Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Recruitment
2.3. Data Collection
2.4. Laboratory Testing
2.5. Data Analysis
3. Results
3.1. Characteristics of Farm Workplace and Study Participants
3.2. Seroprevalence Estimates
3.3. Multivariable Analysis of Risk Factors Associated with Brucella IgG Seropositivity
3.4. Associations Between Brucella Seropositivity and Self-Reported Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| CI | Confidence interval |
| ELISA | enzyme-linked immunosorbent assay |
| IgG | Immunoglobulin G |
| OR | Odds ratio |
| ODK | Open data kit |
| RBT | Rose Bengal test |
| RVF | Rift Valley Fever |
References
- O’Callaghan, D. Human brucellosis: Recent advances and future challenges. Infect. Dis. Poverty 2020, 9, 101. [Google Scholar] [CrossRef]
- Laine, C.G.; Johnson, V.E.; Scott, H.M.; Arenas-Gamboa, A.M. Global estimate of human brucellosis incidence. Emerg. Infect. Dis. 2023, 29, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. 2013, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Corbel, M.J. Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Yousefi-Nooraie, R.; Mortaz-Hejri, S.; Mehrani, M.; Sadeghipour, P. Antibiotics for treating human brucellosis. Cochrane Database Syst. Rev. 2012, 10, CD007179. [Google Scholar] [CrossRef]
- Schrire, T. Human brucellosis in South Africa. S. Afr. Med. J. 1962, 36, 342–349. [Google Scholar]
- Küstner, H.G.V. Brucellosis in the eastern Orange Free State. Epidemiol. Comments 1985, 12, 2–23. [Google Scholar]
- Wojno, J.M.; Moodley, C.; Pienaar, J.; Beylis, N.; Jacobsz, L.; Nicol, M.P.; Rossouw, J.; Bamford, C. Human brucellosis in South Africa: Public health and diagnostic pitfalls. S. Afr. Med. J. 2016, 106, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Frean, J.; Cloete, A.; Rossouw, J.; Blumberg, L. Brucellosis in South Africa—a notifiable medical condition. NICD Commun. Dis. Surveill. Bull. 2018, 16, 110–117. [Google Scholar]
- Pal, M.; Gizaw, F.; Fekadu, G.; Alemayehu, G.; Kandi, V. Public health and economic importance of bovine brucellosis: An overview. Am. J. Epidemiol. Infect. Dis. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Department of Agriculture, Forestry and Fisheries (DAFF). Discussion Paper on the Review of Bovine Brucellosis Control in South Africa; DAFF: Pretoria, South Africa, 2017.
- Cloete, A.; Gerstenberg, C.; Mayet, N.; Tempia, S. Brucellosis knowledge, attitudes and practices of a South African communal cattle keeper group. Onderstepoort J. Vet. Res. 2019, 86, a1671. [Google Scholar] [CrossRef]
- Simpson, G.J.; Quan, V.; Frean, J.; Knobel, D.L.; Rossouw, J.; Weyer, J.; Marcotty, T.; Godfroid, J.; Blumberg, L.H. Prevalence of selected zoonotic diseases and risk factors at a human-wildlife-livestock interface in Mpumalanga Province, South Africa. Vector Borne Zoonotic Dis. 2018, 18, 303–310. [Google Scholar] [CrossRef]
- Govindasamy, V.; Etter, E.M.C.; Harris, B.N.; Rossouw, J.; Abernethy, D.A.; Thompson, P.N. Knowledge of brucellosis, health-seeking behaviour, and risk factors for Brucella infection amongst workers on cattle farms in Gauteng, South Africa. Pathogens 2021, 10, 1484. [Google Scholar] [CrossRef]
- Govindasamy, V.; Thompson, P.N.; Harris, B.N.; Rossouw, J.; Abernethy, D.A.; Etter, E.M.C. Bovine brucellosis in Gauteng, South Africa: Seroprevalence amongst cattle handlers and variables associated with seropositive cattle herds, 2014–2016. Pathogens 2021, 10, 1547. [Google Scholar] [CrossRef]
- Kolo, F.B.; Adesiyun, A.A.; Fasina, F.O.; Harris, B.N.; Rossouw, J.; Byaruhanga, C.; Geyer, H.D.W.; Blumberg, L.; Frean, J.; van Heerden, H. Brucellosis seropositivity using three serological tests and associated risk factors in abattoir workers in Gauteng Province, South Africa. Pathogens 2024, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, C.G.; Burt, F.J.; Van Heerden, N.; Van Zyl, W.; Anthonissen, T.; Musoke, J. Prevalence and occupational exposure to zoonotic diseases in high-risk populations in the Free State Province, South Africa. Front. Microbiol. 2023, 14, 1196044. [Google Scholar] [CrossRef] [PubMed]
- Msimang, V.; Thompson, P.N.; Jansen van Vuren, P.; Tempia, S.; Cordel, C.; Kgaladi, J.; Khosa, J.; Burt, F.J.; Liang, J.; Rostal, M.K.; et al. Rift Valley fever virus exposure amongst farmers, farm workers, and veterinary professionals in central South Africa. Viruses 2019, 11, 140. [Google Scholar] [CrossRef]
- Bartlett, J.E.; Kotrlik, J.; Higgins, C. Organizational research: Determining appropriate sample size in survey research. Inf. Technol. Learn. Perform. J. 2001, 19, 43–50. [Google Scholar]
- Vircell. Brucella ELISA IgG for In Vitro Diagnostic Use; Vircell, S.L., Ed.; Parque Tecnologico de la Salud, Avicena 8, 18016 Granada, Spain; Vircell: Granada, Spain, 2006. [Google Scholar]
- Pereira, C.R.; Cotrim de Almeida, J.V.F.; Cardoso de Oliveira, I.R.; Faria de Oliveira, L.; Pereira, L.J.; Zangerônimo, M.G.; Lage, A.P.; Dorneles, E.M.S. Occupational exposure to Brucella spp.: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008164. [Google Scholar] [CrossRef]
- Alzuheir, M.; Al Zabadi, H.; Abu Helal, M. Occupational exposure assessment and seroprevalence of Brucella Specific antibodies among veterinarians in the Northern Palestine. Front Med. Sci. 2022, 8, 813900. [Google Scholar] [CrossRef]
- Ducrotoy, M.; Bertu, W.J.; Matope, G.; Cadmus, S.; Conde-Álvarez, R.; Gusi, A.M.; Welburn, S.; Ocholi, R.; Blasco, J.M.; Moriyón, I.; et al. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 2017, 165, 179–193. [Google Scholar] [CrossRef]
- Craighead, L.; Meyer, A.; Chengat, B.; Musallam, I.; Akakpo, J.; Kone, P.; Guitian, J.; Häsler, B. Brucellosis in West and Central Africa: A review of the current situation in a changing landscape of dairy cattle systems. Acta Trop. 2018, 179, 96–108. [Google Scholar] [CrossRef]
- Djangwani, J.; Ooko Abong’, G.; Gicuku Njue, L.; Kaindi, D.W. Brucellosis: Prevalence with reference to East African Community Countries—A Rapid Review. Vet. Med. Sci. 2021, 7, 851–867. [Google Scholar] [CrossRef]
- Tschopp, R.; Gebregiorgis, A.; Tassachew, Y.; Andualem, H.; Osman, M.; Waqjira, M.W.; Hattendorf, J.; Mohammed, A.; Hamid, M.; Molla, W.; et al. Integrated human-animal sero-surveillance of brucellosis in the pastoral Afar and Somali regions of Ethiopia. PLoS Negl. Trop. Dis. 2021, 15, e0009593. [Google Scholar] [CrossRef]
- Mwatondo, A.; Muturi, M.; Akoko, J.; Nyamota, R.; Nthiwa, D.; Maina, J.; Omolo, J.; Gichuhi, S.; Mureithi, M.W.; Bett, B. Seroprevalence and related risk factors of Brucella spp. in livestock and humans in Garbatula Subcounty, Isiolo County, Kenya. PLoS Negl. Trop. Dis. 2023, 17, e0011682. [Google Scholar] [CrossRef]
- Cadmus, S.; Salam, S.P.; Adesokan, H.K.; Akporube, K.; Ola-Daniel, F.; Awosanya, E.J. Seroprevalence of brucellosis and Q fever infections amongst pastoralists and their cattle herds in Sokoto State, Nigeria. PLoS ONE 2021, 16, e0254530. [Google Scholar] [CrossRef] [PubMed]
- Efrem, G.H.; Mihreteab, B.; Ghebremariam, M.K.; Tesfai, B.; Mor, S.M.; Mamo, G. Seroprevalence of brucellosis, knowledge, and risky practices in dairy cattle owners and workers in Maekel and Debub Regions, Eritrea. Am. J. Trop. Med. Hyg. 2024, 111, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Awah-Ndukum, J.; Mouiche, M.M.M.; Kouonmo-Ngnoyum, L.; Bayang, H.N.; Manchang, T.K.; Poueme, R.S.N.; Kouamo, J.; Ngu-Ngwa, V.; Assana, E.; Feussom, K.J.M.; et al. Seroprevalence and risk factors of brucellosis among slaughtered indigenous cattle, abattoir personnel and pregnant women in Ngaoundéré, Cameroon. BMC Infect. Dis. 2018, 18, 611. [Google Scholar] [CrossRef] [PubMed]
- Mode, S.; Ketterer, M.; Québatte, M.; Dehio, C. Antibiotic persistence of intracellular Brucella abortus. PLoS Negl. Trop. Dis. 2022, 16, e0010635. [Google Scholar] [CrossRef]
- Ariza, J.; Pellicer, T.; Pallarés, R.; Foz, A.; Gudiol, F. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 1992, 14, 131–140. [Google Scholar] [CrossRef]
- Al Dahouk, S.; Nöckler, K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev. Anti Infect. Ther. 2011, 9, 833–845. [Google Scholar] [CrossRef] [PubMed]

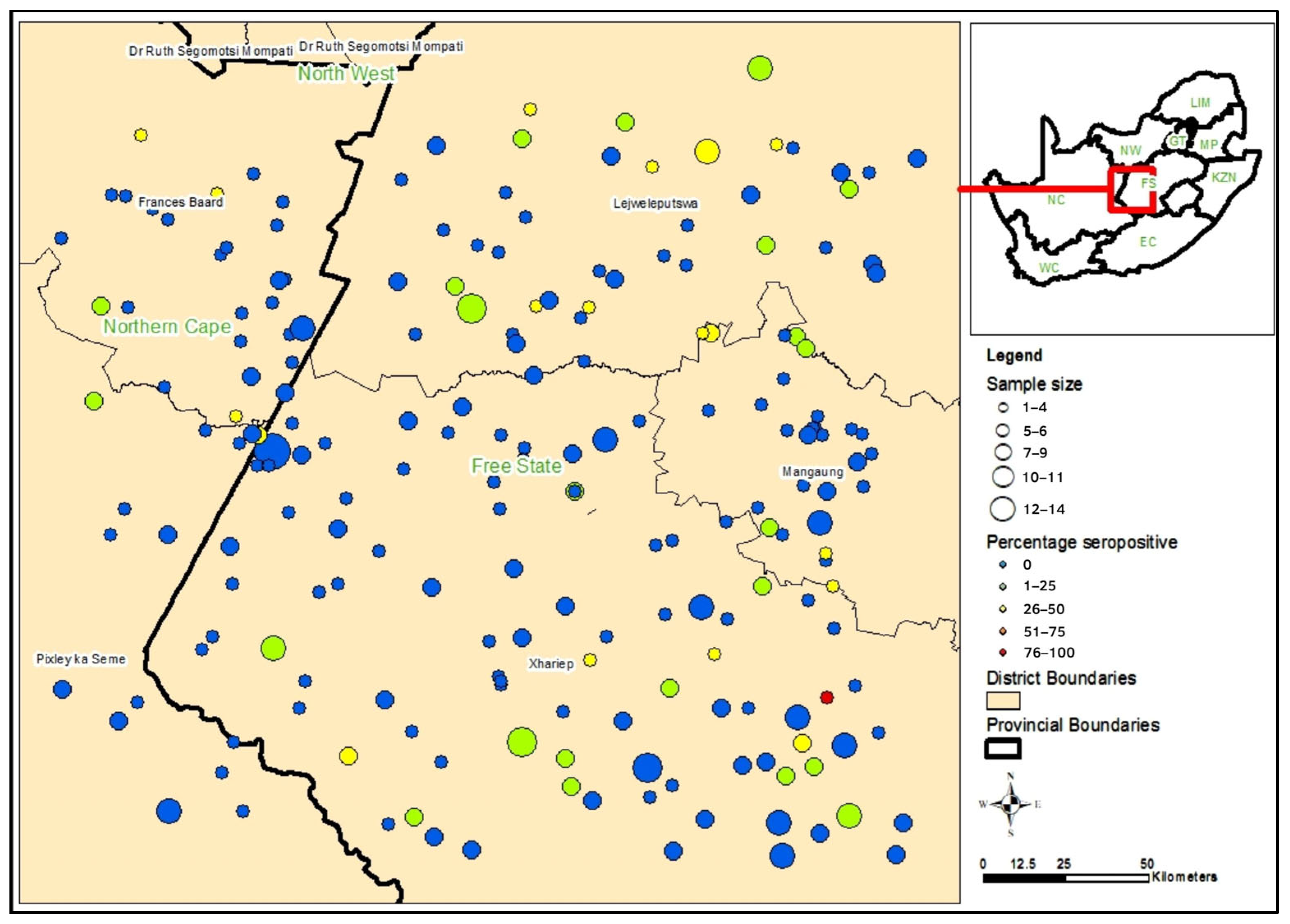

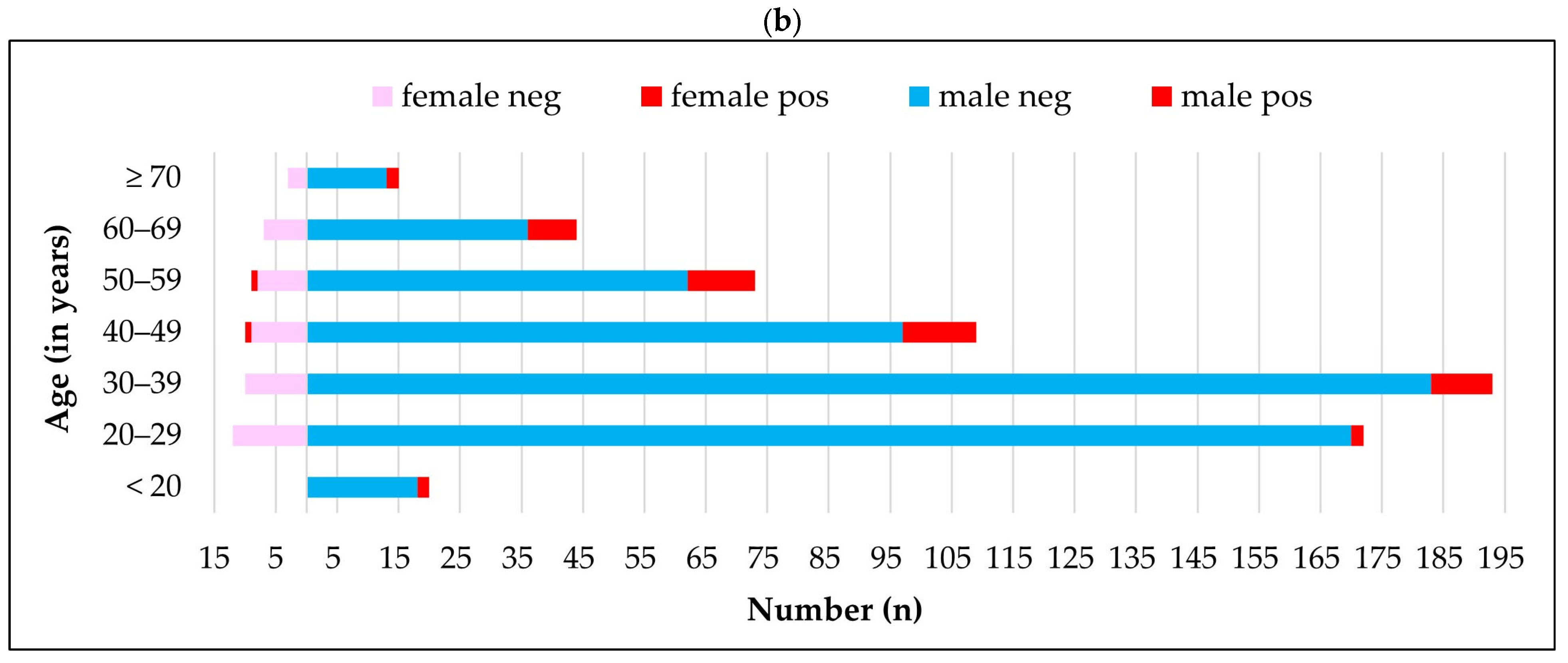
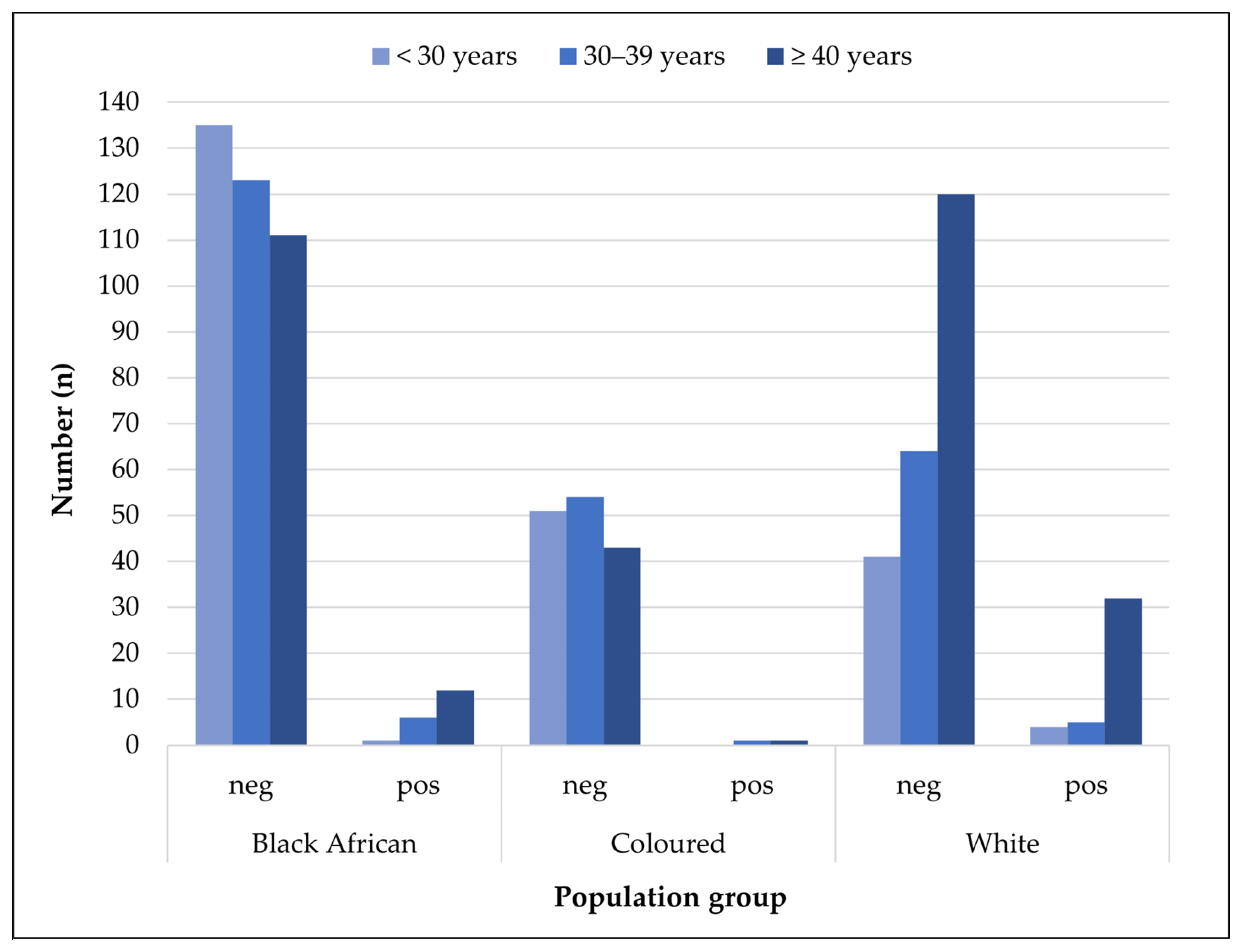
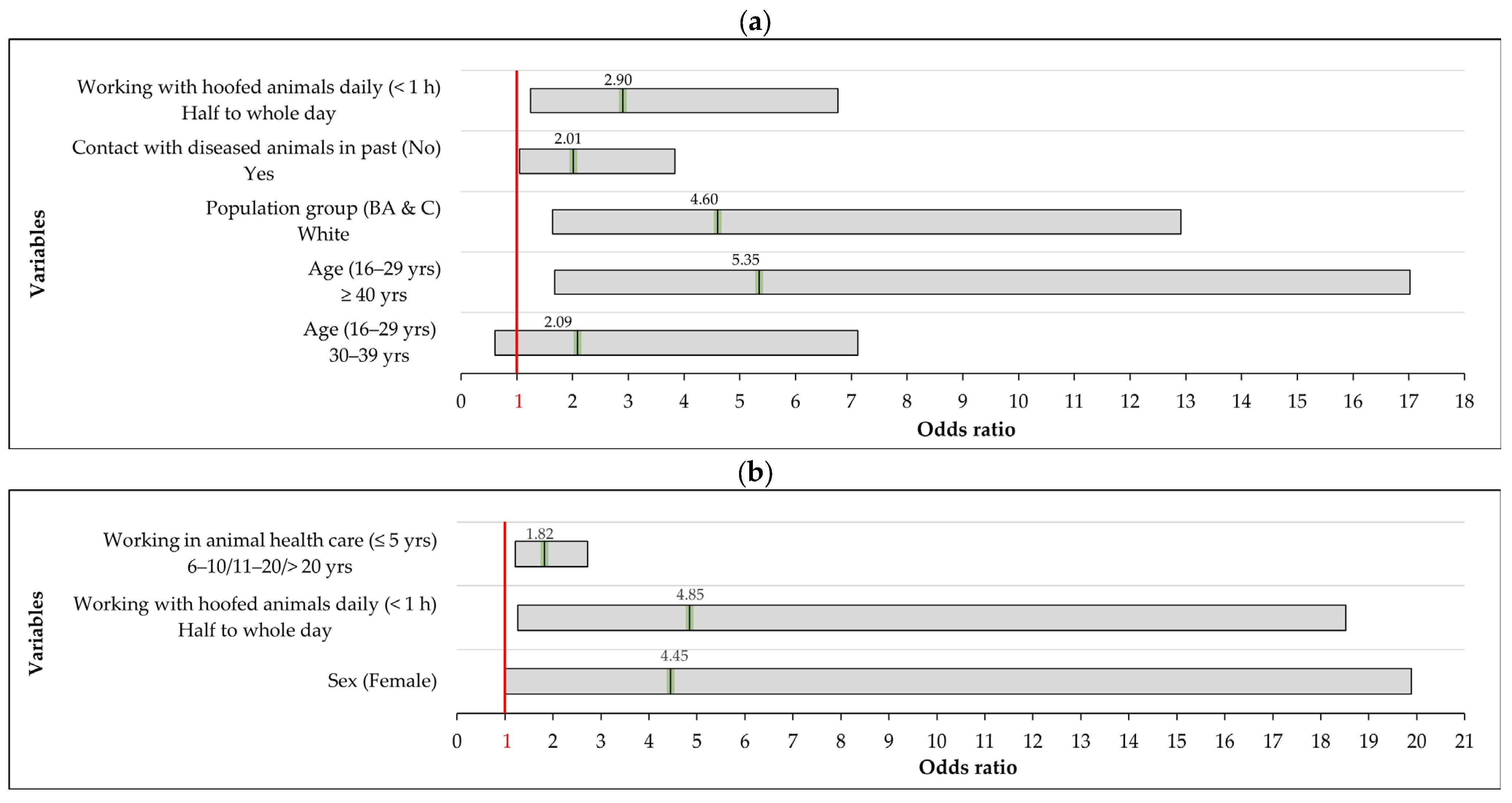
| Farming Population | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Variables | Brucella IgG Seropositive n/N (%) | Odds Ratio (CI 95%) | p-Value (* < 0.2) | Adjusted Odds Ratio (CI 95%) | p-Value (** < 0.05) |
| Sex | |||||
| Female | 2/53 (3.8%) | 1 (base) | - | 1 (base) | - |
| Male | 48/658 (7.3%) | 2.73 (0.73–10.17) | 0.133 * | 2.12 (0.48–9.30) | 0.317 |
| Population group | |||||
| Coloured or Black African | 21/536 (3.9%) | 1 (base) | - | 1 (base) | - |
| White | 29/175 (16.6%) | 5.11 (1.98–13.22) | 0.001 * | 4.60 (1.64–12.91) | 0.004 ** |
| Age (years) | |||||
| 16–29 | 4/204 (2.0%) | 1 (base) | - | 1 (base) | - |
| 30–39 | 10/203 (4.9%) | 2.27 (0.66–7.81) | 0.193 * | 2.09 (0.61–7.12) | 0.237 |
| ≥40 | 35/270 (13.0%) | 5.21 (1.62–16.76) | 0.006 * | 5.35 (1.68–17.02) | 0.005 ** |
| Working on farm (years) | |||||
| ≤5 | 10/320 (3.1%) | 1 (base) | - | 1 (base) | - |
| 6–10 | 6/114 (5.3%) | 1.53 (0.52–4.52) | 0.440 | 1.18 (0.35–4.01) | 0.791 |
| 11–20 | 13/139 (9.4%) | 2.58 (0.99–6.70) | 0.052 * | 1.38 (0.45–4.28) | 0.572 |
| 21–30 | 6/66 (9.1%) | 2.11 (0.73–6.10) | 0.165 * | 1.00 (0.27–3.73) | 0.997 |
| 31–40 | 3/39 (7.7%) | 1.68 (0.44–6.48) | 0.448 | 0.78 (0.18–3.44) | 0.744 |
| 41–75 | 12/49 (24.5%) | 5.60 (2.21–14.20) | 0.000 * | 1.56 (0.44–5.47) | 0.486 |
| Working with animals (years) | |||||
| ≤5 | 7/258 (2.7%) | 1 (base) | - | 1 (base) | - |
| 6–10 | 4/118 (3.4%) | 1.12 (0.31–4.03) | 0.862 | 0.85 (0.19–3.82) | 0.831 |
| 11–20 | 16/156 (10.3%) | 3.30 (1.35–8.08) | 0.009 * | 1.67 (0.48–5.79) | 0.414 |
| >20 | 23/192 (12.0%) | 2.80 (1.16–6.72) | 0.022 * | 0.37 (0.06–2.34) | 0.292 |
| Job description | |||||
| Farmworker/herdsman | 22/505 (4.4%) | 1 (base) | - | 1 (base) | - |
| Farmer/livestock owner; manager | 27/179 (15.1%) | 3.90 (2.21–6.88) | 0.000 * | 0.70 (0.26–1.23) | 0.490 |
| Other (family, domestic staff, driver) | 1/27 (3.7%) | 0.84 (0.11–6.65) | 0.872 | 0.43 (0.05–3.84) | 0.452 |
| Help with transport of animals | |||||
| Yes | 44/526 (8.7%) | 2.04 (0.86–4.81) | 0.103 * | 0.85 (0.29–2.53) | 0.769 |
| No | 6/185 (3.2%) | 1 (base) | - | 1 (base) | - |
| Cleaning of animal equipment | |||||
| Yes | 46/573 (8.0%) | 2.16 (0.77–6.05) | 0.140 * | 1.79 (0.63–5.11) | 0.272 |
| No | 4/138 (2.9%) | 1 (base) | - | 1 (base) | - |
| Assisting with birthing of animals | |||||
| Yes | 46/601 (7.7%) | 2.00 (0.73–5.47) | 0.177 * | 1.04 (0.25–4.29) | 0.916 |
| No | 4/110 (3.6%) | 1 (base) | - | 1 (base) | - |
| Assisting with surgery | |||||
| Yes | 20/144 (13.9%) | 1.88 (0.97–3.67) | 0.062 * | 1.17 (0.50–2.73) | 0.712 |
| No | 30/567 (5.3%) | 1 (base) | - | 1 (base) | - |
| Slaughter animals | |||||
| Yes | 44/585 (7.5%) | 1.80 (0.76–4.28) | 0.183 * | 0.78 (0.24–2.53) | 0.675 |
| No | 6/126 (4.8%) | 1 (base) | - | 1 (base) | - |
| Performing post-mortem | |||||
| Yes | 23/159 (14.5%) | 2.18 (1.11–4.27) | 0.024 * | 1.93 (0.91–4.1) | 0.087 |
| No | 27/552 (4.9%) | 1 (base) | - | 1 (base) | - |
| Use of protective gear when working with animals | |||||
| Yes | 9/254 (3.5%) | 0.58 (0.27–1.25) | 0.161 * | 0.44 (0.18–1.09) | 0.076 |
| No | 41/456 (9.0%) | 1 (base) | - | 1 (base) | - |
| Working with hoofed animals | |||||
| Half to whole day | 44/595 (7.4%) | 2.63 (1.12–6.16) | 0.027 * | 2.90 (1.25–6.76) | 0.014 ** |
| <1 h | 6/116 (5.2%) | 1 (base) | - | 1 (base) | - |
| Contact with diseased animals in the past | |||||
| Yes | 30/257 (11.7%) | 2.32 (1.26–4.27) | 0.007 * | 2.01 (1.05–3.84) | 0.034 ** |
| No | 20/470 (4.3%) | 1 (base) | - | 1 (base) | - |
| Suffered injury from sharp object | |||||
| Yes | 26/251 (10.4%) | 1.68 (0.94–2.99) | 0.079 * | 1.08 (0.51–2.31) | 0.839 |
| No | 24/476 (5.0%) | 1 (base) | - | 1 (base) | - |
| Drink milk 1 | |||||
| Yes | 48/604 (7.9%) | 4.19 (0.98–18.04) | 0.054 * | - | - |
| No | 2/107 (1.9%) | 1 (base) | - | - | - |
| Drinking raw milk | |||||
| Yes | 26/309 (8.4%) | 2.09 (0.96–4.56) | 0.064 * | 1.53 (0.66–3.56) | 0.320 |
| Sometimes | 7/58 (12.1%) | 2.22 (0.85–5.76) | 0.102 * | 1.73 (0.51–5.83) | 0.375 |
| Always boiled or pasteurised | 15/237 (6.3%) | 1 (base) | - | 1 (base) | - |
| Suffered headache | |||||
| Yes past 2 weeks | 28/413 (6.8%) | 0.64 (0.26–1.59) | 0.331 | 0.67 (0.23–1.94) | 0.456 |
| Yes past year | 16/143 (11.2%) | 1.55 (0.80–3.00) | 0.189 * | 1.24 (0.44–3.43) | 0.684 |
| No | 28/413 (6.8%) | 1 (base) | - | 1 (base) | - |
| Chronic heart condition | |||||
| Yes | 7/35 (20%) | 2.31 (0.88–6.10) | 0.089 * | 1.98 (0.47–8.33) | 0.347 |
| No | 43/676 (6.4%) | 1 (base) | - | 1 (base) | - |
| Chronic liver disease | |||||
| Yes | 0/3 (0%) | ||||
| No | 50/708 (7.1%) | 1 (base) | - | 1 (base) | - |
| Communal versus private land use 1 | |||||
| Yes | 1/45 (2.2%) | 0.17 (0.02–1.34) | 0.093 * | - | - |
| No | 50/673 (7.4%) | 1 (base) | - | - | - |
| Animals in the house | |||||
| Yes | 48/650 (7.9%) | 0.09 (0.01–1.43) | 0.087 * | 0.09 (0.01–1.50) | 0.093 |
| No | 0/52 (0%) | 1 (base) | - | 1 (base) | - |
| Access to dam on farm | |||||
| Yes | 45/563 (8.0%) | 2.69 (0.99–7.33) | 0.053 * | 0.83 (0.30–2.32) | 0.718 |
| No | 5/141 (3.5%) | 1 (base) | - | 1 (base) | - |
| Administration antibiotics new animals | |||||
| Yes | 2/91 (2.2%) | 0.28 (0.07–1.17) | 0.080 * | 0.27 (0.06–1.15) | 0.077 |
| No | 49/627 (7.8%) | 1 (base) | 1 (base) | - | |
| People employed on farm | |||||
| 0–2 | 8/187 (4.3%) | 1 (base) | - | 1 (base) | - |
| 3–5 | 24/256 (9.4%) | 2.88 (1.10–7.50) | 0.031 * | 2.96 (0.95–9.22) | 0.061 |
| 6–10 | 11/134 (8.2%) | 2.79 (0.93–8.40) | 0.067 * | 2.32 (0.70–7.62) | 0.166 |
| 11–50 | 7/125 (5.6%) | 1.80 (0.56–5.85) | 0.324 | 1.57 (0.35–7.12) | 0.556 |
| Sheep or cattle on farm | |||||
| No sheep (alone or with cattle/goats) | 8/199 (4.0%) | 1 (base) | - | 1 (base) | - |
| Yes sheep | 5/89 (5.6%) | 1.42 (0.46–4.39) | 0.538 | 1.48 (0.47–4.72) | 0.502 |
| Yes sheep and cattle | 38/430 (8.8%) | 2.31 (1.05–5.09) | 0.038 * | 1.95 (0.88–4.35) | 0.100 |
| Goats on the farm | |||||
| Yes | 7/146 (4.8%) | 0.38 (0.14–1.01) | 0.051 * | 0.37 (0.14–0.99) | 0.216 |
| No | 44/572 (7.7%) | 1 (base) | - | 1 (base) | - |
| Wildlife on the farm | |||||
| Yes | 25/246 (10.2%) | 2.23 (1.18–4.20) | 0.014 * | 1.84 (0.96–3.55) | 0.066 |
| No | 26/472 (5.5%) | 1 (base) | - | 1 (base) | - |
| Brucellosis on farm past | |||||
| Yes | 9/84 (10.7%) | 1.99 (0.85–4.66) | 0.113 * | 2.22 (0.89–5.55) | 0.087 |
| No | 41/627 (6.5%) | 1 (base) | - | 1 (base) | - |
| Animals vaccinated against brucellosis | |||||
| Yes | 24/267 (9.0%) | 1.85 (0.98–3.47) | 0.056 * | 1.77 (0.89–3.52) | 0.101 |
| No | 26/444 (5.9%) | 1 (base) | - | 1 (base) | - |
| Veterinary Professionals | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Variables | Brucella IgG Seropositive n/N (%) | Odds Ratio (CI 95%) | p-Value (* < 0.2) | Penalized Odds Ratio (CI 95%) | p-Value (** < 0.05) |
| Sex | |||||
| Female | 2/70 (2.9%) | 1 (base) | - | 1 (base) | |
| Male | 14/68 (20.6%) | 8.60 (1.83–40.4) | 0.007 * | 4.45 (1.00–19.89) | 0.05 ** |
| Population group | |||||
| Coloured or Black African | 0/36 (0%) | 1 (base) | - | 1 (base) | |
| White | 16/102 (15.7%) | 11.48 (0.65–203) | 0.096 * | 46.0 (0.07–30545) | 0.248 |
| Age (years) | |||||
| 22–29 | 1/28 (3.6%) | 1 (base) | - | 1 (base) | |
| 30–39 | 2/50 (4.0%) | 1.35 (0.11–16.58) | 0.812 | 1.07 (0.22–5.21) | 0.933 |
| 40–49 | 3/20 (15.0%) | 4.95 (0.44–55.18) | 0.191 * | ||
| 50–59 | 3/20 (15.0%) | 5.37 (0.46–63.14) | 0.179 * | ||
| ≥60 | 4/9 (44.4%) | 24.3 (1.87–315) | 0.015 * | ||
| Working as animal healthcare worker (years) | |||||
| ≤5 | 1/38 (2.6%) | 1 (base) | - | 1 (base) | |
| 6–10 | 1/24 (4.2%) | 1.62 (0.85–30.6) | 0.747 | 1.82 (1.21–2.72) | 0.004 ** |
| 11–20 | 2/23 (8.7%) | 3.29 (0.28–39.2) | 0.343 | ||
| >20 | 12/37 (32.4%) | 18.54 (2.01–171) | 0.011 * | ||
| Job description | |||||
| Animal health technician | 3/37 (8.1%) | 1 (base) | |||
| Veterinarian | 13/66 (19.7%) | 0.91 (0.65–1.27) | 0.584 | 0.85 (0.53–1.38) | 0.513 |
| Other (incl. vet nurse, researcher, wildlife capturers) | 0/19 (0%) | ||||
| Assisting with birthing of animals 1 | |||||
| Yes | 16/98 (16.3%) | 15.6 (0.86–282) | 0.063 * | - | - |
| No | 0/40 (0%) | 1 (base) | - | - | |
| Touching of aborted foetus | |||||
| Yes | 16/106 (15.1%) | 10.6 (0.58–192) | 0.111 * | 10.2 (0.40–264) | 0.160 |
| No | 0/32 (0%) | 1 (base) | - | 1 (base) | |
| Assisting with surgery | |||||
| Yes | 16/106 (15.1%) | 10.6 (0.58–192) | 0.111 * | 0.32 (0.00–53.2) | 0.661 |
| No | 0/32 (0%) | 1 (base) | 1 (base) | ||
| Working with hoofed animals | |||||
| Half to whole day | 13/71 (18.3%) | 5.08 (1.33–19.42) | 0.018 * | 4.85 (1.27–18.52) | 0.021 ** |
| <1 h | 3/67 (4.5%) | 1 (base) | - | 1 (base) | |
| Wash hands after touching animals | |||||
| Always | 15/103 (14.6%) | 4.45 (0.80–24.63) | 0.088 * | 1.62 (0.06–41.7) | 0.772 |
| Sometimes | 1/33 (3.0%) | ||||
| Rarely | 0/2 (0%) | 1 (base) | - | 1 (base) | - |
| Contact with Brucella-positive animals in the past | |||||
| Yes | 16/76 (21.1) | 24.5 (1.43–418) | 0.027 * | 14.1 (0.77–258) | 0.075 |
| No | 0/46 (0%) | 1 (base) | - | 1 (base) | |
| Suffered needlestick | |||||
| Yes | 14/90 (15.6%) | 2.73 (0.57–13.0) | 0.205 * | 0.04 (0.00–1.50) | 0.078 |
| No | 2/48 (4.2%) | 1 (base) | 1 (base) | ||
| Chronic heart disease | |||||
| Yes | 4/13 (30.8%) | 3.67 (0.91–14.8) | 0.067 * | 0.04 (0.00–33.1) | 0.344 |
| No | 12/125 (9.6%) | 1 (base) | 1 (base) | ||
| Chronic diabetes | |||||
| Yes | 3/6 (50.0%) | 8.0 (1.63–393) | 0.011 * | 110 (0.16–76656) | 0.159 |
| No | 13/132 (9.8%) | 1 (base) | - | 1 (base) | - |
| No chronic condition | |||||
| Yes | 11/116 (9.5%) | 0.36 (0.11–1.16) | 0.085 * | 0.49 (0.00–335) | 0.829 |
| No | 5/22 (22.7%) | 1 (base) | 1 (base) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossouw, J.; Trataris-Rebisz, A.N.; Tempia, S.; Rostal, M.K.; Karesh, W.B.; Msimang, V. Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016. Trop. Med. Infect. Dis. 2025, 10, 302. https://doi.org/10.3390/tropicalmed10110302
Rossouw J, Trataris-Rebisz AN, Tempia S, Rostal MK, Karesh WB, Msimang V. Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016. Tropical Medicine and Infectious Disease. 2025; 10(11):302. https://doi.org/10.3390/tropicalmed10110302
Chicago/Turabian StyleRossouw, Jennifer, Anastasia N. Trataris-Rebisz, Stefano Tempia, Melinda K. Rostal, William B. Karesh, and Veerle Msimang. 2025. "Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016" Tropical Medicine and Infectious Disease 10, no. 11: 302. https://doi.org/10.3390/tropicalmed10110302
APA StyleRossouw, J., Trataris-Rebisz, A. N., Tempia, S., Rostal, M. K., Karesh, W. B., & Msimang, V. (2025). Seroprevalence and Associated Risk Factors of Human Brucellosis in a Farming and Animal Health Community in South Africa, 2015–2016. Tropical Medicine and Infectious Disease, 10(11), 302. https://doi.org/10.3390/tropicalmed10110302







