted but not validated. | Differentially expressed during infection; potential regulatory roles in host–pathogen interactions. |
| ncRNA Type | Biomarker(s) | Sample/Clinical Context | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| miRNA | miR-155-5p, miR-5011-5p, miR-6785-5p, miR-4795-3p [55] | Lesion biopsies (L. major, L. tropica) | 86–100% | 100% | 0.92–1.00 |
| miRNA | miR-361-3p [54] | Lesion biopsies (L. braziliensis) | 81.20% | 100% | Not reported |
| lncRNA | MALAT1, NUTM2A-AS1, LINC00963, others [56] | Serum (visceral or cutaneous leishmaniasis patients) | Not quantified | Not quantified | Not quantified |
| ncRNA Type | Biomarker | Sample/Clinical Context | Sensitivity | Specificity | AUC (ROC) |
|---|---|---|---|---|---|
| miRNA [60] | miR-19a-3p | Serum | 67% | 80% | 0.77 |
| miR-21-5p | 57% | 60% | 0.54 | ||
| miR-29b-3p | 60% | 70% | 0.7 | ||
| miR-199b-5p | 67% | 57% | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Juárez, E.; Pérez-Campos Mayoral, E.; Pérez-Campos Mayoral, L.; Moreno Rodríguez, A.; Romero-Díaz, C.; Avendaño-Villegas, M.E.; Santiago Ramírez, T.S.; Martínez Cruz, M.; Hernández-Morales, J.L.; Bolaños-Hilario, L.G.; et al. Non-Coding RNAs as Emerging Biomarkers in Leishmaniasis and Chagas Disease. Trop. Med. Infect. Dis. 2025, 10, 319. https://doi.org/10.3390/tropicalmed10110319
Ramos Juárez E, Pérez-Campos Mayoral E, Pérez-Campos Mayoral L, Moreno Rodríguez A, Romero-Díaz C, Avendaño-Villegas ME, Santiago Ramírez TS, Martínez Cruz M, Hernández-Morales JL, Bolaños-Hilario LG, et al. Non-Coding RNAs as Emerging Biomarkers in Leishmaniasis and Chagas Disease. Tropical Medicine and Infectious Disease. 2025; 10(11):319. https://doi.org/10.3390/tropicalmed10110319
Chicago/Turabian StyleRamos Juárez, Eduardo, Eduardo Pérez-Campos Mayoral, Laura Pérez-Campos Mayoral, Adriana Moreno Rodríguez, Carlos Romero-Díaz, Miriam Emily Avendaño-Villegas, Tania Sinaí Santiago Ramírez, Margarito Martínez Cruz, José Luis Hernández-Morales, Lilian Guadalupe Bolaños-Hilario, and et al. 2025. "Non-Coding RNAs as Emerging Biomarkers in Leishmaniasis and Chagas Disease" Tropical Medicine and Infectious Disease 10, no. 11: 319. https://doi.org/10.3390/tropicalmed10110319
APA StyleRamos Juárez, E., Pérez-Campos Mayoral, E., Pérez-Campos Mayoral, L., Moreno Rodríguez, A., Romero-Díaz, C., Avendaño-Villegas, M. E., Santiago Ramírez, T. S., Martínez Cruz, M., Hernández-Morales, J. L., Bolaños-Hilario, L. G., Suárez Luna, I. K., Elizarrarás-Rivas, J., García González, A. A., Cabrera-Fuentes, H. A., Hernández-Huerta, M. T., & Pérez-Campos, E. (2025). Non-Coding RNAs as Emerging Biomarkers in Leishmaniasis and Chagas Disease. Tropical Medicine and Infectious Disease, 10(11), 319. https://doi.org/10.3390/tropicalmed10110319










