Web-Based Dashboard for Tracking Cryptococcosis-Related Deaths in Brazil (2000–2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population, Study Area, and Period
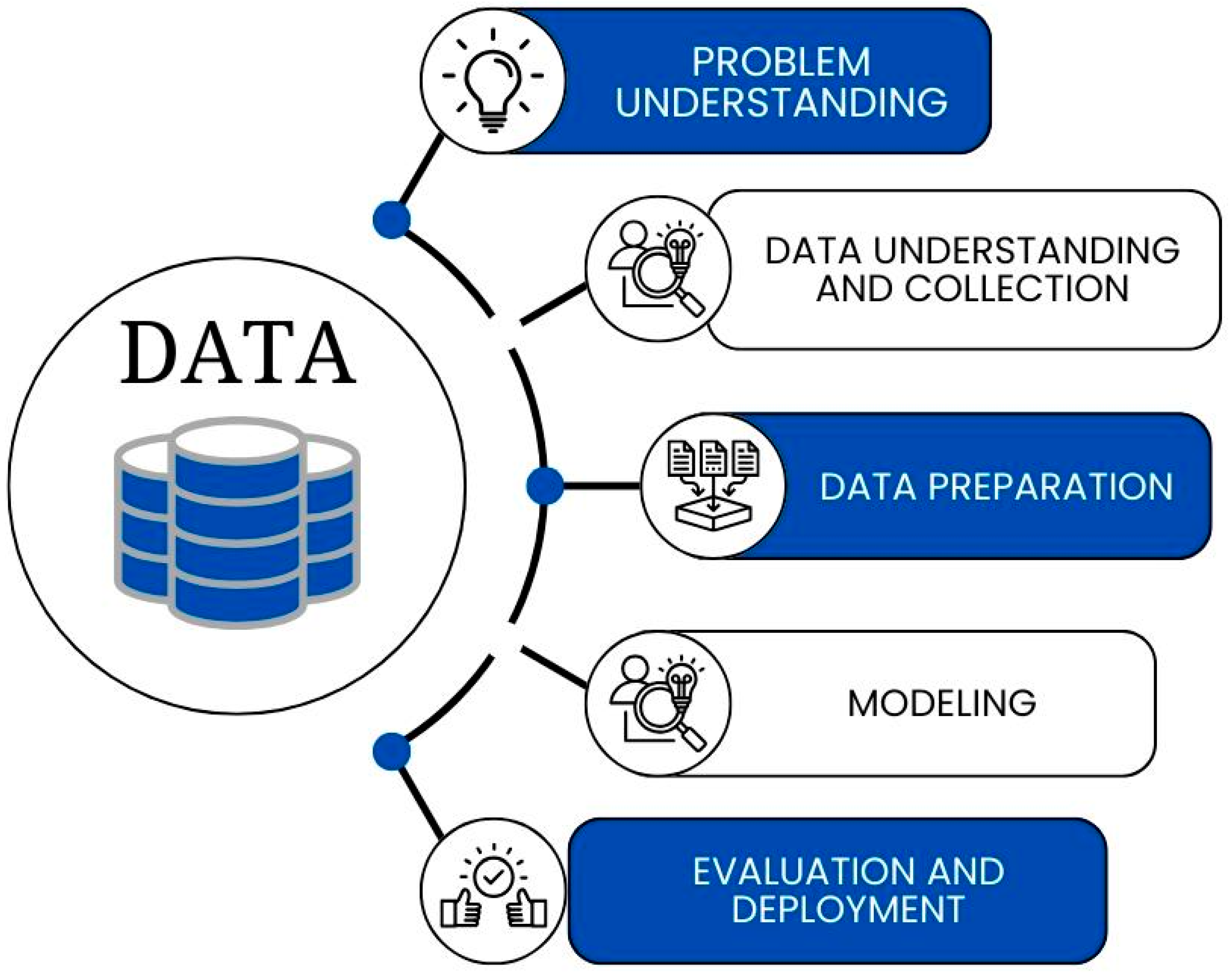
2.3. Problem Understanding
2.4. Data Acquisition and Initial Processing
2.5. Data Preparation and Study Variables
2.6. Modeling
2.7. Evaluation and Deployment
3. Results
3.1. Data Completeness and Quality
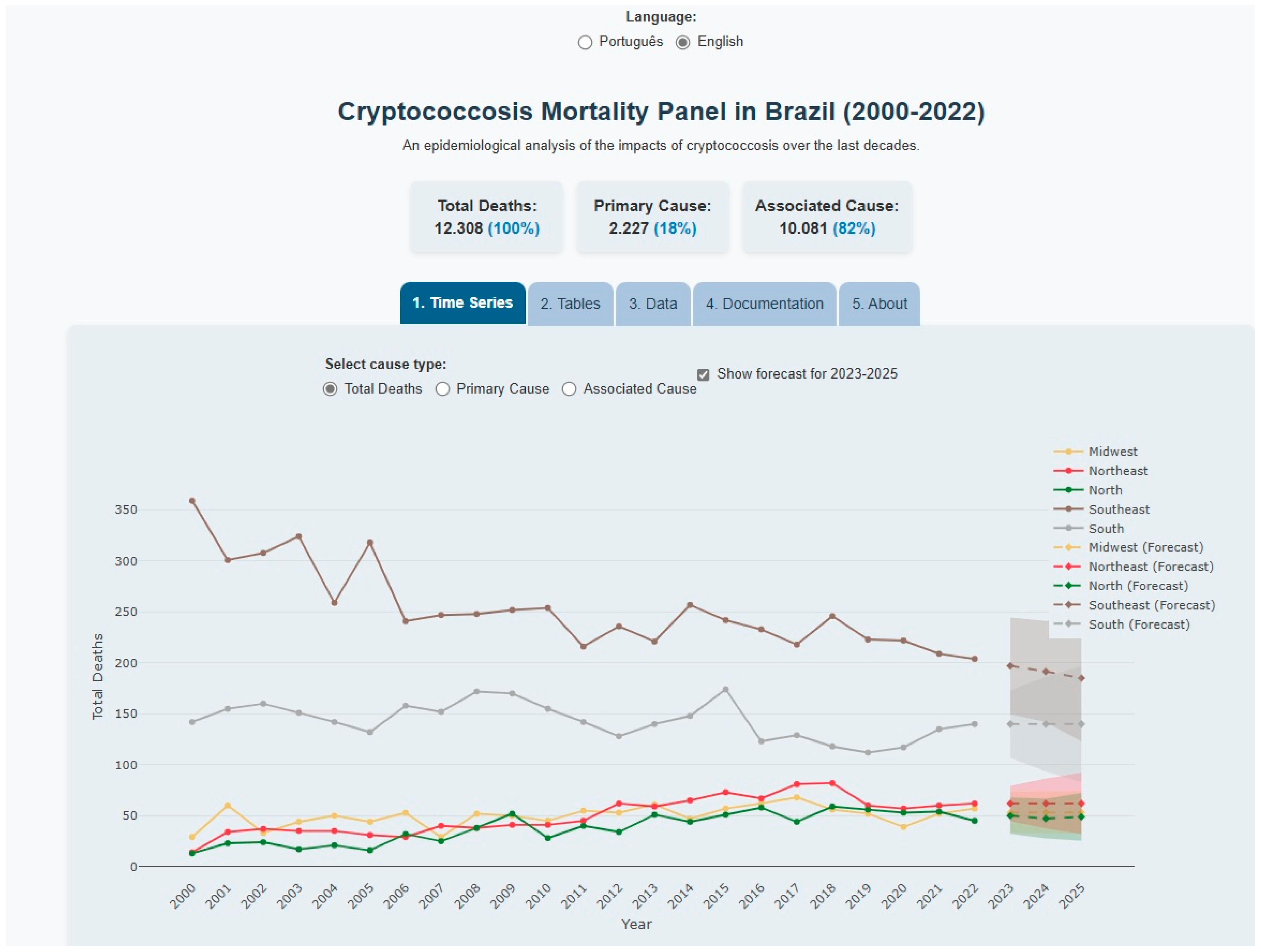
3.2. Dashboard Functionality and Regional Mortality Patterns
3.3. ARIMA Model Specifications and Forecasting Performance
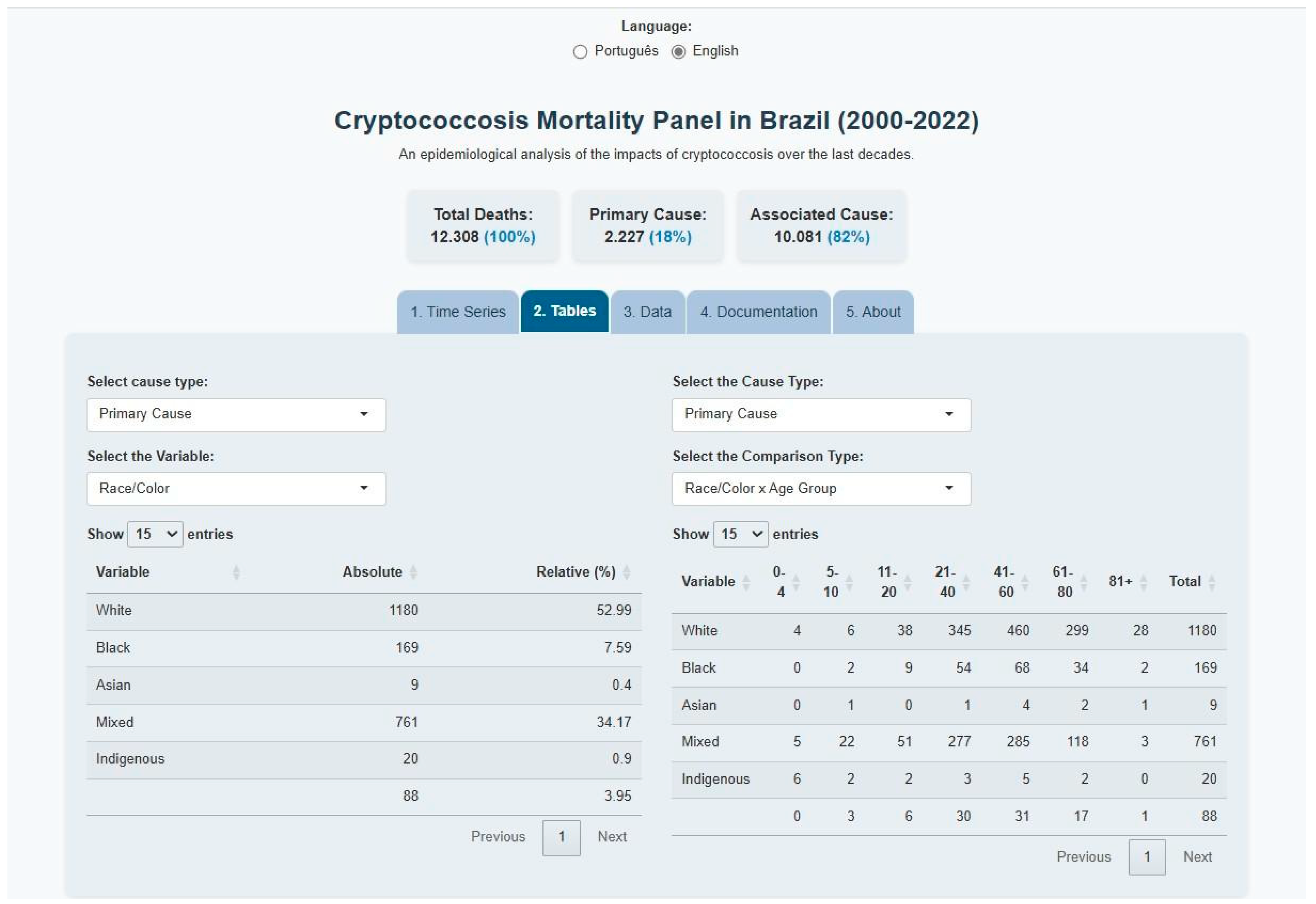
3.4. Sociodemographic Characteristics of Mortality
3.5. Data Export and Tool Accessibility
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIM | Mortality Information System |
| CRISP-DM | Cross-Industry Standard Process for Data Mining |
| DATASUS | Department of Informatics of the United Health Systems |
| UI | User Interface |
| ARIMA | AutoRegressive Integrated Moving Average |
| AR | Order of the autoregressive component |
| AM | Moving Average |
| AIC | Akaike Information Criterion |
| FAIR | Findable, Accessible, Interoperable, Reusable |
References
- Dollo, I.; Menu, E.; Dudouet, P.; Aubry, C.; L’Ollivier, C.; Ranque, S. Cryptococcosis at the university hospital of Marseille: A case series. J. Med. Mycol. 2024, 34, 101500. [Google Scholar] [CrossRef]
- Maciel, I.F.; de Freitas-Xavier, R.S.; Vicentini, A.P.; Apoliano, C.F.; Fernandes, J.R.; dos Santos Dias, A.; Gimenes, V.F.M.; Benard, G.; Vasconcelos, D.M. Evaluation of IFN-γ secretion after stimulation with C. neoformans and C. gattii antigens in individuals with frequent exposure to the fungus. J. Med. Mycol. 2022, 32, 101230. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Nielsen, K.; De Obaldia, A.L.; Heitman, J. Cryptococcus neoformans Mates on Pigeon Guano: Implications for the Realized Ecological Niche and Globalization. Eukaryot. Cell 2007, 6, 949–959. [Google Scholar] [CrossRef]
- Góralska, K.; Blaszkowska, J.; Dzikowiec, M. Neuroinfections caused by fungi. Infection 2018, 46, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.A.D.F.d.; Pedroso, R.d.S.; Borges, A.S.; Moreira, T.d.A.; Araújo, L.B.d.; Röder, D.V.D.d.B. The epidemiology of cryptococcosis and the characterization of Cryptococcus neoformans isolated in a Brazilian University Hospital. Rev. Inst. Med. Trop. São Paulo 2017, 59, e13. [Google Scholar] [CrossRef] [PubMed]
- Alves Soares, E.; Lazera, M.D.S.; Wanke, B.; Faria Ferreira, M.D.; Carvalhaes de Oliveira, R.V.; Oliveira, A.G.; Coutinho, Z.F. Mortality by cryptococcosis in Brazil from 2000 to 2012: A descriptive epidemiological study. PLoS Negl. Trop. Dis. 2019, 13, e0007569. [Google Scholar] [CrossRef]
- Corrêa Pinheiro, M.; Dos Reis, D.S.T.; de Brito, M.T.F.M.; Simões Quaresma, J.A. Cryptococcosis in the Amazon: A current overview and future perspectives. Acta Trop. 2019, 197, 105023. [Google Scholar] [CrossRef]
- dos Reis, D.S.T.; de Brito, M.T.F.M.; Guimarães, R.J.d.P.S.; Quaresma, J.A.S. Cryptococcosis: Identification of Risk Areas in the Brazilian Amazon. Microorganisms 2022, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Friedrichson, B.; Ketomaeki, M.; Jasny, T.; Old, O.; Grebe, L.; Nürenberg-Goloub, E.; Adam, E.H.; Zacharowski, K.; Kloka, J.A. Web-based Dashboard on ECMO Utilization in Germany: An Interactive Visualization, Analysis, and Prediction Based on Real-life Data. J. Med. Syst. 2024, 48, 48. [Google Scholar] [CrossRef]
- Poppe, J.A.; Smorenburg, R.S.; Goos, T.G.; Taal, H.R.; Reiss, I.K.M.; Simons, S.H.P. Development of a Web-Based Oxygenation Dashboard for Preterm Neonates: A Quality Improvement Initiative. J. Med. Syst. 2024, 48, 46. [Google Scholar] [CrossRef]
- Kahn, R.A.; Egorova, N.; Ouyang, Y.; Burnett, G.W.; Hofer, I.; Wax, D.B.; Trinh, M. Influence of Practitioner Dashboard Feedback on Anesthetic Greenhouse Gas Emissions: A Prospective Performance Improvement Investigation. J. Med. Syst. 2025, 49, 12. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.G.; Donlon, K.; Siewert, J.D.; Franken, T.; Lewis, N.E. Improving the Efficiency and Ease of Healthcare Analysis Through Use of Data Visualization Dashboards. Big Data 2016, 4, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Brand, F.; Geppert, J.; Böl, G.F. Digital Dashboards Visualizing Public Health Data: A Systematic Review. Front Public Health. 4 May 2023. Available online: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2023.999958/full (accessed on 15 February 2025).
- Concannon, D.; Herbst, K.; Manley, E. Developing a Data Dashboard Framework for Population Health Surveillance: Widening Access to Clinical Trial Findings. JMIR Form. Res. 2019, 3, e11342. [Google Scholar] [CrossRef]
- Dasgupta, N.; Kapadia, F. The Future of the Public Health Data Dashboard. Am. J. Public Health 2022, 112, 886–888. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, H.; Joshi, M.C.; Sharma, A. Digital dashboards with paradata can improve data quality where disease surveillance relies on real-time data collection. Digit. Health 2023, 9, 20552076231164098. [Google Scholar] [CrossRef]
- Kenigsberg, T.A.; Hause, A.M.; McNeil, M.M.; Nelson, J.C.; Ann Shoup, J.; Goddard, K.; Lou, Y.; Hanson, K.E.; Glenn, S.C.; Weintraub, E.S. Dashboard development for near real-time visualization of COVID-19 vaccine safety surveillance data in the Vaccine Safety Datalink. Vaccine 2022, 40, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Statistical Modeling: The Two Cultures (with comments and a rejoinder by the author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Ferraz, V.C.d.A.B.; Ferezin, V.V.; Knoch, M.; Durovni, B.; Saraceni, V.; Lahdo, V.; dos Santos, M.L.d.M.; De-Carli, A.D. Painéis de monitoramento de dados epidemiológicos como estratégia de gestão da vigilância e da atenção à saúde. Ciência Saúde Coletiva 2024, 29, e04142024. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/estatisticas/downloads-estatisticas.html (accessed on 21 January 2025).
- Saldanha, R.d.F.; Bastos, R.R.; Barcellos, C. Microdatasus: Pacote para download e pré-processamento de microdados do Departamento de Informática do SUS (DATASUS). Cad. Saúde Pública 2019, 35, e00032419. [Google Scholar] [CrossRef]
- Morais, R.M.d.; Costa, A.L. Uma avaliação do Sistema de Informações sobre Mortalidade. Saúde Em Debate 2017, 41, 101–117. [Google Scholar] [CrossRef]
- Rebouças, P.; Alves, F.J.; Ferreira, A.; Marques, L.; Guimarães, N.S.; Souza, G.R.d.; Pinto, P.F.P.S.; Teixeira, C.; Ortelan, N.; Silva, N.; et al. Avaliação da qualidade do Sistema Brasileiro de Informações sobre Mortalidade (SIM): Uma scoping review. Ciência Saúde Coletiva 2025, 30, e08462023. [Google Scholar] [CrossRef]
- Schenkman, S.; Bousquat, A. Intersectional Equity in Brazil’s Remote Rural Municipalities: The Road to Efficiency and Effectiveness in Local Health Systems. Front Public Health. 10 September 2024. Available online: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1401193/full (accessed on 20 February 2025).
- Boing, A.F.; Boing, A.C.; Subramanian, S.V. Inequalities in the access to healthy urban structure and housing: An analysis of the Brazilian census data. Cad. Saúde Pública 2021, 37, e00233119. [Google Scholar] [CrossRef]
- Roman, A. A Closer Look Into Brazil’s Healthcare System: What Can We Learn? Cureus 2023, 15, e38390. [Google Scholar] [CrossRef]
- de Souza, H.P.; de Oliveira, W.T.G.H.; dos Santos, J.P.C.; Toledo, J.P.; Ferreira, I.P.S.; de Sousa Esashika, S.N.G.; de Lima, T.F.P.; de Sousa Delácio, A. Doenças infecciosas e parasitárias no Brasil de 2010 a 2017: Aspectos para vigilância em saúde. Rev. Panam. Salud Pública 2020, 44, e10. [Google Scholar] [CrossRef]
- Segurado, A.C.; Cassenote, A.J.; Luna Ede, A. Saúde nas metrópoles-Doenças infecciosas. Estud. Avançados 2016, 30, 29–49. [Google Scholar] [CrossRef]
- Pasquier, E.; Kunda, J.; De Beaudrap, P.; Loyse, A.; Temfack, E.; Molloy, S.F.; Harrison, T.S.; Lortholary, O. Long-term Mortality and Disability in Cryptococcal Meningitis: A Systematic Literature Review. Clin. Infect. Dis. 2018, 66, 1122–1132. [Google Scholar] [CrossRef]
- Dao, A.; Kim, H.Y.; Garnham, K.; Kidd, S.; Sati, H.; Perfect, J.; Sorrell, T.C.; Harrison, T.; Rickerts, V.; Gigante, V.; et al. Cryptococcosis—A systematic review to inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae043. [Google Scholar] [CrossRef]
- Hurtado, J.C.; Castillo, P.; Fernandes, F.; Navarro, M.; Lovane, L.; Casas, I.; Quintó, L.; Marco, F.; Jordao, D.; Ismail, M.R.; et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: An autopsy study. Sci. Rep. 2019, 9, 7493. [Google Scholar] [CrossRef]
- Barreto, M.; Ichihara, M.; Almeida, B.; Barreto, M.; Cabral, L.; Fiaccone, R.; Carreiro, R.P.; Teles, C.A.S.; Pitta, R.; Penna, G.O.; et al. The Centre for Data and Knowledge Integration for Health (CIDACS): Linking Health and Social Data in Brazil. Int. J. Popul. Data Sci. 2019, 4, 1140. [Google Scholar]
- Coelho Neto, G.C.; Andreazza, R.; Chioro, A. Integration among national health information systems in Brazil: The case of e-SUS Primary Care. Rev. Saúde Pública 2021, 55, 93. [Google Scholar]
- Chor, D.; Pereira, A.; Pacheco, A.G.; Santos, R.V.; Fonseca, M.J.M.; Schmidt, M.I.; Duncan, B.B.; Barreto, S.M.; Aquino, E.M.L.; Mill, J.G.; et al. Context-dependence of race self-classification: Results from a highly mixed and unequal middle-income country. PLoS ONE 2019, 14, e0216653. [Google Scholar] [CrossRef] [PubMed]
- da Conceição, J.R.; Lopes, C.P.G.; Ferreira, E.I.; Epiphanio, S.; Giarolla, J. Neglected tropical diseases and systemic racism especially in Brazil: From socio-economic aspects to the development of new drugs. Acta Trop. 2022, 235, 106654. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Harrison, T.S.; Bicanic, T.A.; Chayakulkeeree, M.; Sorrell, T.C.; Warris, A.; Hagen, F.; Spec, A.; Oladele, R.; Govender, N.P.; et al. Global guideline for the diagnosis and management of cryptococcosis: An initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect. Dis. 2024, 24, e495–e512. [Google Scholar] [CrossRef]
- Tugume, L.; Ssebambulidde, K.; Kasibante, J.; Ellis, J.; Wake, R.M.; Gakuru, J.; Lawrence, D.S.; Abassi, M.; Rajasingham, R.; Meya, D.B.; et al. Cryptococcal meningitis. Nat. Rev. Dis. Primer. 2023, 9, 1–18. [Google Scholar] [CrossRef]
- Lin, S.; Tao, S.; Chou, W.C.; Zhang, G.Q.; Li, X. VisualSphere: A Web-based Interactive Visualization System for Clinical Research Data. AMIA Summits Transl. Sci. Proc. 2024, 2024, 603–612. [Google Scholar]
- Carroll, L.N.; Au, A.P.; Detwiler, L.T.; Fu Tchieh Painter, I.S.; Abernethy, N.F. Visualization and analytics tools for infectious disease epidemiology: A systematic review. J. Biomed. Inform. 2014, 51, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, A.; Rullo, A.; Vocaturo, E. Process mining applications in the healthcare domain: A comprehensive review. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2022, 12, e1442. [Google Scholar] [CrossRef]
- Sinaci, A.A.; Gencturk, M.; Teoman, H.A.; Erturkmen, G.B.L.; Alvarez-Romero, C.; Martinez-Garcia, A.; Poblador-Plou, B.; Carmona-Pírez, J.; Löbe, M.; Parra-Calderon, C.L. A Data Transformation Methodology to Create Findable, Accessible, Interoperable, and Reusable Health Data: Software Design, Development, and Evaluation Study. J. Med. Internet Res. 2023, 25, e42822. [Google Scholar] [CrossRef]
- Alvarez-Romero, C.; Martínez-García, A.; Sinaci, A.A.; Gencturk, M.; Méndez, E.; Hernández-Pérez, T.; Liperoti, R.; Angioletti, C.; Löbe, M.; Ganapathy, N.; et al. FAIR4Health: Findable, Accessible, Interoperable and Reusable data to foster Health Research. Open Res. Eur. 2022, 2, 34. [Google Scholar]
- Mahajan, S. Design and development of an open-source framework for citizen-centric environmental monitoring and data analysis. Sci. Rep. 2022, 12, 14416. [Google Scholar] [CrossRef]
- Uelmen, J.A.; Clark, A.; Palmer, J.; Kohler, J.; Van Dyke, L.C.; Low, R.; Mapes, C.D.; Carney, R.M. Global mosquito observations dashboard (GMOD): Creating a user-friendly web interface fueled by citizen science to monitor invasive and vector mosquitoes. Int. J. Health Geogr. 2023, 22, 28. [Google Scholar] [CrossRef]
- Chokki, A.P.; Simonofski, A.; Frénay, B.; Vanderose, B. Engaging Citizens with Open Government Data: The Value of Dashboards Compared to Individual Visualizations. Digit. Gov. Res. Pract. 2022, 3, 1–20. [Google Scholar] [CrossRef]
- Sato, R.C. Gerenciamento de doenças utilizando séries temporais com o modelo ARIMA. Einstein São Paulo 2013, 11, 128–131. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Jiang, B.; Yang, W. Forecasting Incidence of Hemorrhagic Fever with Renal Syndrome in China Using ARIMA Model. BMC Infect. Dis. 2011, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, J.; Liu, G. Application of Seasonal Time Series Model in the Precipitation Forecast. Math. Comput. Model. 2013, 58, 677–683. [Google Scholar] [CrossRef]
- Ramos, P.I.P.; Marcilio, I.; Bento, A.I.; Penna, G.O.; Oliveira, J.F.d.; Khouri, R.; Andrade, R.F.S.; Carreiro, R.P.; Oliveira, V.d.A.; Galvão, L.A.C.; et al. Combining Digital and Molecular Approaches Using Health and Alternate Data Sources in a Next-Generation Surveillance System for Anticipating Outbreaks of Pandemic Potential. JMIR Public Health Surveill. 2024, 10, e47673. [Google Scholar] [CrossRef]
- Miranda, A.E.; Golub, J.E.; Lucena, F.d.F.; Maciel, E.N.; Gurgel, M.d.F.; Dietze, R. Tuberculosis and AIDS Co-Morbidity in Brazil: Linkage of the Tuberculosis and AIDS Databases. Braz. J. Infect. Dis. 2009, 13, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, M.V.; Sakabe, S.; Waldman, E.A. Mortality and Survival of Tuberculosis Coinfected Patients Living with AIDS in São Paulo, Brazil: A 12-Year Cohort Study. BMC Infect. Dis. 2022, 22, 223. [Google Scholar] [CrossRef]
- Ali, M.S.; Ichihara, M.Y.; Lopes, L.C.; Barbosa, G.C.G.; Pita, R.; Carreiro, R.P.; dos Santos, D.B.; Ramos, D.; Bispo, N.; Raynal, F.; et al. Administrative Data Linkage in Brazil: Potentials for Health Technology Assessment. Front. Pharmacol. 2019, 10, 984. [Google Scholar] [CrossRef]
- de Lima, L.V.; Pavinati, G.; de Oliveira, R.R.; Couto, R.d.M.; Alves, K.B.A.; Magnabosco, G.T. Temporal Trend in the Incidence of Tuberculosis-HIV Coinfection in Brazil, by Macro-Region, Federative Unit, Sex and Age Group, 2010–2021. Epidemiol. E Serviços Saúde 2024, 33, e2023522. [Google Scholar] [CrossRef]
- Studer, S.; Bui, T.B.; Drescher, C.; Hanuschkin, A.; Winkler, L.; Peters, S.; Müller, K.-R. Towards CRISP-ML(Q): A Machine Learning Process Model with Quality Assurance Methodology. Mach. Learn. Knowl. Extr. 2021, 3, 392–413. [Google Scholar] [CrossRef]
- Elkabalawy, M.; Al-Sakkaf, A.; Mohammed Abdelkader, E.; Alfalah, G. CRISP-DM-Based Data-Driven Approach for Building Energy Prediction Utilizing Indoor and Environmental Factors. Sustainability 2024, 16, 7249. [Google Scholar] [CrossRef]
- Bulstra, C.A.; Hontelez, J.A.C.; Otto, M.; Stepanova, A.; Lamontagne, E.; Yakusik, A.; El-Sadr, W.M.; Apollo, T.; Rabkin, M.; Atun, R.; et al. Integrating HIV Services and Other Health Services: A Systematic Review and Meta-Analysis. PLoS Med. 2021, 18, e1003836. [Google Scholar] [CrossRef]
- Brazil, Conselho Nacional de Saúde. Resolução n 466, de 12 de dezembro de 2012. Diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Of. União República Fed. Bras. 2013, 150, 59–62. [Google Scholar]
- Brazil, Conselho Nacional de Saúde. Resolução n 738, de 01 de Fevereiro de 2024. Dispõe Sobre o uso de Bancos de Dados com Finalidade de Pesquisa Científica Envolvendo seres Humanos. Diário Of. União República Fed. Bras. 2024, 1–12. Available online: https://www.gov.br/conselho-nacional-de-saude/pt-br/atos-normativos/resolucoes/2024/resolucao-no-738.pdf/view (accessed on 12 August 2025).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, E.R.L.; Nielsen, L.; Melo-Neto, J.S.d.; Miranda, C.d.S.C.; Gonçalves, N.V.; Sousa, R.C.M.; Rodrigues, A.R. Web-Based Dashboard for Tracking Cryptococcosis-Related Deaths in Brazil (2000–2022). Trop. Med. Infect. Dis. 2025, 10, 304. https://doi.org/10.3390/tropicalmed10110304
Figueiredo ERL, Nielsen L, Melo-Neto JSd, Miranda CdSC, Gonçalves NV, Sousa RCM, Rodrigues AR. Web-Based Dashboard for Tracking Cryptococcosis-Related Deaths in Brazil (2000–2022). Tropical Medicine and Infectious Disease. 2025; 10(11):304. https://doi.org/10.3390/tropicalmed10110304
Chicago/Turabian StyleFigueiredo, Eric Renato Lima, Lucca Nielsen, João Simão de Melo-Neto, Claudia do Socorro Carvalho Miranda, Nelson Veiga Gonçalves, Rita Catarina Medeiros Sousa, and Anderson Raiol Rodrigues. 2025. "Web-Based Dashboard for Tracking Cryptococcosis-Related Deaths in Brazil (2000–2022)" Tropical Medicine and Infectious Disease 10, no. 11: 304. https://doi.org/10.3390/tropicalmed10110304
APA StyleFigueiredo, E. R. L., Nielsen, L., Melo-Neto, J. S. d., Miranda, C. d. S. C., Gonçalves, N. V., Sousa, R. C. M., & Rodrigues, A. R. (2025). Web-Based Dashboard for Tracking Cryptococcosis-Related Deaths in Brazil (2000–2022). Tropical Medicine and Infectious Disease, 10(11), 304. https://doi.org/10.3390/tropicalmed10110304





