Epidemiology and Risks Survey of Onchocerca volvulus Infection in Igbo-Eze North Local Government Area, Enugu State, Nigeria
Abstract
1. Background
2. Methods
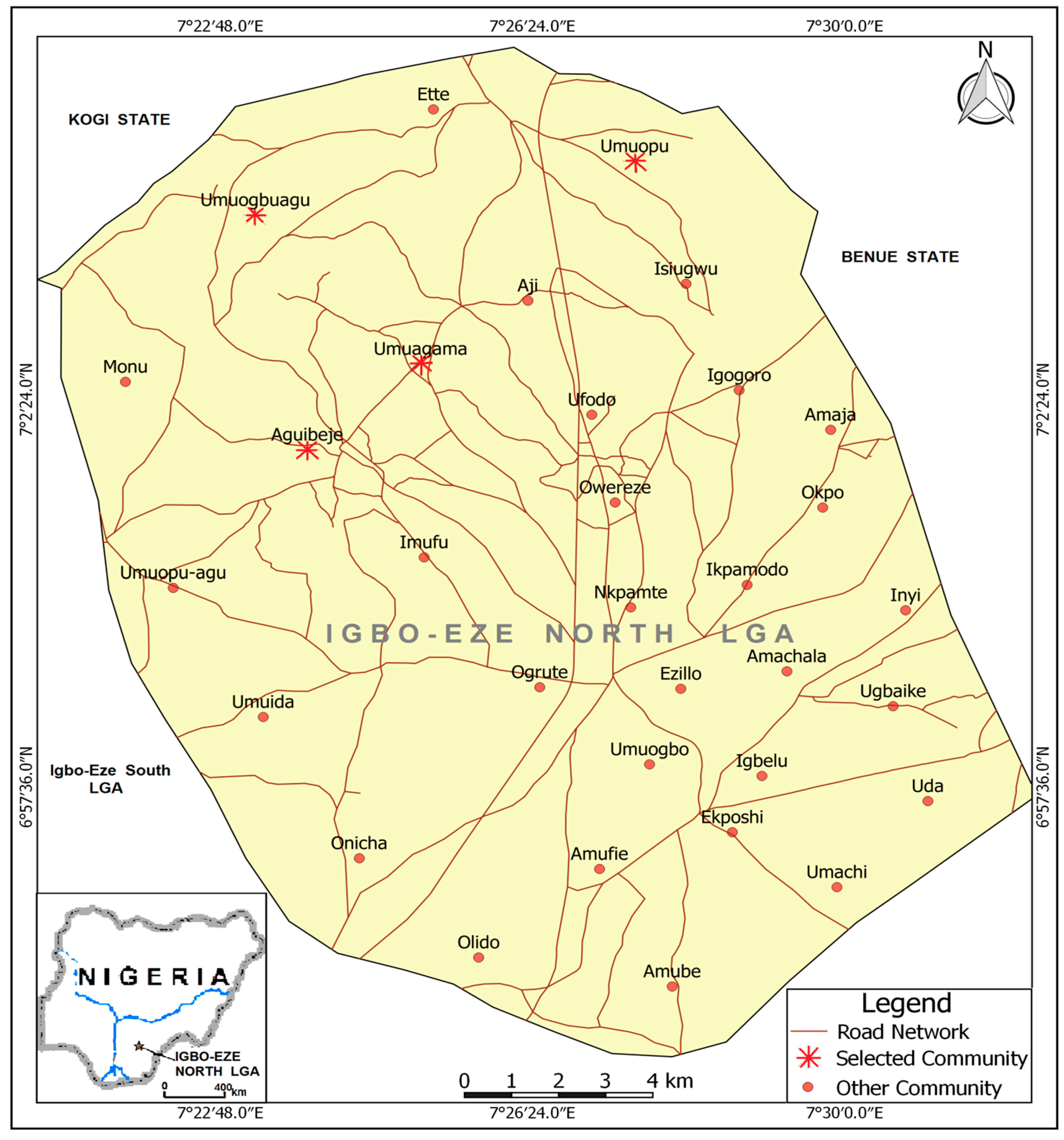
2.1. Study Area
2.2. Ethical Clearance
2.3. Study Design
2.4. Sample Size Determination
2.5. Examination of Study Participants
2.6. Administration of Questionnaire
2.7. Statistical Analysis
3. Results
3.1. Prevalence of Onchocerciasis in Igbo-Eze North LGA
3.2. Risk Factors for Onchocerciasis in Igbo-Eze North LGA
4. Discussion
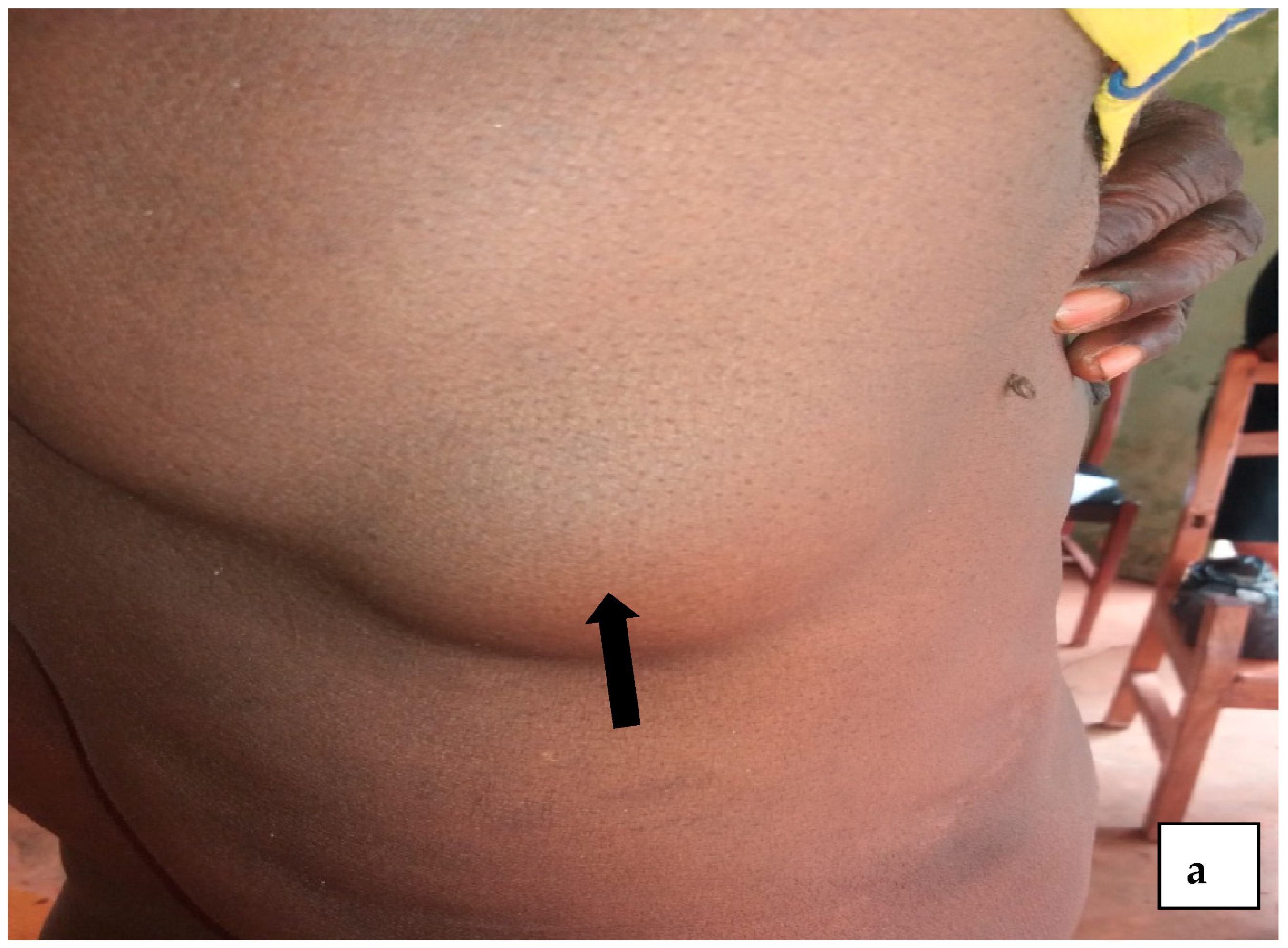
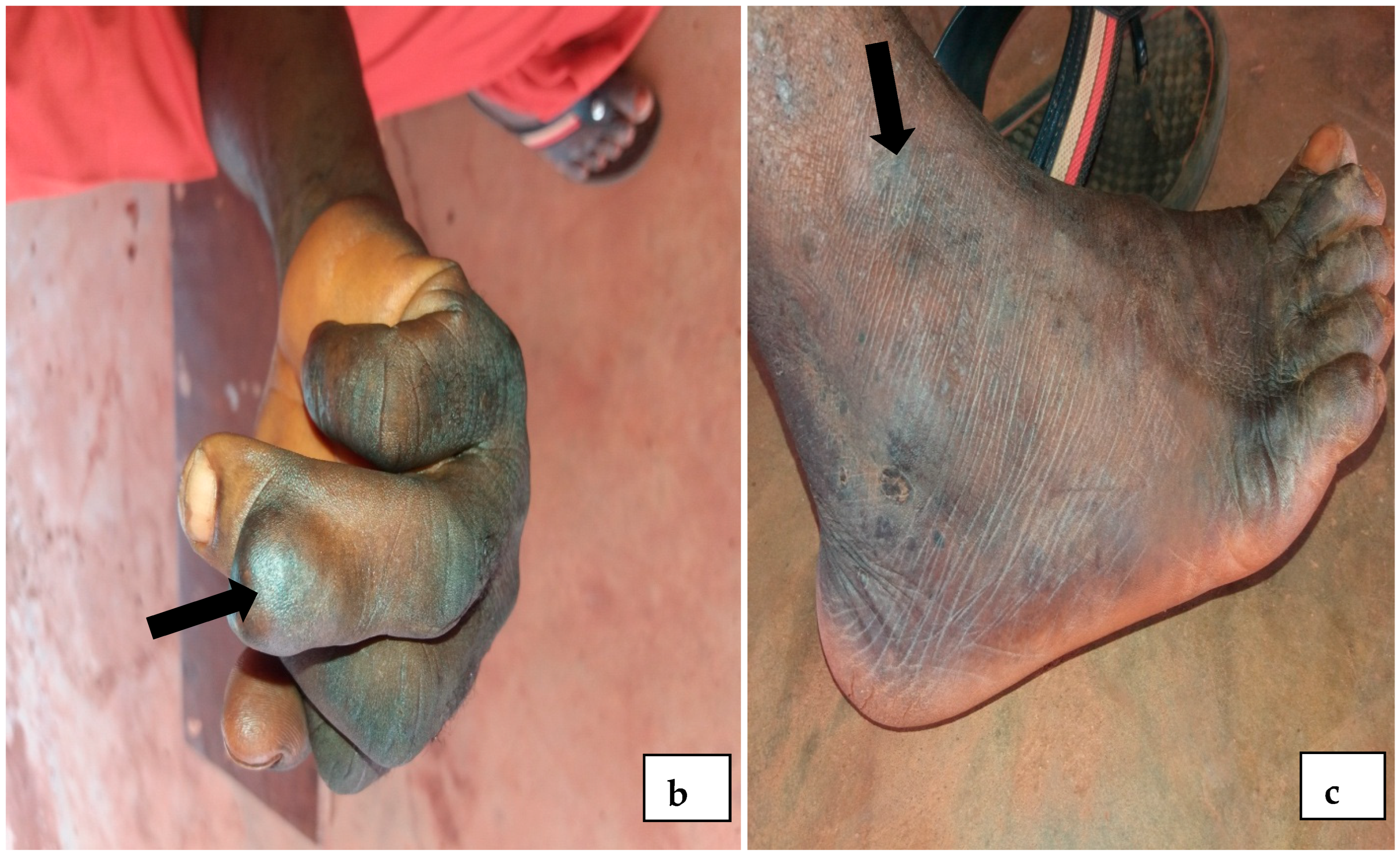
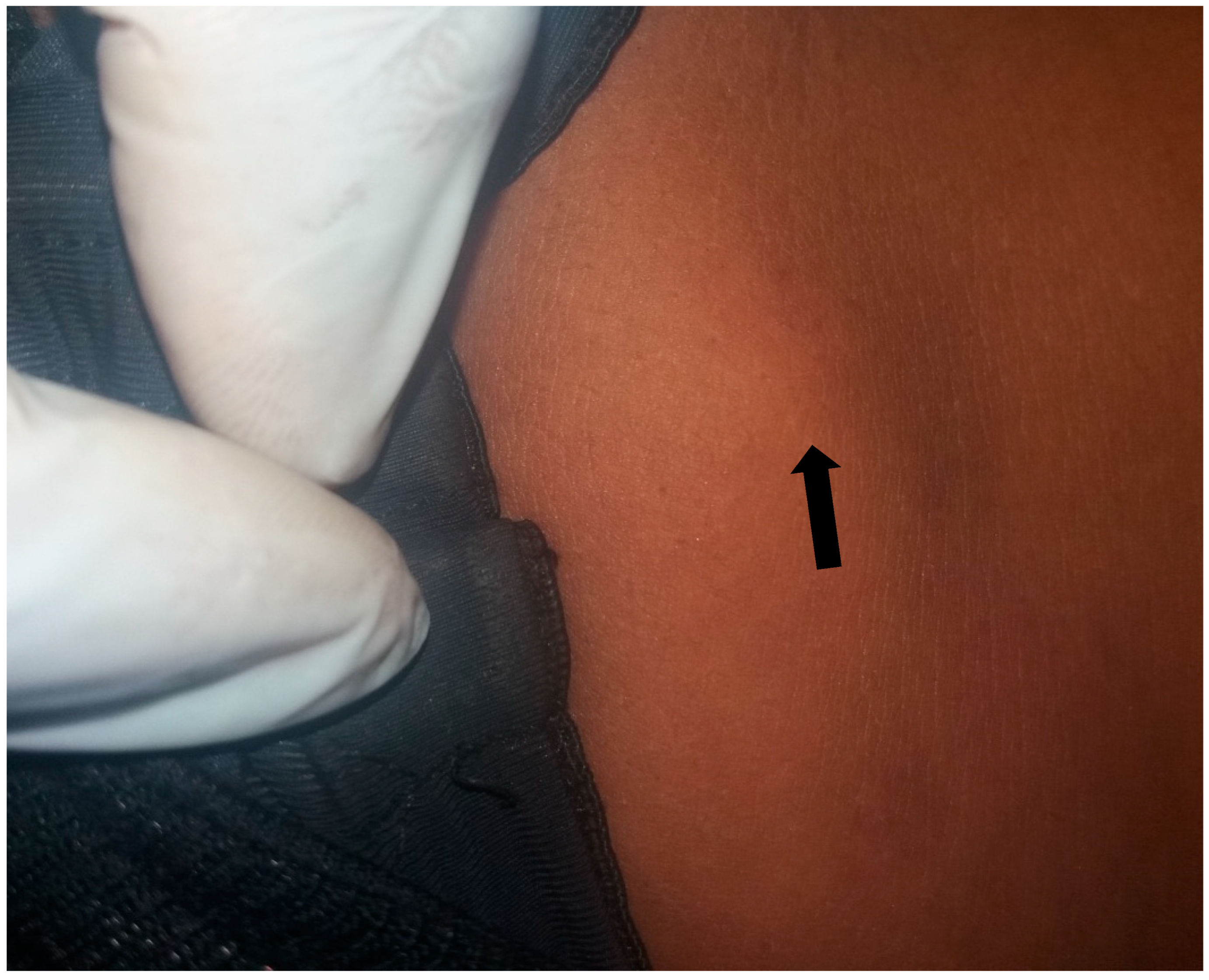
4.1. Prevalence of Onchocerciasis in Igbo-Eze North LGA
4.2. Risk Factors for Onchocerciasis in Igbo-Eze North LGA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, D.R.; Arora, B.B. Medical Parasitology, 3rd ed.; CBS Publishers and Distributors: New Delhi, India, 2014. [Google Scholar]
- Ubachukwu, P.O. Socio-economic impact of onchocerciasis with particular reference to females and children: A review. Anim. Res. Int. 2006, 3, 494–505. [Google Scholar] [CrossRef]
- Okoye, I.C.; Ubachukwu, P.O.; Okeke, V.; Obiezue, R.N.N.; Onyishi, G.C. Reassessment of onchocerciasis prevalence in Etteh, Nigeria, after a decade of mass Mectizan chemotherapeutic intervention: Preliminary report. Anim. Res. Int. 2008, 5, 827–830. [Google Scholar] [CrossRef]
- Ekpo, U.F.; Eneanya, O.A.; Nwankwo, E.N.; Soneye, I.Y.; Weil, G.J.; Fischer, P.U.; Nwaorgu, O.C. Persistence of onchocerciasis in villages in Enugu and Ogun states in Nigeria following many rounds of mass distribution of ivermectin. BMC Infect. Dis. 2022, 22, 832. [Google Scholar] [CrossRef] [PubMed]
- Sarmukaddam, S.; Garad, S. On validity of assumptions while determining sample size. Indian J. Community Med. 2004, 29, 87–91. [Google Scholar]
- Ngomou, P.; Walsh, J.E. A Manual for Rapid Epidemiological Mapping of Onchocerciasis; UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases & WHO Programme for the Prevention of Blindness: Geneva, Switzerland, 1993. [Google Scholar]
- Nwosu, C.G.; Ivoke, N.; Okoye, I.C.; Obiezue, N.R.; Onyishi, C.G.; Ugokwe, U.C.; Ezewudo, B.I.; Olasoji, O.O.; Anunobi, T.J.; Ozioko, U.K.; et al. Assessment of the obstetric, demographic and economic burden on Falciparum-malaria parasitaemia among women of child-bearing age receiving antenatal services in Nsukka, Nigeria. Int. J. Sci. Technoledge 2018, 6, 193–200. [Google Scholar]
- Okonkwo, C.I.; Iroha, I.R.; Ayogu, T.E.; Oji, A.E.; Onwa, N.C. Epidemiology of human onchocerciasis among farmers in Ebonyi State, Nigeria. Int. J. Med. Med. Sci. 2010, 2, 246–250. [Google Scholar]
- Usip, L.P.E.; Opara, K.N.; Ibanga, E.S.; Atting, I.A.; Uttah, E. Clinical onchocerciasis in Ini Local Government Area, Akwa Ibom State, Nigeria. Niger. J. Parasitol. 2006, 27, 36–40. [Google Scholar] [CrossRef]
- Uttah, E.U. Onchocerciasis in the Upper Imo River Basin, Nigeria: Prevalence and comparative study of waist and shoulder snips from mesoendemic communities. Iran. J. Parasitol. 2010, 5, 33–41. [Google Scholar] [PubMed]
- Akinboye, D.O.; Okwong, E.; Ajiteru, N.; Fawole, O.; Agbolade, O.M.; Ayinde, O.O.; Amosu, A.M.; Atulomah, N.O.; Oduola, O.; Owodunni, B.M.; et al. Onchocerciasis among inhabitants of Ibarapa Local Government community of Oyo State, Nigeria. Biomed. Res. 2010, 21, 174–178. [Google Scholar]
- Njenga, S.M.; Wamae, C.N.; Mwandawiro, C.S.; Molyneux, D.H. Immuno-parasitological assessment of bancroftian filariasis in a highly endemic area along the river Sabaki, in Malindi district, Kenya. Ann. Trop. Med. Parasitol. 2007, 101, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, W.; Kebede, T.; Graves, P.M.; Golasa, M.; Gebre, T.; Mosher, A.W.; Tadesse, A.; Sime, H.; Lambiyo, T.; Panicker, K.N.; et al. Lymphatic filariasis in Western Ethiopia with special emphasis on prevalence of Wuchereria bancrofti antigenaemis in and around onchocerciasis endemic areas. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Basáñez, M.G.; Boussinesq, M. Population biology of human onchocerciasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Eyo, J.; Onyishi, G.; Ugokwe, C. Rapid epidemiological assessment of onchocerciasis in a tropical semi urban community, Enugu State, Nigeria. Iran. J. Parasitol. 2013, 8, 145–151. [Google Scholar] [PubMed]
- Oparaocha, E.T.; Odaibo, A.B.; Nwoke, B.E.B. Clinoco-pathological studies of human onchocerciasis in Imo River Basin Nigeria and its implication for the control of the disease in the area. Glob. J. Pure Appl. Sci. 2000, 6, 5–9. [Google Scholar]
- Okuliez, J.F.; Elston, D.M.; Schwartz, R.A. Onchocerciasis (River Blindness). 2007. Available online: http://www.emedicine.com (accessed on 15 December 2017).
- Abdullahi, Y.; Oyeyi, T.I. Current status of onchocerciasis in Tudun Wada and Doguwa local government areas of Kano State. Niger. J. Parasitol. 2003, 24, 77–88. [Google Scholar]

| Community | Number Examined | Number Infected | Prevalence (%) |
|---|---|---|---|
| Aguibege | 54 | 3 | 5.6 |
| Umuopu | 46 | 2 | 4.3 |
| Umuogbuagu | 52 | 1 | 1.9 |
| Umuagama | 49 | 1 | 2.0 |
| Overall | 201 | 7 | 3.5 |
| F = 1.484, df = 3, p = 0.750 | |||
| Variable | Category | Number Examined | Number Infected | Prevalence (%) | p-Value |
|---|---|---|---|---|---|
| Sex | Male | 71 | 3 | 4.2 | 0.699 |
| Female | 130 | 4 | 3.1 | ||
| Total | 201 | 7 | 3.5 | ||
| Age (years) | 0–9 | 9 | 0 | 0.0 | 0.493 |
| 10–19 | 19 | 0 | 0.0 | ||
| 20–29 | 21 | 0 | 0.0 | ||
| 30–39 | 29 | 3 | 10.3 | ||
| 40–49 | 43 | 1 | 2.3 | ||
| ≥50 | 80 | 3 | 3.8 | ||
| Total | 201 | 7 | 3.5 | ||
| Occupation | None | 8 | 0 | 0.0 | 0.013 |
| Schooling | 25 | 0 | 0.0 | ||
| Farmer/Fisherman | 29 | 5 | 17.2 | ||
| Civil Servant | 17 | 0 | 0.0 | ||
| Artisan | 27 | 1 | 3.7 | ||
| Trader | 95 | 1 | 1.1 | ||
| Total | 201 | 7 | 3.5 |
| Variable | Category | df | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Knowledge of onchocerciasis | Yes | 1 | 0.744 (0.114–15.857) | 0.814 |
| No | 1 | |||
| Knowledge of possible causes of onchocerciasis | Charm | 2 | 0.073 (0.674–277.123) | 0.089 |
| Witchcraft | 0.200 (0.240–104.147) | |||
| Genetic | 1 | |||
| Seen onchocerciasis patient with signs/symptoms | Yes | 1 | 0.517 (0.166–22.497) | 0.599 |
| No | 1 | |||
| Knowledge of onchocerciasis vector | Yes | 1 | 29.000 (0.002–0.773) | 0.034 |
| No | 1 | |||
| Do you visit water bodies? | Yes | 1 | 4.417 (0.018–2.884) | 0.253 |
| No | 1 | |||
| Proximity of water body to the house | Yes | 1 | 29.000 (0.002–0.773) | 0.034 |
| No | 1 | |||
| Do you make use of mosquito nets? | Yes | 1 | 0.452 (0.190–25.769) | 0.526 |
| No | 1 | |||
| What is your preferred drug of choice? | Hospital/Orthodox | 1 | 1.105 (0.077–10.578) | 0.936 |
| Traditional | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniaku, I.E.; Onyishi, G.C.; Nwosu, C.G.; Ngwu, G.I.; Okeke, C.J.; Oraneli, U.B.; Otuu, C.A.; Akobe, N.A.; Nnama, A.U.; Onah, K.I. Epidemiology and Risks Survey of Onchocerca volvulus Infection in Igbo-Eze North Local Government Area, Enugu State, Nigeria. Trop. Med. Infect. Dis. 2025, 10, 285. https://doi.org/10.3390/tropicalmed10100285
Aniaku IE, Onyishi GC, Nwosu CG, Ngwu GI, Okeke CJ, Oraneli UB, Otuu CA, Akobe NA, Nnama AU, Onah KI. Epidemiology and Risks Survey of Onchocerca volvulus Infection in Igbo-Eze North Local Government Area, Enugu State, Nigeria. Tropical Medicine and Infectious Disease. 2025; 10(10):285. https://doi.org/10.3390/tropicalmed10100285
Chicago/Turabian StyleAniaku, Ifeoma Esther, Grace Chinenye Onyishi, Chigozie Godwin Nwosu, Godwin Ikechukwu Ngwu, Chioma Janefrances Okeke, Uche Boniface Oraneli, Chidiebere Agha Otuu, Nicholas Arome Akobe, Augustine Uchechukwu Nnama, and Kyrian Ikenna Onah. 2025. "Epidemiology and Risks Survey of Onchocerca volvulus Infection in Igbo-Eze North Local Government Area, Enugu State, Nigeria" Tropical Medicine and Infectious Disease 10, no. 10: 285. https://doi.org/10.3390/tropicalmed10100285
APA StyleAniaku, I. E., Onyishi, G. C., Nwosu, C. G., Ngwu, G. I., Okeke, C. J., Oraneli, U. B., Otuu, C. A., Akobe, N. A., Nnama, A. U., & Onah, K. I. (2025). Epidemiology and Risks Survey of Onchocerca volvulus Infection in Igbo-Eze North Local Government Area, Enugu State, Nigeria. Tropical Medicine and Infectious Disease, 10(10), 285. https://doi.org/10.3390/tropicalmed10100285








