Risk Factors for Foodborne Zoonoses Among Populations With and Without a Migration Background in Berlin, Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Questionnaire, and Blood Sampling
2.2. Serological Testing
2.3. Data Analysis
3. Results
3.1. Descriptive Analysis
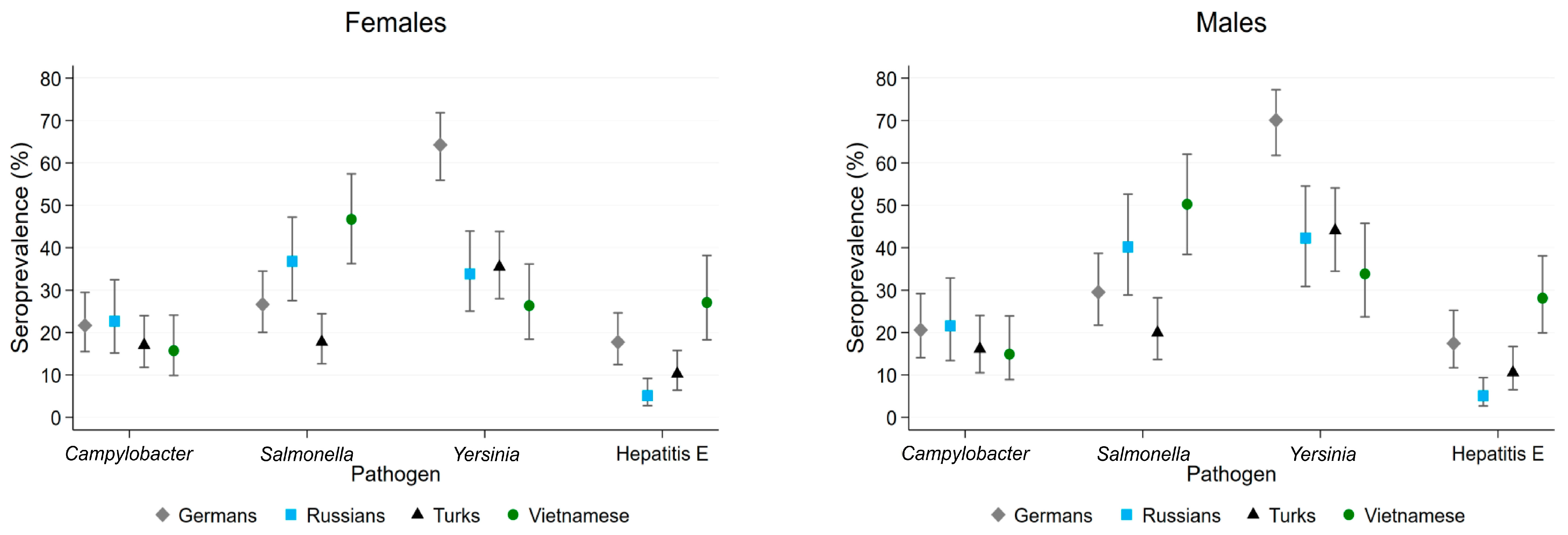
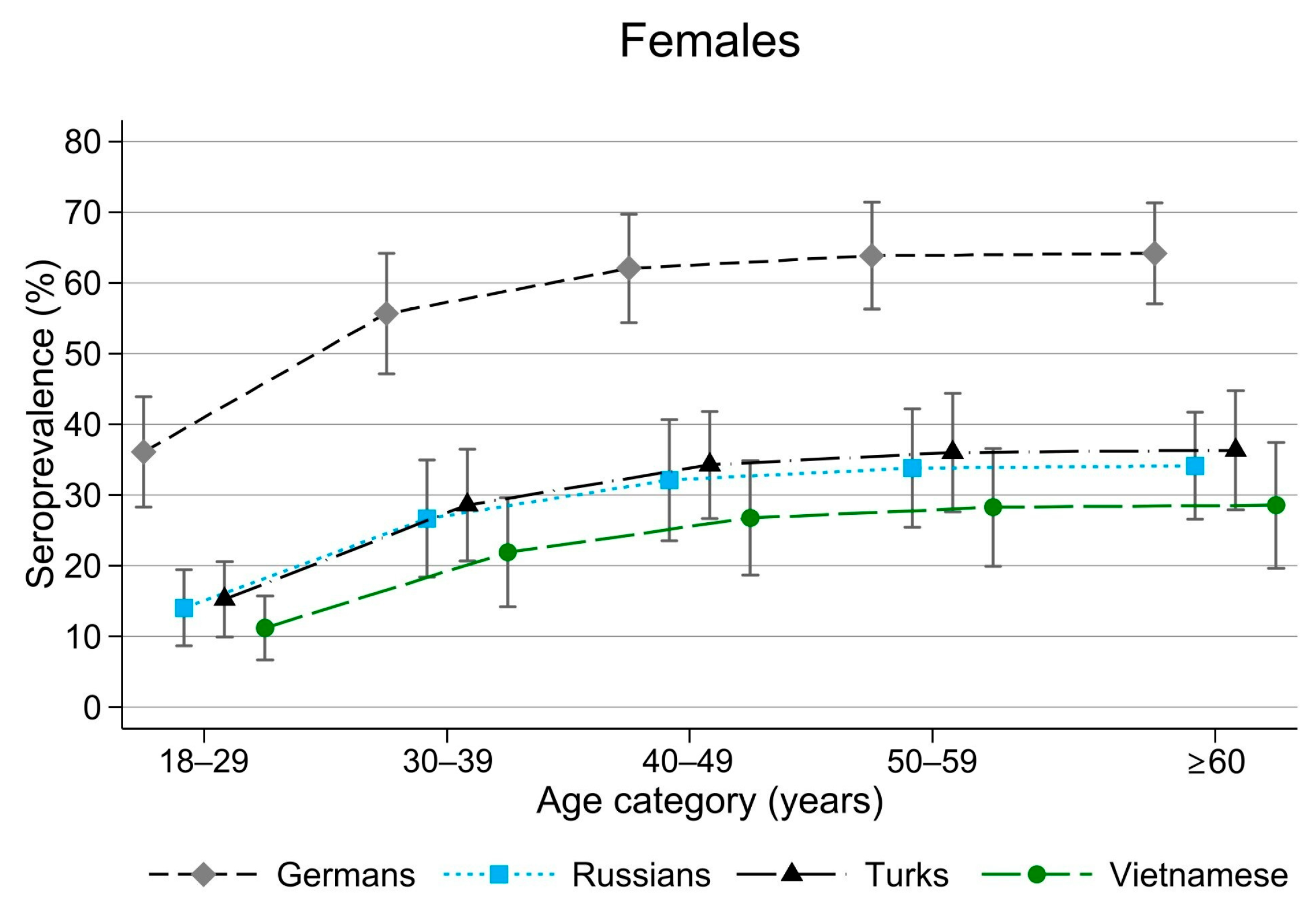
3.2. Seroprevalence
3.3. Association Between Seropositivity and Potential Risk Factors
3.3.1. Campylobacter
3.3.2. Salmonella
3.3.3. Yersinia
3.3.4. HEV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| EEA | EU/European Economic Area |
| ELISA | Enzyme-linked immunosorbent assay |
| FBZ | Foodborne zoonoses |
| HEV | Hepatitis E virus |
| OR | Odds ratio |
References
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 5077. [Google Scholar] [CrossRef]
- Robert Koch Institute. SurvStat@RKI 2.0. Available online: https://survstat.rki.de (accessed on 6 August 2024).
- Heymann, D.L. (Ed.) Control of Communicable Diseases Manual, 19th ed.; American Public Health Association: Washington, DC, USA, 2008; p. 746. [Google Scholar]
- Pischke, S.; Behrendt, P.; Bock, C.T.; Jilg, W.; Manns, M.P.; Wedemeyer, H. Hepatitis E in Germany—an under-reported infectious disease. Dtsch. Arztebl. Int. 2014, 111, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- Dietrich, J.; Hammerl, J.A.; Johne, A.; Kappenstein, O.; Loeffler, C.; Nockler, K.; Rosner, B.; Spielmeyer, A.; Szabo, I.; Richter, M.H. Impact of climate change on foodborne infections and intoxications. J. Health Monit. 2023, 8, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Destatis (The Federal Statistical Office). Statistischer Bericht. Einwohnerinnen und Einwohner im Land Berlin am 31. Dezember 2016; Amt für Statistik Berlin-Brandenburg: Potsdam, Germany, 2017. [Google Scholar]
- Sass, A.C.; Grune, B.; Brettschneider, A.K.; Rommel, A.; Razum, O.; Ellert, U. Participation of people with migration background in health surveys of the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2015, 58, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Schenk, L.; Bau, A.-M.; Borde, T.; Butler, J.; Lampert, T.; Neuhauser, H.; Razum, O.; Weilandt, C. Mindestindikatorensatz zur Erfassung des Migrationsstatus. Empfehlungen für die epidemiologische Praxis. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2006, 49, 853–860. [Google Scholar] [CrossRef]
- Al Dahouk, S.; Neubauer, H.; Hensel, A.; Schoneberg, I.; Nockler, K.; Alpers, K.; Merzenich, H.; Stark, K.; Jansen, A. Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerg. Infect. Dis. 2007, 13, 1895–1900. [Google Scholar] [CrossRef]
- Vollmer, T.; Diekmann, J.; Johne, R.; Eberhardt, M.; Knabbe, C.; Dreier, J. Novel approach for detection of hepatitis E virus infection in German blood donors. J. Clin. Microbiol. 2012, 50, 2708–2713. [Google Scholar] [CrossRef]
- Vancelik, S.; Guraksin, A.; Ayyildiz, A. Seroprevalence of human brucellosis in rural endemic areas in eastern Turkey. Trop. Doct. 2008, 38, 42–43. [Google Scholar] [CrossRef]
- Abe, K.; Hayakawa, E.; Sminov, A.V.; Rossina, A.L.; Ding, X.; Huy, T.T.; Sata, T.; Uchaikin, V.F. Molecular epidemiology of hepatitis B, C, D and E viruses among children in Moscow, Russia. J. Clin. Virol. 2004, 30, 57–61. [Google Scholar] [CrossRef]
- Nockler, K.; Reckinger, S.; Broglia, A.; Mayer-Scholl, A.; Bahn, P. Evaluation of a Western Blot and ELISA for the detection of anti-Trichinella-IgG in pig sera. Vet. Parasitol. 2009, 163, 341–347. [Google Scholar] [CrossRef]
- Robert Koch Institute. Falldefinitionen des Robert Koch-Instituts zur Übermittlung von Erkrankungs- oder Todesfällen und Nachweisen von Krankheitserregern; Robert Koch Institute: Berlin, Germany, 2015; Available online: https://www.rki.de/DE/Themen/Infektionskrankheiten/Meldewesen/Falldefinitionen/Archiv/Falldefinitionen_des_RKI.pdf?__blob=publicationFile&v=1 (accessed on 28 September 2025).
- Monge, S.; Teunis, P.; Friesema, I.; Franz, E.; Ang, W.; van Pelt, W.; Mughini-Gras, L. Immune response-eliciting exposure to Campylobacter vastly exceeds the incidence of clinically overt campylobacteriosis but is associated with similar risk factors: A nationwide serosurvey in the Netherlands. J. Infect. 2018, 77, 171–177. [Google Scholar] [CrossRef]
- Linneberg, A.; Ostergaard, C.; Tvede, M.; Andersen, L.P.; Nielsen, N.H.; Madsen, F.; Frolund, L.; Dirksen, A.; Jorgensen, T. IgG antibodies against microorganisms and atopic disease in Danish adults: The Copenhagen Allergy Study. J. Allergy Clin. Immunol. 2003, 111, 847–853. [Google Scholar] [CrossRef]
- Rosner, B.M.; Schielke, A.; Didelot, X.; Kops, F.; Breidenbach, J.; Willrich, N.; Golz, G.; Alter, T.; Stingl, K.; Josenhans, C.; et al. A combined case-control and molecular source attribution study of human Campylobacter infections in Germany, 2011–2014. Sci. Rep. 2017, 7, 5139. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.F.; Chai, L.C.; Mohamad Ghazali, F.; Radu, S.; Haresh, K. Prevalence of Campylobacter spp. in retailed ready-to-eat sushi. Int. Food Res. J. 2008, 15, 331–336. [Google Scholar]
- Gill, C.O. Safety and storage stability of horse meat for human consumption. Meat Sci. 2005, 71, 506–513. [Google Scholar] [CrossRef]
- MacDonald, E.; Einoder-Moreno, M.; Borgen, K.; Thorstensen Brandal, L.; Diab, L.; Fossli, O.; Guzman Herrador, B.; Hassan, A.A.; Johannessen, G.S.; Johansen, E.J.; et al. National outbreak of Yersinia enterocolitica infections in military and civilian populations associated with consumption of mixed salad, Norway, 2014. Eurosurveillance 2016, 21, 30321. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.M.; Stark, K.; Hohle, M.; Werber, D. Risk factors for sporadic Yersinia enterocolitica infections, Germany 2009–2010. Epidemiol. Infect. 2012, 140, 1738–1747. [Google Scholar] [CrossRef]
- Maki-Ikola, O.; Heesemann, J.; Toivanen, A.; Granfors, K. High frequency of Yersinia antibodies in healthy populations in Finland and Germany. Rheumatol. Int. 1997, 16, 227–229. [Google Scholar] [CrossRef]
- Neubauer, H.; Sprague, L.D.; Scholz, H.; Hensel, A. Yersinia enterocolitica infections: 2. Impact on human health. Berl. Munch. Tierarztl. Wochenschr. 2001, 114, 81–87. [Google Scholar] [PubMed]
- Tomaso, H.; Mooseder, G.; Al Dahouk, S.; Bartling, C.; Scholz, H.C.; Strauss, R.; Treu, T.M.; Neubauer, H. Seroprevalence of anti-Yersinia antibodies in healthy Austrians. Eur. J. Epidemiol. 2006, 21, 77–81. [Google Scholar] [CrossRef]
- Faber, M.; Willrich, N.; Schemmerer, M.; Rauh, C.; Kuhnert, R.; Stark, K.; Wenzel, J.J. Hepatitis E virus seroprevalence, seroincidence and seroreversion in the German adult population. J. Viral Hepat. 2018, 25, 752–758. [Google Scholar] [CrossRef]
- Mirzaev, U.K.; Ouoba, S.; Ko, K.; Phyo, Z.; Chhoung, C.; Ataa, A.G.; Sugiyama, A.; Akita, T.; Tanaka, J. Systematic review and meta-analysis of hepatitis E seroprevalence in Southeast Asia: A comprehensive assessment of epidemiological patterns. BMC Infect. Dis. 2024, 24, 525. [Google Scholar] [CrossRef] [PubMed]
- Potemkin, I.A.; Lopatukhina, M.A.; Gadzhieva, O.A.; Prokhorova, E.L.; Diyarrassuba, A.; Isaeva, O.A.; Kozhanova, T.V.; Ivanova, O.E.; Silenova, O.V.; Setdikova, N.; et al. Prevalence of hepatitis E markers in children. Zh. Mikrobiol. Epidemiol. Immunobiol. 2015, 92, 38–46. [Google Scholar]
- Yasar, O.; Karatayli, E.; Cengiz, G.; Kizilpinar, M.; Yurdcu, E.; Albayrak, R.; Guven, A.; Arslan, O.; Karahan, C.; Otlu, B.; et al. HEV seroprevalence in blood donors in Turkey by two commercial total anti-HEV Ab ELISA kits. J. Med. Virol. 2019, 91, 2174–2181. [Google Scholar] [CrossRef]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef]
- Krumbholz, A.; Joel, S.; Dremsek, P.; Neubert, A.; Johne, R.; Durrwald, R.; Walther, M.; Muller, T.H.; Kuhnel, D.; Lange, J.; et al. Seroprevalence of hepatitis E virus (HEV) in humans living in high pig density areas of Germany. Med. Microbiol. Immunol. 2014, 203, 273–282. [Google Scholar] [CrossRef]
- Yazbek, S.; Kreidieh, K.; Ramia, S. Hepatitis E virus in the countries of the Middle East and North Africa region: An awareness of an infectious threat to blood safety. Infection 2016, 44, 11–22. [Google Scholar] [CrossRef]
- Vu Thi, N.; Pozio, E.; Van De, N.; Praet, N.; Pezzotti, P.; Gabriel, S.; Claes, M.; Thuy, N.T.; Dorny, P. Anti-Trichinella IgG in ethnic minorities living in Trichinella-endemic areas in northwest Vietnam: Study of the predictive value of selected clinical signs and symptoms for the diagnosis of trichinellosis. Acta Trop. 2014, 139, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Samkange-Zeeb, F.; Dragano, N.; Keil, T.; Krist, L.; Yesil-Jurgens, R.; Schlaud, M.; Jockel, K.H.; Razum, O.; Reiss, K.; et al. Participation of Turkish migrants in an epidemiological study: Does the recruitment strategy affect the sample characteristics? J. Immigr. Minor. Health 2019, 21, 811–819. [Google Scholar] [CrossRef]

| Location | n | Germans | Russians | Turks | Vietnamese | Total |
|---|---|---|---|---|---|---|
| Clinics (Charité *, Vivantes) | 2 | 298 | 22 | 69 | 7 | 396 |
| Healthcare centres | 4 | 14 | 31 | 66 | 0 | 111 |
| Occupational Health Centre of Charité | 1 | 109 | 3 | 2 | 1 | 115 |
| Migrant associations | 35 | 19 | 157 | 116 | 106 | 398 |
| Ethnic shops | 4 | 3 | 0 | 9 | 54 | 66 |
| Festivals | 3 | 17 | 1 | 4 | 7 | 29 |
| Other § | 5 | 37 | 1 | 7 | 20 | 65 |
| Total | 55 | 497 | 215 | 273 | 195 | 1180 |
| Germans n = 497 | % | Russians n = 215 | % | Turks n = 273 | % | Vietnamese n = 195 | % | |
|---|---|---|---|---|---|---|---|---|
| Sex (female) | 306 | 61.6 | 175 | 81.4 | 194 | 71.1 | 136 | 69.7 |
| Age in years (median, IQR) | 46 (31–60) | 58 (43–65) | 46 (36–53) | 44 (29–54) | ||||
| Generation of migration | ||||||||
| First (born abroad) | - | 215 | 100 | 209 | 77 | 186 | 95 | |
| Second | - | 0 | 0 | 64 | 23 | 9 | 5 | |
| Age in years at migration to Germany (median, IQR) | - | 42 (31–51) | 18 (4–24) | 25 (21–31) |
| Exposure Risks | Campylobacter | Salmonella | Yersinia | Hepatitis E Virus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Migration background (Germans as reference group) | ||||||||||||
| Russian | 1.6 | 1.1–2.3 | 0.008 | 0.4 | 0.2–0.6 | <0.001 | 0.3 | 0.2–0.4 | <0.001 | |||
| Turkish | 0.6 | 0.4–0.9 | 0.008 | 0.5 | 0.3–0.8 | 0.001 | 0.6 | 0.2–0.9 | 0.01 | |||
| Vietnamese | 2.3 | 1.5–3.2 | <0.001 | 0.3 | 0.2–0.4 | <0.001 | 1.9 | 1.2–2.9 | 0.005 | |||
| Age (18–29 years as reference group) | ||||||||||||
| 30–39 | 0.6 | 0.4–1.01 | 0.05 | 2.1 | 1.3–3.4 | 0.002 | 1.8 | 0.9–3.6 | 0.1 | |||
| 40–49 | 0.7 | 0.4–1.1 | 0.1 | 3.3 | 2.1–5.2 | <0.001 | 2.3 | 1.2–4.5 | 0.01 | |||
| 50–59 | 0.4 | 0.3–0.7 | 0.001 | 3.2 | 2.1–5.1 | <0.001 | 4.9 | 2.7–9.0 | <0.001 | |||
| ≥60 | 0.7 | 0.5–1.1 | 0.1 | 3.5 | 2.2–5.4 | <0.001 | 10.1 | 1.2–2.9 | <0.001 | |||
| Sex (female as reference group) | ||||||||||||
| Male | 1.3 | 1.01–1.8 | 0.045 | |||||||||
| Buying meat products in German retail shops | ||||||||||||
| Yes | 1.5 | 1.03–2.0 | 0.03 | |||||||||
| Consumption of chicken (no consumption as reference group) | ||||||||||||
| Rarely | 0.7 | 0.4–1.3 | 0.3 | |||||||||
| Occasionally | 0.6 | 0.3–0.9 | 0.03 | |||||||||
| Often | 0.6 | 0.3–0.9 | 0.02 | |||||||||
| Consumption of raw pork (no consumption as reference group) | ||||||||||||
| Rarely | 1.5 | 0.99–2.2 | 0.06 | |||||||||
| Occasionally | 2.6 | 1.6–4.3 | <0.001 | |||||||||
| Often | 3.4 | 1.6–7.2 | 0.001 | |||||||||
| Consumption of raw fish (no consumption as reference group) | ||||||||||||
| Rarely | 1.6 | 1.1–2.3 | 0.01 | |||||||||
| Occasionally | 1.6 | 1.0–2.4 | 0.048 | |||||||||
| Often | 1.3 | 0.6–2.9 | 0.6 | |||||||||
| Careful when buying animal food products | ||||||||||||
| Yes | 0.6 | 0.5–0.8 | 0.001 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boone, I.; Janßen, S.; Marcotty, T.; Moos, V.; Allers, K.; Geelhaar-Karsch, A.; Schneider, T.; Al Dahouk, S. Risk Factors for Foodborne Zoonoses Among Populations With and Without a Migration Background in Berlin, Germany. Trop. Med. Infect. Dis. 2025, 10, 281. https://doi.org/10.3390/tropicalmed10100281
Boone I, Janßen S, Marcotty T, Moos V, Allers K, Geelhaar-Karsch A, Schneider T, Al Dahouk S. Risk Factors for Foodborne Zoonoses Among Populations With and Without a Migration Background in Berlin, Germany. Tropical Medicine and Infectious Disease. 2025; 10(10):281. https://doi.org/10.3390/tropicalmed10100281
Chicago/Turabian StyleBoone, Idesbald, Sabrina Janßen, Tanguy Marcotty, Verena Moos, Kristina Allers, Anika Geelhaar-Karsch, Thomas Schneider, and Sascha Al Dahouk. 2025. "Risk Factors for Foodborne Zoonoses Among Populations With and Without a Migration Background in Berlin, Germany" Tropical Medicine and Infectious Disease 10, no. 10: 281. https://doi.org/10.3390/tropicalmed10100281
APA StyleBoone, I., Janßen, S., Marcotty, T., Moos, V., Allers, K., Geelhaar-Karsch, A., Schneider, T., & Al Dahouk, S. (2025). Risk Factors for Foodborne Zoonoses Among Populations With and Without a Migration Background in Berlin, Germany. Tropical Medicine and Infectious Disease, 10(10), 281. https://doi.org/10.3390/tropicalmed10100281







