AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence
Abstract
1. Introduction
2. Materials and Methods
2.1. Register and Guidelines
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection Process
2.6. Data Extraction
2.7. Risk-of-Bias Assessment
2.8. Data Synthesis
2.9. Certainty of Evidence Assessment (GRADE)
3. Results
3.1. Meta-Analysis Results
3.2. Meta-Regression Results
4. Discussion
4.1. Principal Findings
4.2. Interpretation of Heterogeneity
4.3. Implications for Policy and Surveillance
4.4. Clinical and Microbiological Implications
4.5. Infection Prevention and Stewardship
4.6. Limitations
4.7. Certainty of Evidence (GRADE)
4.8. Research Priorities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3GCRE | Third-generation cephalosporin-resistant Enterobacterales |
| AmpC | AmpC β-lactamase |
| AMR | Antimicrobial resistance |
| AMS | Antimicrobial stewardship |
| BDM | Broth dilution method |
| CI | Confidence interval |
| CLSI | Clinical and Laboratory Standards Institute |
| CRE | Carbapenem-resistant Enterobacterales |
| ESBL | Extended-spectrum β-lactamase |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| GLMM | Generalized Linear Mixed Model |
| GNB | Gram-negative bacteria |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HC3 | Huber–White cluster-robust standard errors type 3 |
| I2 | I-squared |
| ICU | Intensive Care Unit |
| ICUs | Intensive Care Units |
| JBI | Joanna Briggs Institute |
| LMICs | Low- and middle-income countries |
| MALDI-TOF | Matrix-assisted laser desorption/ionization-time of flight |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| PCR-RFLP | PCR with restriction fragment length polymorphism |
| PCR-SSCP | PCR with single-strand conformational polymorphism |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| Q | Cochran’s Q statistic |
| QM | Omnibus test of moderators |
| REML | Restricted Maximum Likelihood |
| WHO | World Health Organization |
| τ2 | Tau-squared |
References
- WHO Antimicrobial Resistance 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 13 August 2025).
- Eiamphungporn, W.; Schaduangrat, N.; Malik, A.A.; Nantasenamat, C. Tackling the Antibiotic Resistance Caused by Class A β-Lactamases through the Use of β-Lactamase Inhibitory Protein. Int. J. Mol. Sci. 2018, 19, 2222. [Google Scholar] [CrossRef] [PubMed]
- Marsik, F.J.; Nambiar, S. Review of Carbapenemases and AmpC-beta Lactamases. Pediatr. Infect. Dis. J. 2011, 30, 1094–1095. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Ghafourian, S.; Sadeghifard, N.; Soheili, S.; Sekawi, Z. Extended Spectrum Beta-lactamases: Definition, Classification and Epidemiology. Curr. Issues Mol. Biol. 2015, 17, 11–21. [Google Scholar] [CrossRef]
- Philippon, A.; Arlet, G.; Jacoby, G.A. Plasmid-Determined AmpC-Type β-Lactamases. Antimicrob. Agents Chemother. 2002, 46, 1–11. [Google Scholar] [CrossRef]
- Hanson, N.D. AmpC-lactamases: What do we need to know for the future? J. Antimicrob. Chemother. 2003, 52, 2–4. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Hanada, K.; Sugimoto, T. Random-effects meta-analysis via generalized linear mixed models: A Bartlett-corrected approach for few studies. arXiv 2025. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis. In R: A Hands-on Guide; Chapman and Hall/CRC: New York, NY, USA, 2021. [Google Scholar]
- Lin, L.; Chu, H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M. Methods for meta-analysis and meta-regression of binomial data: Concepts and tutorial with Stata command metapreg. Arch. Public Health 2024, 82, 14. [Google Scholar] [CrossRef]
- Kundu, P.; Tang, R.; Chatterjee, N. Generalized meta-analysis for multiple regression models across studies with disparate covariate information. Biometrika 2019, 106, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef]
- Pino, I.C.; Domínguez, Y.M.; González, R.G.; Bello, T.H.; Sepúlveda, A.M.; Mella, M.S.; Zemelman, M.C.; Zemelman, Z.R. Producción de ß-lactamasas de espectro extendido (BLEE) en cepas de Acinetobacter baumannii aisladas en hospitales de la VIIIa Región, Chile. Rev. Chil. Infectol. 2007, 24, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ribas, R.M.; Freitas, C.; Gontijo Filho, P.P. Nosocomial bloodstream infections: Organisms, risk factors and resistant phenotypes in the Brazilian University Hospital. Braz. J. Infect. Dis. 2007, 11, 351–354. [Google Scholar] [CrossRef]
- Bertona, E.; Radice, M.; Rodríguez, C.H.; Barberis, C.; Vay, C.; Famiglietti, A.; Gutkind, G. Caracterización fenotípica y genotípica de la resistencia enzimática a las cefalosporinas de tercera generación en Enterobacter spp. Rev. Argent. Microbiol. 2005, 37, 203–208. [Google Scholar]
- Quinteros, M.; Radice, M.; Gardella, N.; Rodriguez, M.M.; Costa, N.; Korbenfeld, D.; Couto, E.; Gutkind, G. Extended-Spectrum β-Lactamases in Enterobacteriaceae in Buenos Aires, Argentina, Public Hospitals. Antimicrob. Agents Chemother. 2003, 47, 2864–2867. [Google Scholar] [CrossRef]
- Fehlberg, L.C.C.; Xavier, D.E.; Peraro, P.P.; Marra, A.R.; Edmond, M.B.; Gales, A.C. Beta-Lactam Resistance Mechanisms in Pseudomonas aeruginosa Strains Causing Bloodstream Infections: Comparative Results Between Brazilian and American Isolates. Microb. Drug Resist. 2012, 18, 402–407. [Google Scholar] [CrossRef]
- Favier, P.; Raffo, C.; Torres, D.; Serio, E.; Pérez, J.; Primost, I.; Luna, R.; Kumar, L.; Pérez, D. Third-generation cephalosporins programmed restriction in the context of an outbreak of AmpC β-lactamase-producing gram-negative bacilli in critical units: A real-life experience. Rev. Chil. Infectol. 2021, 38, 597–604. [Google Scholar] [CrossRef]
- Silva, A.S.; Da Silva, N.; Do Valle, F.; Da Rocha, J.; Ehrlich, S.; Martins, I. Mortality and Risk Factors of Death in Patients with AmpC β-Lactamase Producing Enterobacterales Bloodstream Infection: A Cohort Study. Infect. Drug Resist. 2024, 17, 4023–4035. [Google Scholar] [CrossRef]
- Ayzanoa, B.; Castillo-Vilcahuaman, C.; Salvatierra, G.; Dávila-Barclay, A.; Huancachoque, J.; Calderón, M.; Rizo-Patrón, E.; Santillán-Salas, C.; Gilman, R.H.; Tsukayama, P. Genomic surveillance of multidrug-resistant E. coli and Klebsiella in clinical and wastewater isolates from a pediatric hospital in lima, peru. Access Microbiol. 2025. [Google Scholar] [CrossRef]
- Rodrigues, S.H.; Nunes, G.D.; Soares, G.G.; Ferreira, R.L.; Damas, M.S.F.; Laprega, P.M.; Shilling, R.E.; Campos, L.C.; da Costa, A.S.; Malavazi, I.; et al. First report of coexistence of blaKPC-2 and blaNDM-1 in carbapenem-resistant clinical isolates of Klebsiella aerogenes in Brazil. Front. Microbiol. 2024, 15, 1352851. [Google Scholar] [CrossRef]
- Faccone, D.; Gomez, S.A.; De Mendieta, J.M.; Sanz, M.B.; Echegorry, M.; Albornoz, E.; Lucero, C.; Ceriana, P.; Menocal, A.; Martino, F.; et al. Emergence of Hyper-Epidemic Clones of Enterobacterales Clinical Isolates Co-Producing KPC and Metallo-Beta-Lactamases during the COVID-19 Pandemic. Pathogens 2023, 12, 479. [Google Scholar] [CrossRef]
- Arns, B.; Sorio, G.G.L.; Vieceli, T.; Pereira, D.; Celestino De Souza, Â.; Lamb Wink, P.; Paes, J.H.; David, L.; Barboza, F.; Hickmann, S.; et al. Evaluation of clinical and microbiological factors related to mortality in patients with Gram-negative bacterial infections treated with ceftazidime–avibactam: A prospective multicentric cohort study. J. Glob. Antimicrob. Resist. 2024, 36, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Cotrina, F.; Condori, D.M.; Gomez, T.O.; Yactayo, K.M.; Barron-Pastor, H. First Isolates of OXA-48-Like Carbapenemase-Producing Enterobacteriaceae in A Specialized Cancer Center. Infect. Chemother. 2022, 54, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Echegorry, M.; Marchetti, P.; Sanchez, C.; Olivieri, L.; Faccone, D.; Martino, F.; Badola, T.S.; Ceriana, P.; Rapoport, M.; Lucero, C.; et al. National Multicenter Study on the Prevalence of Carbapenemase-Producing Enterobacteriaceae in the Post-COVID-19 Era in Argentina: The RECAPT-AR Study. Antibiotics 2024, 13, 1139. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.D.; Alcalde-Rico, M.; Ugalde, J.A.; Olivares-Pacheco, J.; Quiroz, V.; Brito, B.; Rivas, L.M.; Munita, J.M.; García, P.C.; Wozniak, A.; et al. Ceftazidime/avibactam resistance is associated with PER-3-producing ST309 lineage in Chilean clinical isolates of non-carbapenemase producing Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2024, 14, 1410834. [Google Scholar] [CrossRef]
- Aravena, C.; Valencia, B.; Villegas, A.; Ortega, M.; Fernández, R.A.; Araya, R.P.; Araya, R.P.; Saavedra, C.; Del Campo, R. Caracterización de cepas clínicas y ambientales de Salmonella enterica subsp. enterica serovar Heidelberg aisladas en Chile. Rev. Médica Chile 2019, 147, 24–33. [Google Scholar] [CrossRef]
- Cavalcanti, F.L.d.S.; Mirones, C.R.; Paucar, E.R.; Montes, L.Á.; Leal-Balbino, T.C.; de Morais, M.M.C.; Martínez-Martínez, L.; Ocampo-Sosa, A.A.; Cavalcanti, F.L.d.S. Mutational and acquired carbapenem resistance mechanisms in multidrug resistant Pseudomonas aeruginosa clinical isolates from Recife, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 1003–1009. [Google Scholar] [CrossRef]
- Santella, G.; Pollini, S.; Docquier, J.-D.; Almuzara, M.; Gutkind, G.; Rossolini, G.M.; Radice, M. Resistencia a carbapenemes en aislamientos de Pseudomonas aeruginosa: Un ejemplo de interacción entre distintos mecanismos. Rev. Panam. Salud Pública 2011, 30, 545–548. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Villegas, M.-V.; Correa, A.; Quinn, J.P.; Nordmann, P. Wide Dissemination of Pseudomonas aeruginosa Producing β-Lactamase blaKPC-2 Gene in Colombia. Antimicrob. Agents Chemother. 2011, 55, 5350–5353. [Google Scholar] [CrossRef]
- Wozniak, A.; Villagra, N.A.; Undabarrena, A.; Gallardo, N.; Keller, N.; Moraga, M.; Román, J.C.; Mora, G.C.; García, P. Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J. Med. Microbiol. 2012, 61, 1270–1279. [Google Scholar] [CrossRef]
- Delgado, D.Y.C.; Barrigas, Z.P.T.; Astutillo, S.G.O.; Jaramillo, A.P.A.; Ausili, A. Detection and molecular characterization of β-lactamase genes in clinical isolates of Gram-negative bacteria in Southern Ecuador. Braz. J. Infect. Dis. 2016, 20, 627–630. [Google Scholar] [CrossRef]
- Bartoloni, A.; Sennati, S.; Di Maggio, T.; Mantella, A.; Riccobono, E.; Strohmeyer, M.; Revollo, C.; Villagran, A.L.; Pallecchi, L.; Rossolini, G.M. Antimicrobial susceptibility and emerging resistance determinants (blaCTX-M, rmtB, fosA3) in clinical isolates from urinary tract infections in the Bolivian Chaco. Int. J. Infect. Dis. 2016, 43, 1–6. [Google Scholar] [CrossRef]
- De La Cadena, E.; Correa, A.; Muñoz, J.S.; Rojas, L.J.; Hernández-Gómez, C.; Pallares, C.; Perez, F.; Bonomo, R.A.; Villegas, M.V. Molecular characterisation of carbapenem-resistant Enterobacter cloacae complex in Colombia: Bla KPC and the ‘changing landscape’. J. Glob. Antimicrob. Resist. 2018, 13, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Soria-Segarra, C.; Soria-Segarra, C.; Catagua-González, A.; Gutiérrez-Fernández, J. Carbapenemase producing Enterobacteriaceae in intensive care units in Ecuador: Results from a multicenter study. J. Infect. Public Health 2020, 13, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.P.M. β-lactamasas AmpC en bacilos gramnegativos de aislados clínicos en un centro hospitalario de tercer nivel en Colombia. Rev. Cubana Med. Trop. 2021, 73, e503. [Google Scholar]
- Martínez, J.W.; Gutiérrez-Ocampo, E.; Valencia-Arango, D.; Henao-Martínez, J.F.; Sánchez-Duque, J.A. Microbiological characteristics of infections in a group of colombian patients with oncological diagnosis, 2014–2016. Infectio 2020, 24, 182. [Google Scholar] [CrossRef]
- Morales, S.; AGallego, M.; Vanegas, J.M.; Jiménez, J.N. Detection of carbapenem resistance genes in Pseudomonas aeruginosa isolates with several phenotypic susceptibility profiles. Ces Med. 2018, 32, 203–214. [Google Scholar] [CrossRef]
- Arango, J.J.; Leal, A.L.; Montilla, M.D.P.; Camacho Moreno, G. Inference of the phenotypic resistance profile of Pseudomonas aeruginosa through an interpretative reading of the antibiogram in a pediatric hospital. 2006–2014. Rev. Fac. Med. 2016, 64, 409. [Google Scholar] [CrossRef]
- Cacci, L.C.; Chuster, S.G.; Martins, N.; Carmo, P.R.; Girão, V.B.d.C.; Nouér, S.A.; Freitas, W.V.D.; Matos, J.A.D.; Magalhães, A.C.D.G.; Ferreira, A.L.P.; et al. Mechanisms of carbapenem resistance in endemic Pseudomonas aeruginosa isolates after an SPM-1 metallo-β-lactamase producing strain subsided in an intensive care unit of a teaching hospital in Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 551–558. [Google Scholar] [CrossRef]
- Vasques, M.R.G.; Bello, A.R. β-lactamase producing Enterobacteria isolated from surveillance swabs of patients in a Cardiac Intensive Care Unit in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2011, 15, 28–33. [Google Scholar]
- Cunha Ferreira, T.D.; Martins, I.S. Risk Factors of Death in Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacterales in Patients with Neoplasia. Infect. Drug Resist. 2021, 14, 3083–3097. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.A.C.; Campos, J.C.; Passadore, L.F.; Sampaio, S.C.F.; Nicodemo, A.C.; Sampaio, J.L.M. Frequency of Plasmid-Mediated AmpC β-Lactamases in Escherichia coli Isolates from Urine Samples in São Paulo, Brazil. Microb. Drug Resist. 2016, 22, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Carvalhaes, C.G.; da Silva, G.L.; Soares, S.P.T.; de Alcåntara, L.R.; Elias, L.S.; Sandri, A.M.; Gales, A.C. Outbreak of Carbapenem-Resistant Providencia stuartii in an Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2012, 33, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.P.; Koong, J.; Wozniak, A.; Opazo-Capurro, A.; To, J.; Garcia, P.; Hamidian, M. Genomic Analysis of Carbapenem-Resistant Acinetobacter baumannii Strains Recovered from Chilean Hospitals Reveals Lineages Specific to South America and Multiple Routes for Acquisition of Antibiotic Resistance Genes. Microbiol. Spectr. 2022, 10, e02463-22. [Google Scholar] [CrossRef]
- Cejas, D.; Fernández, C.L.; Quinteros, M.; Giovanakis, M.; Vay, C.L.S.; Mutti, D.; Pagniez, G.; Almuzara, M.; Gutkind, G.; Radice, M. Plasmid-Encoded AmpC (pAmpC) in Enterobacteriaceae: Epidemiology of microorganisms and resistance markers. Rev. Argent. Microbiol. 2012, 44, 182–186. [Google Scholar]
- Cruz, G.R.; Radice, M.; Sennati, S.; Pallecchi, L.; Rossolini, G.M.; Gutkind, G.; Conza, J.A.D. Prevalence of plasmid-mediated quinolone resistance determinants among oxyiminocephalosporin-resistant Enterobacteriaceae in Argentina. Mem. Inst. Oswaldo Cruz 2013, 108, 924–927. [Google Scholar] [CrossRef]
- Beirão, E.M.; Rodrigues, S.; de Andrade, T.K.; Serra, F.B.; de Paula, M.D.N.; Polis, T.J.B.; Gales, A.C. Activity of ceftolozane-tazobactam and comparators against gram-negative bacilli: Results from the study for monitoring antimicrobial resistance trends (SMART—Brazil; 2016–2017). Braz. J. Infect. Dis. 2020, 24, 310–321. [Google Scholar] [CrossRef]
- Tuon, F.F.; Cieslinski, J.; Rodrigues, S.D.S.; Serra, F.B.; Paula, M.D.-N.D. Evaluation of in vitro activity of ceftolozane–tazobactam against recent clinical bacterial isolates from Brazil—The EM200 study. Braz. J. Infect. Dis. 2020, 24, 96–103. [Google Scholar] [CrossRef]
- Varon, F.; Torres-Caro, C.; Herrera-Diaz, C.; Ali, A.; Hernández-Parra, A.; Aguirre-Franco, C.; Uribe-Hernández, A.M. Microbiological characterization of severe exacerbations in Chronic Obstructive Pulmonary Disease (COPD) in patients admitted to the ICU with or without associated pneumonia: A retrospective cross-sectional study. Infectio 2019, 23, 307. [Google Scholar] [CrossRef]
- Gómez-González, J.F.; Sánchez-Duque, J.A. Perfil microbiológico y resistencia bacteriana en una unidad de cuidados intensivos de Pereira, Colombia, 2015. Rev. Médicas UIS 2018, 31, 9–15. [Google Scholar] [CrossRef]
- De La Lastra, V.; Rivas, L.M.; Silva, F.; Ulloa, M.T.; Pinto, M.E.; Vidal, M. Detección de serinocarbapenemasas de clase A y otros mecanismos de resistencia enzimática a β-lactámicos en cepas de enterobacterias con susceptibilidad disminuida a carbapenémicos, aisladas de pacientes de un hospital universitario de Santiago, Chile. Rev. Chil. Infectol. 2014, 31, 682–688. [Google Scholar] [CrossRef]
- Campana, E.H.; Barbosa, P.P.; Fehlberg, L.C.C.; Gales, A.C. Frequency of plasmid-mediated AmpC in Enterobacteriaceae isolated in a Brazilian Teaching Hospital. Braz. J. Microbiol. 2013, 44, 477–480. [Google Scholar] [CrossRef]
- Hoyos, Á.; Serna, L.; Ortiz, G.; Aguirre, J. Infección urinaria adquirida en la comunidad en pacientes pediátricos: Clínica, factores de riesgo, etiología, resistencia a los antibióticos y respuesta a la terapia empírica. Infectio 2012, 16, 94–103. [Google Scholar] [CrossRef]
- Nastro, M.; Piazza, L.M.; Saposnik, E.; García, S.; Barberis, C.; Vay, C.; Carlos, R.; Carlos, H.; Famiglietti, A. Resistencia a cefalosporinas de espectro extendido en enterobacterias sin AmpC inducible: Evaluación de los nuevos puntos de corte. Rev. Argent. Microbiol. 2012, 44, 30–35. Available online: https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0325-75412012000100007&lng=es (accessed on 13 August 2025). [PubMed]
- Marcano, D.; Jesús, A.D.; Hernández, L. Frecuencia de enzimas asociadas a sensibilidad disminuida a betalactámicos en aislados de enterobacterias, Caracas, Venezuela. Rev. Panam. Salud Publica 2011, 30, 529–534. Available online: https://www.scielosp.org/pdf/rpsp/2011.v30n6/529-534/es#:~:text=Determinar%20la%20frecuencia%20de%20los%20mecanismos%20enzim%C3%A1ticos%20asociados,obtenidos%20de%20centros%20hospitalarios%20de%20Caracas%2C%20Venezuela.%20M%C3%A9todos (accessed on 13 August 2025).
- JuRe, M.A.; Presti, C.; Cudmani, N.M.; Grellet, L.M.; López, C.; Musa, E.H.; Aulet, O.C.; Peñalver, C.N.; Saavedra, L.; de Castillo, M.C. β-lactamasas AmpC plasmídicas tipo CMY-2 emergentes en Tucumán, Argentina. Rev. Argent. Microbiol. 2011, 43, 24–27. Available online: https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0325-75412011000100005&lng=es (accessed on 13 August 2025).
- Cantarelli, V.V.; Inamine, E.; Brodt, T.C.Z.; Secchi, C.; Cavalcante, B.C.; Pereira, F.D.S. Utility of the ceftazidime-imipenem antagonism test (CIAT) to detect and confirm the presence of inducible AmpC beta-lactamases among Enterobacteriaceae. Braz. J. Infect. Dis. 2007, 11, 237–239. [Google Scholar] [CrossRef]
- Romero, I.A.G.; Saavedra, C.H.; Castro, A.L.L.; Schmalbac, J.E.; Anaya, J.R.M. Caracterización molecular de aislamientos de Enterobacter cloacae multirresistentes, productores â-lactamasas provenientes de pacientes de un hospital de tercer nivel de Bogotá. Rev. Fac. Med. 2005, 53, 148–159. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-00112005000300001&lng=en (accessed on 13 August 2025).
- Cezário, R.C.; Ribas, R.M.; Abdallah, V.O.S.; Carneiro, C.L.; Gontijo Filho, P.P. Infection and colonization by Gram-negative bacilli in neonates hospitalized in High Risk Nursery at Uberlandia Federal University Hospital: Etiology, resistant phenotypes and risk factors. Braz. J. Microbiol. 2004, 35, 193–198. [Google Scholar] [CrossRef]
- Mojica, M.F.; De La Cadena, E.; García-Betancur, J.C.; Porras, J.; Novoa-Caicedo, I.; Páez-Zamora, L.; Pallares, C.; Appel, T.M.; Radice, M.A.; Paulo Castañeda-Méndez, G.; et al. Molecular Mechanisms of Resistance to Ceftazidime/Avibactam in Clinical Isolates of Enterobacterales and Pseudomonas aeruginosa in Latin American Hospitals. mSphere 2023, 8, e00651-22. [Google Scholar] [CrossRef]
- Lee, A.H.Y.; Porto, W.F.; De Faria, C., Jr.; Dias, S.C.; Alencar, S.A.; Pickard, D.J.; Hancock, R.E.; Franco, O.L. Genomic insights into the diversity, virulence and resistance of Klebsiella pneumoniae extensively drug resistant clinical isolates. Microb Genomics 2021, 7, 613. [Google Scholar] [CrossRef]
- Perez, L.R.R.; Carniel, E.; Narvaez, G.A. High minimum inhibitory concentrations among derepressed AmpC-beta-lactamase–producing Enterobacter cloacae complex isolates for ceftolozane with tazobactam. Infect. Control Hosp. Epidemiol. 2020, 41, 631–633. [Google Scholar] [CrossRef]
- Papa-Ezdra, R.; Grill Diaz, F.; Vieytes, M.; García-Fulgueiras, V.; Caiata, L.; Ávila, P.; Brasesco, M.; Christophersen, I.; Cordeiro, N.F.; Algorta, G.; et al. First three Escherichia coli isolates harbouring mcr-1 in Uruguay. J. Glob. Antimicrob. Resist. 2020, 20, 187–190. [Google Scholar] [CrossRef]
- Nocua-Báez, L.C.; Cortés, J.A.; Leal, A.L.; Arias, G.F.; Ovalle-Guerro, M.V.; Saavedra-Rojas, S.Y.; Buitrago, G.; Escobar-Pérez, J.A.; Castro-Cardozo, B. Antimicrobial susceptibility profile in urinary pathogens causing community-acquired infections in diabetic patients in Colombia. Biomédica 2017, 37, 353. [Google Scholar] [CrossRef] [PubMed]
- Pavez, M.; Vieira, C.; De Araujo, M.R.; Cerda, A.; De Almeida, L.M.; Lincopan, N.; Mamizuka, E.M. Molecular mechanisms of membrane impermeability in clinical isolates of Enterobacteriaceae exposed to imipenem selective pressure. Int. J. Antimicrob. Agents 2016, 48, 78–85. [Google Scholar] [CrossRef]
- Martins, H.; Bomfim, M.; França, R.; Farias, L.; Carvalho, M.; Serufo, J.; Santos, S. Resistance Markers and Genetic Diversity in Acinetobacter baumannii Strains Recovered from Nosocomial Bloodstream Infections. Int. J. Environ. Res. Public Health 2014, 11, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.L.; Cortés, J.A.; Arias, G.; Ovalle, M.V.; Saavedra, S.Y.; Buitrago, G.; Escobar, J.A.; Castro, B.E. Emergencia de fenotipos resistentes a cefalosporinas de tercera generación en Enterobacteriaceae causantes de infección del tracto urinario de inicio comunitario en hospitales de Colombia. Enfermedades Infecc. Microbiol. Clínica 2013, 31, 298–303. [Google Scholar] [CrossRef]
- Nogueira-Miranda, K.D.S.; Palmeiro, J.K.; Conte, D.; Maia, F.V.; Reason, I.T.D.M.; Monteiro, C.L.; Dalla-Costa, L.M. Detection of Extended-Spectrum β-Lactamase in Enterobacter spp.—Evaluation of Six Phenotypic Tests. Microb. Drug Resist. 2012, 18, 66–70. [Google Scholar] [CrossRef]
- Picão, R.C.; Poirel, L.; Gales, A.C.; Nordmann, P. Diversity of β-Lactamases Produced by Ceftazidime-Resistant Pseudomonas aeruginosa Isolates Causing Bloodstream Infections in Brazil. Antimicrob. Agents Chemother. 2009, 53, 3908–3913. [Google Scholar] [CrossRef]
- Pavez, M.; Neves, P.; Dropa, M.; Matté, M.H.; Grinbaum, R.S.; Elmor De Araújo, M.R.; Mamizuka, E.M.; Lincopan, N. Emergence of carbapenem-resistant Escherichia coli producing CMY-2-type AmpC β-lactamase in Brazil. J. Med. Microbiol. 2008, 57, 1590–1592. [Google Scholar] [CrossRef]
- Da Silva Dias, R.C.; Borges-Neto, A.A.; D’Almeida Ferraiuoli, G.I.; de-Oliveira, M.P.; Riley, L.W.; Moreira, B.M. Prevalence of AmpC and other β-lactamases in Enterobacteria at a large urban university hospital in Brazil. Diagn. Microbiol. Infect. Dis. 2008, 60, 79–87. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N.; Dowzicky, M.J.; Fritsche, T.R. Antimicrobial activity of tigecycline tested against nosocomial bacterial pathogens from patients hospitalized in the intensive care unit. Diagn. Microbiol. Infect. Dis. 2005, 52, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Casellas, J.M.; Tomé, G.; Bantar, C.; Bertolini, P.; Blázquez, N.; Borda, N.; Couto, E.; Cudmani, N.; Guerrera, J.; Juárez, M.J.; et al. Argentinean collaborative multicenter study on the in vitro comparative activity of piperacillin-tazobactam against selected bacterial isolates recovered from hospitalized patients. Diagn. Microbiol. Infect. Dis. 2003, 47, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, C.; Hsiung, A.; Oplustil, C.; Sampaio, J.; Sakagami, E.; Turner, P.; Mendes, C. Antimicrobial susceptibility of Gram-negative bacteria in Brazilian hospitals: The MYSTIC Program Brazil 2003. Braz. J. Infect. Dis. 2005, 9, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Cortes, J.; Arango, Á.; Correa, C.; Leal, A. Resistencia Antimicrobiana en Unidades de Cuidado Intensivo de Bogotá, Colombia, 2001–2003. Rev. Salud Pública 2006, 8, 86–101. [Google Scholar] [CrossRef]
- Velandia, D.; Gutierrez, L.; Quiroga, C.; Caycedo, M. Molecular Characterization of Multidrug-Resistant Bacteria Isolated from a Boyacá Hospital, Colombia. Internet J. Microbiol. 2018, 15, 1–2. Available online: https://www.academia.edu/download/82235827/53024.pdf (accessed on 13 August 2025).
- Sennati, S.; Santella, G.; Di Conza, J.; Pallecchi, L.; Pino, M.; Ghiglione, B.; Rossolini, G.M.; Radice, M.G. Gutkind. Changing Epidemiology of Extended-Spectrum β-Lactamases in Argentina: Emergence of CTX-M-15. Antimicrob. Agents Chemother. 2012, 56, 6003–6005. [Google Scholar] [CrossRef]
- Scheffer, M.C.; Bazzo, M.L.; Steindel, M.; Darini, A.L.; Clímaco, E.; Dalla-Costa, L.M. Intrahospital spread of carbapenem-resistant Pseudomonas aeruginosa in a University Hospital in Florianópolis, Santa Catarina, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 367–371. [Google Scholar] [CrossRef]
- Cuicapuza, D.; Loyola, S.; Velásquez, J.; Fernández, N.; Llanos, C.; Ruiz, J.; Tsukayama, P.; Tamariz, J. Molecular characterization of carbapenemase-producing Enterobacterales in a tertiary hospital in Lima, Peru. Microbiol. Spectr. 2024, 12, e02503-23. [Google Scholar] [CrossRef]
- Martínez, P.; Mattar, S. Imipenem-resistant Acinetobacter baumannii carrying the ISAba1-bla OXA-23, 51 and ISAba1-bla ADC-7 genes in Monteria, Colombia. Braz. J. Microbiol. 2012, 43, 1274–1280. [Google Scholar] [CrossRef]
- Picão, R.C.; Carrara-Marroni, F.E.; Gales, A.C.; Venâncio, E.J.; Xavier, D.E.; Cristina, M.; Pelayo, J.S. Metallo-β-lactamase-production in meropenem-susceptible Pseudomonas aeruginosa isolates: Risk for silent spread. Mem. Inst. Oswaldo Cruz 2012, 107, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antimicrobial Resistance Threats in the United States, 2021-2022 [Internet]. Antimicrobial Resistance. 2024. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/update-2022.html (accessed on 13 August 2025).
- Rodríguez-Guerrero, E.; Callejas-Rodelas, J.C.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Systematic Review of Plasmid AmpC Type Resistances in Escherichia coli and Klebsiella pneumoniae and Preliminary Proposal of a Simplified Screening Method for ampC. Microorganisms 2022, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Blanco, M.; Labarca, J.A.; Villegas, M.V.; Gotuzzo, E. Extended spectrum β-lactamase producers among nosocomial Enterobacteriaceae in Latin America. Braz. J. Infect. Dis. 2014, 18, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Mohamudha, P.R.; Harish, B.N.; Parija, S.C. AmpC beta lactamases among Gram negative clinical isolates from a tertiary hospital, South India. Braz. J. Microbiol. 2010, 41, 596–602. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Romero-Alvarez, D.; Valdez-Vélez, V.; Morales, R.D.; Montalvo-Hernández, A.; Gomes-Dias, C.; Gomes-Dias, C.; Calvopina, M. Extended-Spectrum Beta-Lactamases Producing Escherichia coli in South America: A Systematic Review with a One Health Perspective. Infect. Drug Resist. 2022, 15, 5759–5779. [Google Scholar] [CrossRef]
- Hardy, M.E.; Kenney, R.M.; Tibbetts, R.J.; Shallal, A.B.; Veve, M.P. Leveraging stewardship to promote ceftriaxone use in severe infections with low- and no-risk AmpC Enterobacterales. Antimicrob. Agents Chemother. 2023, 67, e00826-23. [Google Scholar] [CrossRef]
- Cheo, H.M.; Ho, D.Y.K.; Koh, L.P.; Koh, M.C.Y.; Ngiam, J.N.; Lye, D.C.B. Cefepime Versus Carbapenem Therapy for the Treatment of Invasive Infections With Inducible Chromosomal AmpC-Producing Enterobacterales: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2025, 12, ofaf413. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Fabre, V.; Cosgrove, S.E.; Secaira, C.; Tapia Torrez, J.C.; Lessa, F.C.; Patel, T.S.; Quiros, R. Antimicrobial stewardship in Latin America: Past, present, and future. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e68. [Google Scholar] [CrossRef]
- Latin American and Caribbean Network for Antimicrobial Resistance Surveillance—ReLAVRA+—PAHO/WHO|Pan American Health Organization [Internet]. Available online: https://www.paho.org/en/topics/antimicrobial-resistance/latin-american-and-caribbean-network-antimicrobial-resistance (accessed on 13 August 2025).
- Hegewisch-Taylor, J.; Dreser-Mansilla, A.; Romero-Mónico, J.; Levy-Hara, G. Antimicrobial stewardship in hospitals in Latin America and the Caribbean: A scoping review. Rev. Panam. Salud Pública 2020, 44, e68. [Google Scholar] [CrossRef]
- Pallares, C.J.; Porras, J.; De La Cadena, E.; García-Betancur, J.C.; Restrepo-Arbeláez, N.; Viveros, S.M.C.; María, S.; Cornistein, W.; Castañeda-Méndez, P.; Cuellar, L.; et al. Antimicrobial stewardship programs in seven Latin American countries: Facing the challenges. BMC Infect. Dis. 2023, 23, 463. [Google Scholar] [CrossRef]
- PAHO and GARDP Will Collaborate to Tackle Antibiotic Resistance in Latin America and the Caribbean [Internet]. Paho.org. 2024. Available online: https://www.paho.org/en/news/26-9-2024-paho-and-gardp-will-collaborate-tackle-antibiotic-resistance-latin-america-and (accessed on 13 August 2025).
- ReLAVRA+: Leading the Fight Against Antimicrobial Resistance in Latin America and the Caribbean. Paho.org. 2025. Available online: https://www.paho.org/en/documents/relavra-leading-fight-against-antimicrobial-resistance-latin-america-and-caribbean (accessed on 13 August 2025).
- Tebano, G.; Zaghi, I.; Cricca, M.; Cristini, F. Antibiotic Treatment of Infections Caused by AmpC-Producing Enterobacterales. Pharmacy 2024, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, R.; Bähr, T.; Gatermann, S.G. Effect of ampC derepression on cefepime MIC in Enterobacterales with chromosomally encoded inducible AmpC β-lactamase. Clin. Microbiol. Infect. 2019, 25, 1158.e1–1158.e4. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Girdwood, S.C.T.; Gopaul, R.; Tekle, T.; Roberts, A.A.; Harris, A.D.; Cosgrove, S.E.; Carroll, K.C. The Use of Cefepime for Treating AmpC β-Lactamase–Producing Enterobacteriaceae. Clin. Infect. Dis. 2013, 57, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Meije, Y.; Pigrau, C.; Fernández-Hidalgo, N.; Clemente, M.; Ortega, L.; Sanz, X.; Loureiro-Amigo, J.; Sierra, M.; Ayestarán, A.; Morales-Cartagena, A.; et al. Non-intravenous carbapenem-sparing antibiotics for definitive treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase: A propensity score study. Int. J. Antimicrob. Agents 2019, 54, 189–196. [Google Scholar] [CrossRef]
- Mashru, J.; Elligsen, M.; Daneman, N.; Granger, M.-F.; Kozak, R.A.; Li, X.X.; Leis, J.A.; Lam, P.W. Impact of two antimicrobial susceptibility reporting strategies on treatment of bacteremia caused by low-risk AmpC inducible organisms. Antimicrob. Steward. Healthc. Epidemiol. 2025, 5, e70. [Google Scholar] [CrossRef]
- Rayner, B.; Verderosa, A.D.; Ferro, V.; Blaskovich, M.A.T. Siderophore conjugates to combat antibiotic-resistant bacteria. RSC Med. Chem. 2023, 14, 800–822. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef]
- Rahman, M.S.; Koh, Y.-S. A Novel Antibiotic Agent, Cefiderocol, for Multidrug-Resistant Gram-Negative Bacteria. J. Bacteriol. Virol. 2020, 50, 218–226. [Google Scholar] [CrossRef]
- Maseda, E.; Suárez De La Rica, A. The role of cefiderocol in clinical practice. Rev. Esp. Quimioter. 2022, 35, 39–44. [Google Scholar] [CrossRef]
- Smith, H.G.; Basak, S.; Aniebok, V.; Beech, M.J.; Alshref, F.M.; Allen, M.D.; Farley, M.; Schofield, C.J. Structural basis of Pseudomonas aeruginosa penicillin binding protein 3 inhibition by the siderophore-antibiotic cefiderocol. Chem. Sci. 2024, 15, 16928–16937. [Google Scholar] [CrossRef]
- El Ghali, A.; Kunz Coyne, A.J.; Lucas, K.; Tieman, M.; Xhemali, X.; Lau, S.; Iturralde, G.; Purdy, A.; Holger, D.J.; Garcia, E.; et al. Cefiderocol: Early clinical experience for multi-drug resistant gram-negative infections. Microbiol. Spectr. 2024, 12, e03108-23. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Khazaal, M.T.; El-Hendawy, H.H.; Mabrouk, M.I.; Faraag, A.H.I.; Bakkar, M.R. Antibiotic resistance and siderophores production by clinical Escherichia coli strains. BioTechnologia 2022, 103, 169–184. [Google Scholar] [CrossRef]
- Thompson, M.G.; Corey, B.W.; Si, Y.; Craft, D.W.; Zurawski, D.V. Antibacterial Activities of Iron Chelators against Common Nosocomial Pathogens. Antimicrob. Agents Chemother. 2012, 56, 5419–5421. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Huber, D.C.; Dichtl, S.; Steixner, S.; Nogler, M.; Weiss, G. Iron chelation destabilizes bacterial biofilms and potentiates the antimicrobial activity of antibiotics against coagulase-negative Staphylococci. Pathog. Dis. 2018, 76, fty052. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hassan, M.A.; Britigan, B.E.; Narayanasamy, P. Antimicrobial Activity of Gallium(III) Compounds: Pathogen-Dependent Targeting of Multiple Iron/Heme-Dependent Biological Processes. Curr. Issues Mol. Biol. 2024, 46, 9149–9161. [Google Scholar] [CrossRef] [PubMed]
- Zelaya-Molina, L.X.; Chávez-Díaz, I.F.; Urrieta-Velázquez, J.A.; Aragón-Magadan, M.A.; Puente-Valenzuela, C.O.; Blanco-Camarillo, M.; de los Santos-Villalobos, S.; Ramos-Garza, J. Microbial Metallophores in the Productivity of Agroecosystems. Microbiol. Res. 2025, 16, 67. [Google Scholar] [CrossRef]
- McFarlane, J.S.; Zhang, J.; Wang, S.; Lei, X.; Moran, G.R.; Lamb, A.L. Staphylopine and pseudopaline dehydrogenase from bacterial pathogens catalyze reversible reactions and produce stereospecific metallophores. J. Biol. Chem. 2019, 294, 17988–18001. [Google Scholar] [CrossRef]
- Stelitano, G.; Bettoni, C.; Mori, M.; Cocorullo, M.; Tresoldi, A.; Meneghetti, F.; Villa, S.; Chiarelli, L.R. Repurposing of FDA-Approved Drugs to Disrupt Iron Uptake in Mycobacterium abscessus: Targeting Salicylate Synthase as a Novel Approach. Chem. Biol. Drug Des. 2025, 106, e70162. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal Resistance and Its Association with Antibiotic Resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
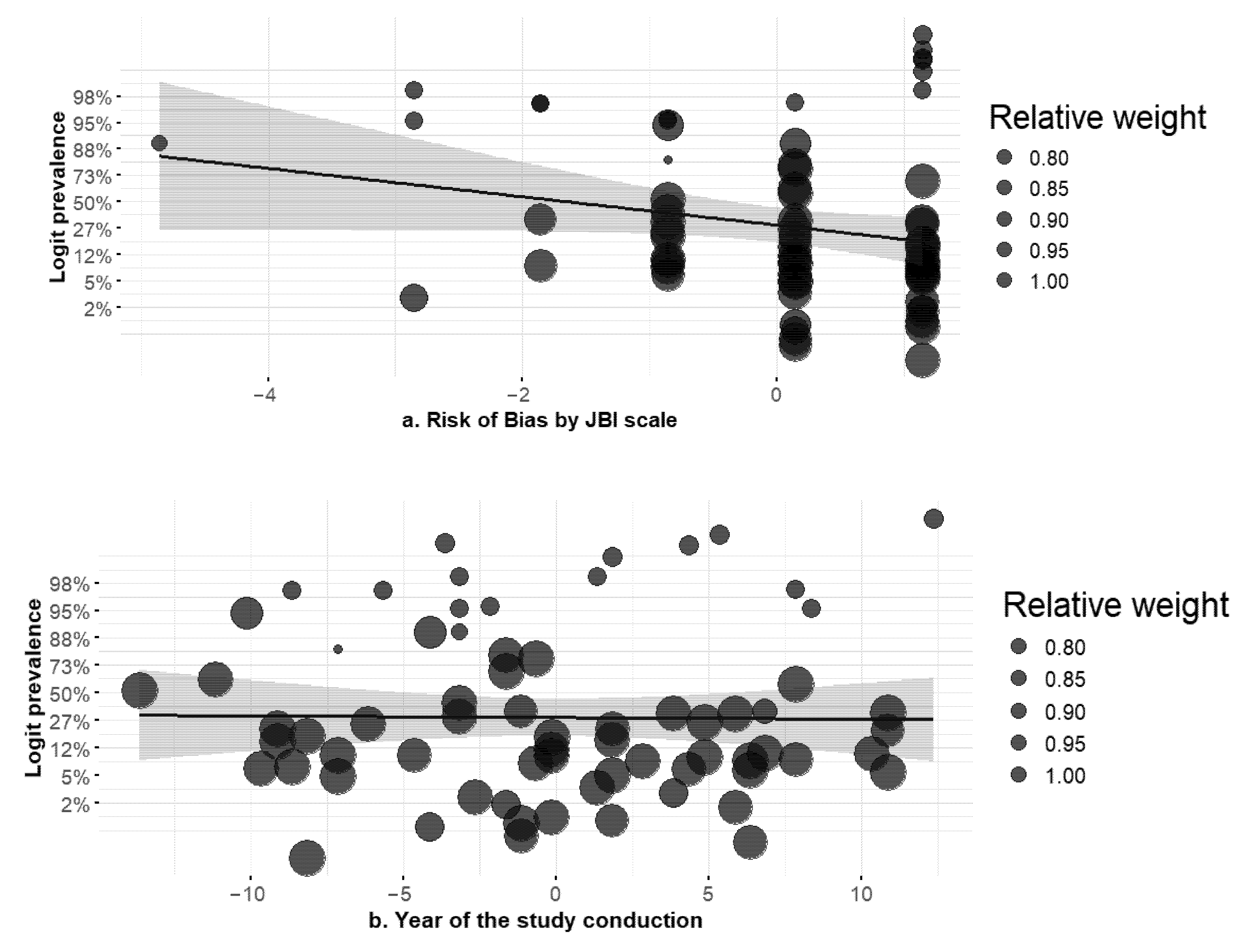
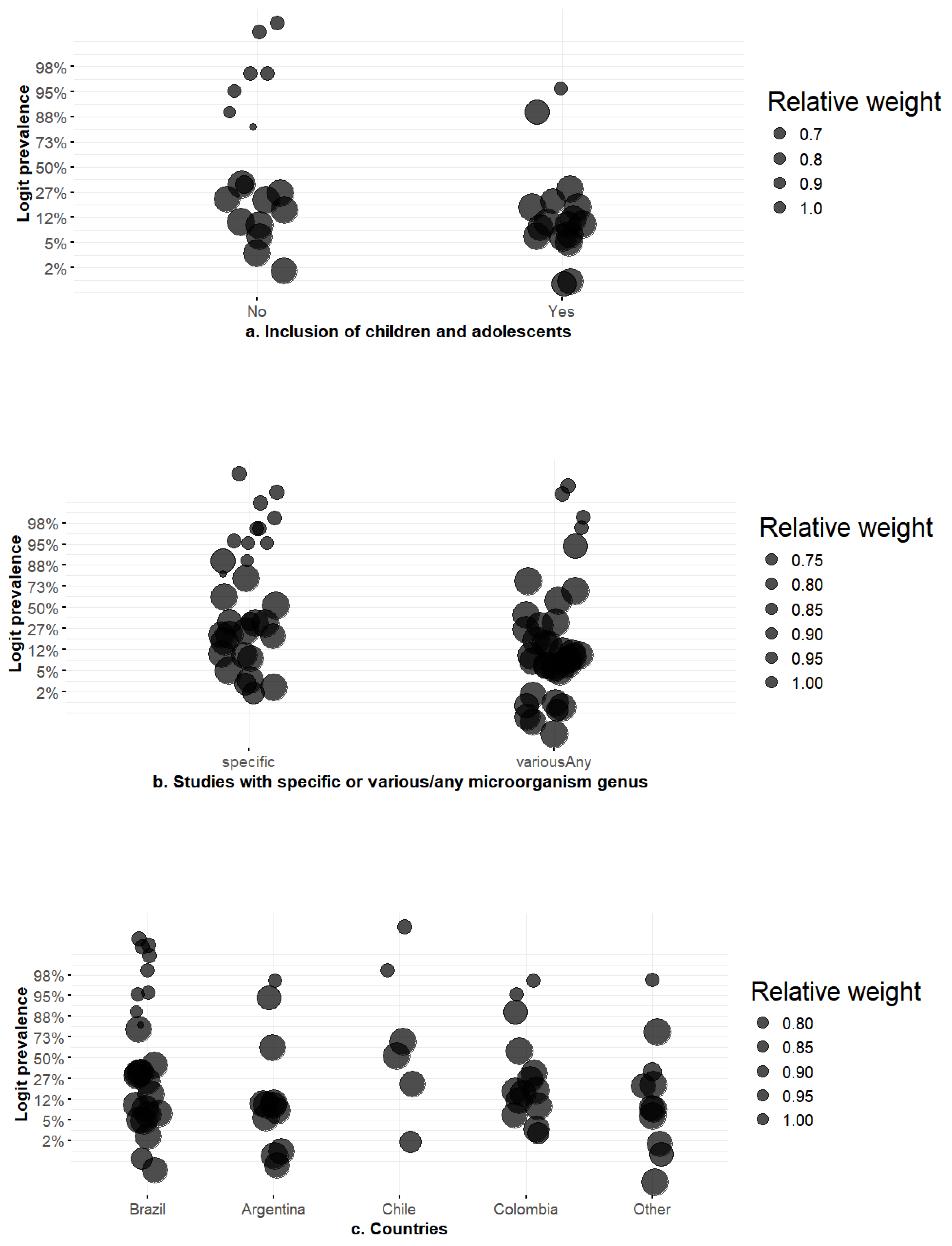
| Study | Country | Data Collection Period (Years) | Design | No. of Centers | Care Setting (s) | Pediatric Patients | Outpatients | Case Type (Colonization vs. Infection) | AmpC Test Type |
|---|---|---|---|---|---|---|---|---|---|
| Favier (2021) [20] | Argentina | 2016–2018 | Quasi-experimental before–after | 1 | ICU, plus hospital-wide intervention | No | No | Infection | Phenotypic |
| Mora Moreno (2021) [38] | Colombia | 2018 | Cross-sectional | 1 | Multiple wards (ICU, emergency, surgery, internal medicine, orthopedics, pediatric hospitalization, pediatric emergency, gynecology) | NR | NR | NR | Both |
| Martínez (2020) [39] | Colombia | 2014–2016 | Cross-sectional descriptive | 4 | Hospitalization, ICU, operating rooms | No | Yes | NR | Phenotypic |
| Beirão (2020) [50] | Brazil | 2016–2017 | Prospective multicenter surveillance | 8 | Hospital (various wards: intra-abdominal, respiratory, urinary tract infections) | NR | No | Infection | Genotypic |
| Tuon (2020) [51] | Brazil | 2016–2017 | Multicenter prospective surveillance | 10 | ICUs and non-ICU wards | NR | No | Infection | Genotypic |
| Varon (2019) [52] | Colombia | 2012 | Retrospective cross-sectional | 1 | ICU | No | No | NR | Phenotypic |
| Aravena (2019) [29] | Chile | 2006–2011 | Cross-sectional | 1 | Hospital wards (specific wards NR) | NR | NR | NR | Both |
| Morales (2018) [40] | Colombia | 2012–2016 | Cross-sectional laboratory | Multiple | Hospital wards (specific wards NR) | NR | NR | Infection | Genotypic |
| Gómez-González (2018) [53] | Colombia | 2015 | Retrospective observational | 1 | ICU | No | No | Both | Phenotypic |
| Cacci (2016) [42] | Brazil | 2007–2008 | Prospective cohort | 1 | ICU | NR | NR | NR | Phenotypic |
| Arango (2016) [41] | Colombia | 2006–2014 | Descriptive cross-sectional | 1 | (PICU), NICU, oncology, burn unit, General Hospitalization, emergency room, surgery, Hematopoietic Transplant Unit | Yes | No | Infection | Phenotypic |
| Cavalcanti (2015) [30] | Brazil | 2008–2010 | Observational, laboratory-based | 3 | ICU, cardiology unit, oncology unit, adult isolation facility | NR | NR | NR | Genotypic |
| de la Lastra (2014) [54] | Chile | 2010–2014 | Cross-sectional laboratory surveillance | 2 | Hospital wards (specific wards NR) | No | No | Infection | Phenotypic |
| Campana (2013) [55] | Brazil | 2006 | Cross-sectional laboratory surveillance | 1 | Hospital wards (specific wards NR) | NR | No | Infection | Both |
| Cejas (2012) [48] | Argentina | 2009 | Prospective multicenter surveillance | 7 | Hospital wards (specific wards NR) | NR | Yes | Infection | Both |
| Hoyos (2012) [56] | Colombia | 2009–2011 | Cross-sectional | 1 | Pediatric emergency, neonatology, NICU, general pediatric ward | Yes | No | Infection | Phenotypic |
| Nastro (2012) [57] | Argentina | 2009–2010 | Prospective cross-sectional laboratory | 1 | Hospital wards (specific wards NR) | NR | Yes | Infection | Both |
| Marcano (2011) [58] | Venezuela | 2009–2010 | Cross-sectional, multicenter laboratory surveillance | 8 | Multiple wards | NR | Yes | Both | Both |
| Santella (2011) [31] | Argentina | 2004–2005 | Retrospective outbreak | 1 | Hospital wards (specific wards NR) | No | No | Infection | Genotypic |
| Jure (2011) [59] | Argentina | 2009 | Cross-sectional laboratory surveillance | 3 | Hospital wards (specific wards NR) | NR | Yes | Infection | Both |
| Vasques (2011) [43] | Brazil | 2007 | Prospective cohort | 1 | Cardiac ICU | Yes | No | Colonization | Genotypic |
| Ribas (2007) [16] | Brazil | 2000–2001 | Prospective cross-sectional surveillance | 1 | Tertiary acute care hospital | Yes | No | Infection | Phenotypic |
| Cantarelli (2007) [60] | Brazil | NR | Cross-sectional laboratory-based investigation | 1 | Hospital wards (specific wards NR) | NR | NR | NR | Both |
| Pino (2007) [15] | Chile | 1995–1998 | Laboratory surveillance | Multiple | Hospital wards (specific wards NR) | NR | No | Infection | Phenotypic |
| Bertona (2005) [17] | Argentina | 1999 | Cross-sectional laboratory surveillance | 1 | Hospital wards (specific wards NR) | NR | No | Infection | Phenotypic |
| García Romero (2005) [61] | Colombia | 2001–2002 | Cross-sectional | 1 | Multiple wards (ICU, surgical ward, nephrology, emergency, internal medicine, coronary care unit, orthopedics, neurology) | No | No | Both | Phenotypic |
| Cezário (2004) [62] | Brazil | 2001–2002 | Cross-sectional prevalence surveys | 1 | Multiple wards (NICU, High-Risk Unit, Intermediate Care, etc.) | Yes | NR | Both | Phenotypic |
| Silva (2024) [21] | Brazil | 2013–2018 | Prospective cohort | 1 | Surgical and clinical wards, ICU, coronary unit, ER, outpatient hemodialysis clinic | No | NR | Infection | Phenotypic |
| Soto (2024) [28] | Chile | 2021–2024 | Cross-sectional laboratory-based of cohort | 1 | Multiple wards | NR | NR | NR | Both |
| Rodrigues (2024) [23] | Brazil | 2017–2020 | Cross-sectional | 1 | ICU and NICU | No | No | Both | Both |
| Arns (2024) [25] | Brazil | 2020–2022 | Prospective multicentric cohort | 5 | Hospital wards (specific wards NR) | No | No | Infection | Phenotypic |
| Mojica (2023) [63] | Multiple countries | 2016–2017 | Cross-sectional laboratory-based surveillance | Multiple | Hospital wards (specific wards NR) | Yes | NR | NR | Genotypic |
| Villanueva-Cotrina (2022) [26] | Peru | 2021 | Case series | 1 | Specialized cancer center (inpatient, outpatient, emergency) | Yes | Yes | Infection | Phenotypic |
| Lee (2021) [64] | Brazil | 2010–2014 | Cross-sectional | 12 | Hospital wards (specific wards NR) | NR | NR | Infection | Genotypic |
| Ferreira (2021) [44] | Brazil | 2012–2017 | Cohort | 1 | Cancer hospital (inpatient wards, ICU, clinical/surgical wards) | No | No | Infection | Phenotypic |
| Perez (2020) [65] | Brazil | 2016– 2017 | Cross-sectional | 1 | Hospital wards (specific wards NR) | NR | No | Infection | Phenotypic |
| Papa-Ezdra (2020) [66] | Uruguay | 2017 | Case series | 2 | Hospital A (ICU, general ward), Hospital B (primary care clinic) | No | Yes | Both | Genotypic |
| Nocua-Báez (2017) [67] | Colombia | 2014–2015 | Cross-sectional | 9 | Emergency department and inpatient wards (adults, UTI) | No | NR | Infection | Both |
| Pavez (2016) [68] | Brazil | 2005–2009 | Laboratory-based | 3 | Hospital wards (specific wards NR) | NR | NR | NR | Both |
| Rocha (2016) [45] | Brazil | 2012 | Prospective cross-sectional laboratory | 7 | Hospital wards (specific wards NR) | Yes | NR | NR | Both |
| Martins (2014) [69] | Brazil | 2008–2009 | Laboratory-based surveillance | 5 | General hospitals (with ICU, emergency, outpatient) | NR | NR | Infection | Both |
| Leal (2013) [70] | Colombia | 2011–2012 | Analytical case–control | 9 | Hospital, mainly emergency services | No | No | Infection | Both |
| Zavascki (2012) [46] | Brazil | 2008 | Case series (Outbreak investigation) | 1 | ICU | Yes | No | Both | Both |
| Fehlberg (2012) [19] | Multiple countries | 2000–2002 | Comparative cross-sectional | 2 | Hospital wards (specific wards NR) | No | No | Infection | Genotypic |
| Nogueira-Miranda (2012) [71] | Brazil | 2005–2008 | Cross-sectional laboratory-based evaluation | 1 | Hospital wards (specific wards NR) | NR | NR | NR | Phenotypic |
| Cuzon (2011) [32] | Colombia | 2006–2010 | Cross-sectional laboratory surveillance | Multiple | ICU, general wards | NR | NR | Infection | Genotypic |
| Picão (2009) [72] | Brazil | 2005 | Retrospective survey | 1 | ICU, emergency room, pediatric oncology unit, bone marrow transplant, hemodialysis, surgery | Yes | NR | Infection | Phenotypic |
| Lincopan (2008) [73] | Brazil | 2007 | Case report | 1 | Surgical ward, transplant unit, | No | No | Infection | Both |
| Dias (2008) [74] | Brazil | 2001 | Retrospective cohort laboratory investigation | 1 | Hospital wards (specific wards NR) | NR | No | Both | Both |
| Sader (2005) [75] | Multiple countries | 2000–2004 | Multicenter laboratory surveillance | 93 | ICU | NR | No | Infection | Phenotypic |
| Casellas (2003) [76] | Argentina | 2001–2002 | Multicenter cross-sectional | 17 | Hospital wards (specific wards NR) | Yes | NR | NR | Phenotypic |
| Quinteros (2003) [18] | Argentina | 2000 | Multicenter cross-sectional survey | 17 | Hospital wards (specific wards NR) | NR | NR | NR | Both |
| Soria-Segarra (2020) [37] | Ecuador | 2016 | Observational prospective cohort | 7 | ICU | No | NR | NR | Phenotypic |
| Bartoloni (2016) [35] | Bolivia | 2010–2014 | Prospective surveillance | 1 | Hospital wards (specific wards NR) | Yes | Yes | Both | Both |
| Kiffer (2005) [77] | Brazil | 2003 | Multicenter laboratory surveillance | 20 | ICU, neutropenic units, general wards | NR | NR | NR | Phenotypic |
| Ayzanoa (2025) [22] | Peru | 2017–2019 | Cross-sectional genomic surveillance | 1 | Pediatric inpatient wards | Yes | No | Both | Phenotypic |
| Alvarez (2006) [78] | Colombia | 2001–2003 | Multicenter surveillance, observational | 14 | ICU (adult, pediatric, neonatal, burn unit, surgical, medical, cardiovascular) | Yes | NR | Both | Phenotypic |
| Calva Delgado (2016) [34] | Ecuador | 2013 | Cross-sectional laboratory surveillance | 2 | Hospital wards (specific wards NR) | NR | NR | NR | Both |
| Brito (2022) [47] | Chile | 2010–2013 | Cross-sectional molecular epidemiology | 2 | Multiple wards (ICU, surgical wards, etc.) | NR | Yes | Infection | Genotypic |
| Wozniak (2012) [33] | Chile | 2006–2011 | Cross-sectional, laboratory-based, multicenter surveillance | 2 | Hospital wards (specific wards NR) | NR | NR | Infection | Both |
| Velandia (2018) [79] | Colombia | 2014–2014 | Cross-sectional | 1 | Hospital wards (specific wards NR) | NR | Yes | Both | NR |
| Sennati (2012) [80] | Argentina | 2010 | Cross-sectional | 15 | Hospital wards (specific wards NR) | Yes | Yes | Both | Phenotypic |
| Rincon Cruz (2013) [49] | Argentina | 2010 | Multicenter point-prevalence surveillance | 15 | Hospital wards (specific wards NR) | NR | Yes | Infection | Both |
| Scheffer (2010) [81] | Brazil | 2003–2005 | Cross-sectional surveillance | 1 | ICU, internal medicine units, surgical units, emergency, other inpatient units | NR | No | NR | Phenotypic |
| Cuicapuza (2024) [82] | Peru | 2018 | Cross-sectional laboratory-based | 1 | Hospital wards (specific wards NR) | NR | Yes | Infection | Genotypic |
| Martínez (2012) [83] | Colombia | 2005–2007 | Cross-sectional surveillance | 1 | ICU, NICU | Yes | No | Infection | Genotypic |
| Echegorry (2024) [27] | Argentina | 2021 | Prospective, open, multicenter prevalence | 182 | Critical and non-critical areas | NR | NR | Infection | Genotypic |
| Faccone (2023) [24] | Argentina | 2020–2021 | Cross-sectional molecular surveillance | 28 | Multiple wards (ICU, medical/surgical wards, etc.) | Yes | No | Both | Genotypic |
| Picão (2012) [84] | Brazil | 2003 | Case series | 1 | Surgical ward, urology ward | No | No | Infection | Genotypic |
| Bacterial Group | Pooled Prevalence % (95% CI) | 95% PI | n Isolates (k) | Heterogeneity (τ2 [logit], I2 %, Q, p) | GRADE Quality (Reasons) |
|---|---|---|---|---|---|
| Overall (all microorganisms) | 11.7% (11.4–12.0 %) | 11.4–12.0% | 48 801 (69) | τ2 = 0; I2 = 97.2%; Q = 2 429.55; p < 0.001 | Low—risk of bias, high heterogeneity, potential publication bias |
| Enterobacter spp. | 46.0% (43.7–48.4 %) | 43.6–48.5% | 1 684 (30) | τ2 = 0; I2 = 30.9%; Q = 41.96; p = 0.057 | Moderate—some risk of bias, moderate heterogeneity |
| Salmonella spp. | 28.5% (21.2–37.0 %) | 19.6–39.3% | 123 (7) | τ2 = 0; I2 = 0.0%; Q ≈ 0; p = 1.000 | Moderate—imprecision due to few studies; some risk of bias |
| Morganella spp. | 20.2% (14.8–27.0 %) | 14.5–27.6% | 168 (18) | τ2 = 0; I2 = 0.0%; Q = 1.01; p = 1.000 | Low—imprecision and potential study bias |
| Serratia spp. | 20.0% (17.2–23.0 %) | 17.1–23.2% | 736 (21) | τ2 = 0; I2 = 0.0%; Q = 10.30; p = 0.962 | Moderate—moderate evidence, low heterogeneity |
| Pseudomonas spp. † | 19.3% (18.0–20.7 %) | 18.0–20.7% | 3 434 (28) | τ2 = 0; I2 = 83.4%; Q = 162.31; p < 0.001 | Low—high heterogeneity and possible publication bias |
| Citrobacter spp. | 18.9% (14.0–25.2 %) | 13.7–25.6% | 190 (19) | τ2 = 0; I2 = 0.0%; Q = 10.96; p = 0.896 | Low—imprecision, moderate risk of bias |
| Acinetobacter spp. † | 16.7% (14.8–18.7 %) | 14.6–19.0% | 1 410 (12) | τ2 = 0; I2 = 0.0%; Q = 1.06; p = 1.000 | Moderate—limited number of studies, mild small-study bias |
| Klebsiella spp. | 7.05% (6.60–7.53 %) | 6.6–7.6% | 11 583 (42) | τ2 = 0; I2 = 78.7%; Q = 192.15; p < 0.001 | Low—high heterogeneity, possible small-study bias |
| Proteus spp. | 5.23% (3.87–7.03 %) | 3.8–7.1% | 784 (27) | τ2 = 0; I2 = 0.0%; Q = 19.87; p = 0.798 | Low—imprecision and few events |
| Escherichia spp. | 4.48% (4.03–4.98 %) | 4.0–5.0% | 7 388 (39) | τ2 = 0; I2 = 93.4%; Q = 575.38; p < 0.001 | Low—very high heterogeneity and possible small-study bias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastely-Junior, V.N.; Rocha, H.S.N.; Reis, M.G. AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence. Trop. Med. Infect. Dis. 2025, 10, 280. https://doi.org/10.3390/tropicalmed10100280
Rastely-Junior VN, Rocha HSN, Reis MG. AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence. Tropical Medicine and Infectious Disease. 2025; 10(10):280. https://doi.org/10.3390/tropicalmed10100280
Chicago/Turabian StyleRastely-Junior, Valmir Nascimento, Hosanea Santos Nascimento Rocha, and Mitermayer Galvão Reis. 2025. "AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence" Tropical Medicine and Infectious Disease 10, no. 10: 280. https://doi.org/10.3390/tropicalmed10100280
APA StyleRastely-Junior, V. N., Rocha, H. S. N., & Reis, M. G. (2025). AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence. Tropical Medicine and Infectious Disease, 10(10), 280. https://doi.org/10.3390/tropicalmed10100280








