Three Complementary Sampling Approaches Provide Comprehensive Characterization of Pesticide Contamination in Urban Stormwater
Abstract
1. Introduction
1.1. Water, Biofilm, and Passive Sampling for Monitoring Urban Pesticide Contamination
1.2. Objectives and Hypotheses
2. Methods
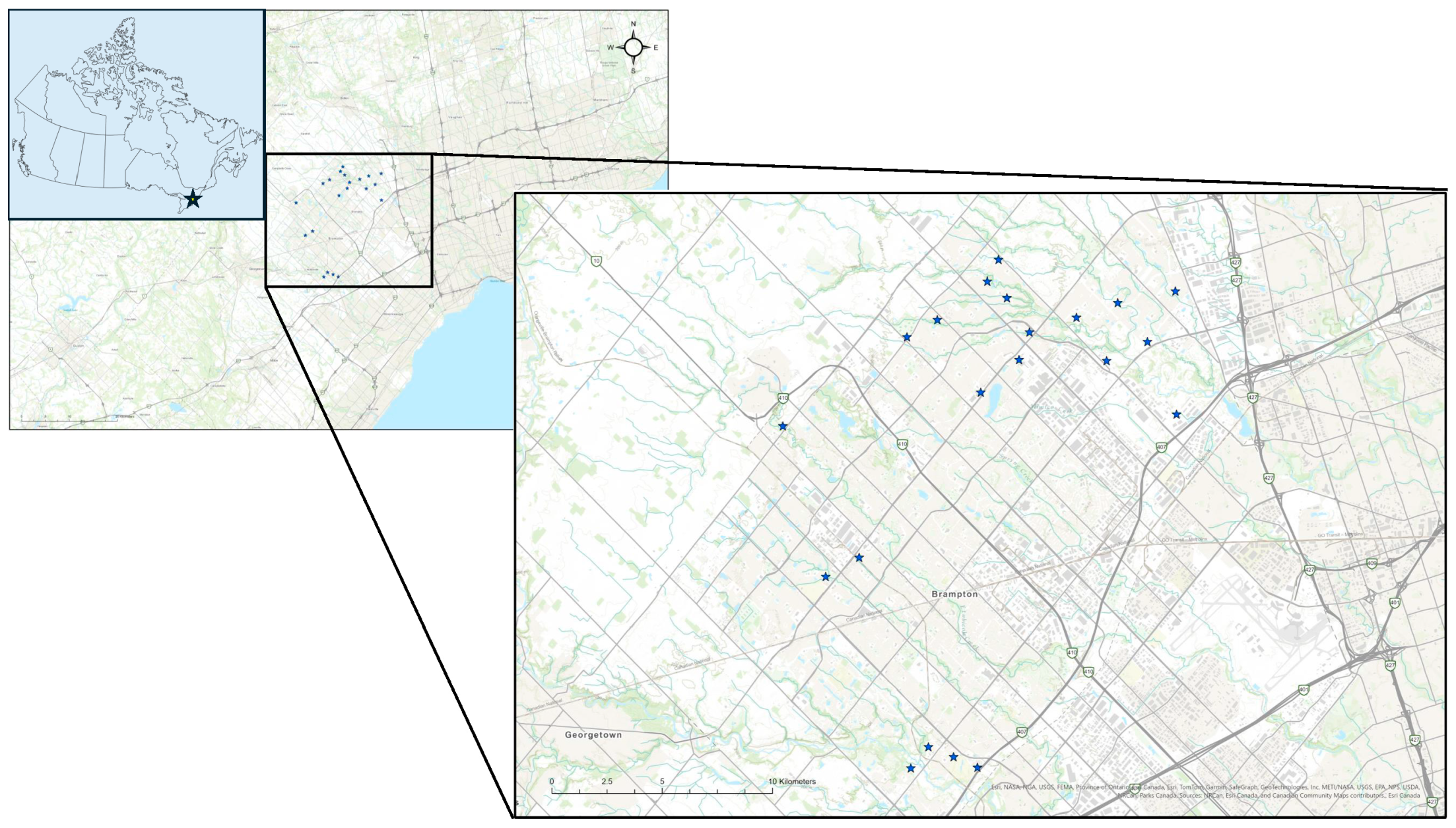
2.1. Study Context
2.2. Study Area
2.3. Sampling
2.3.1. Water
2.3.2. Biofilm
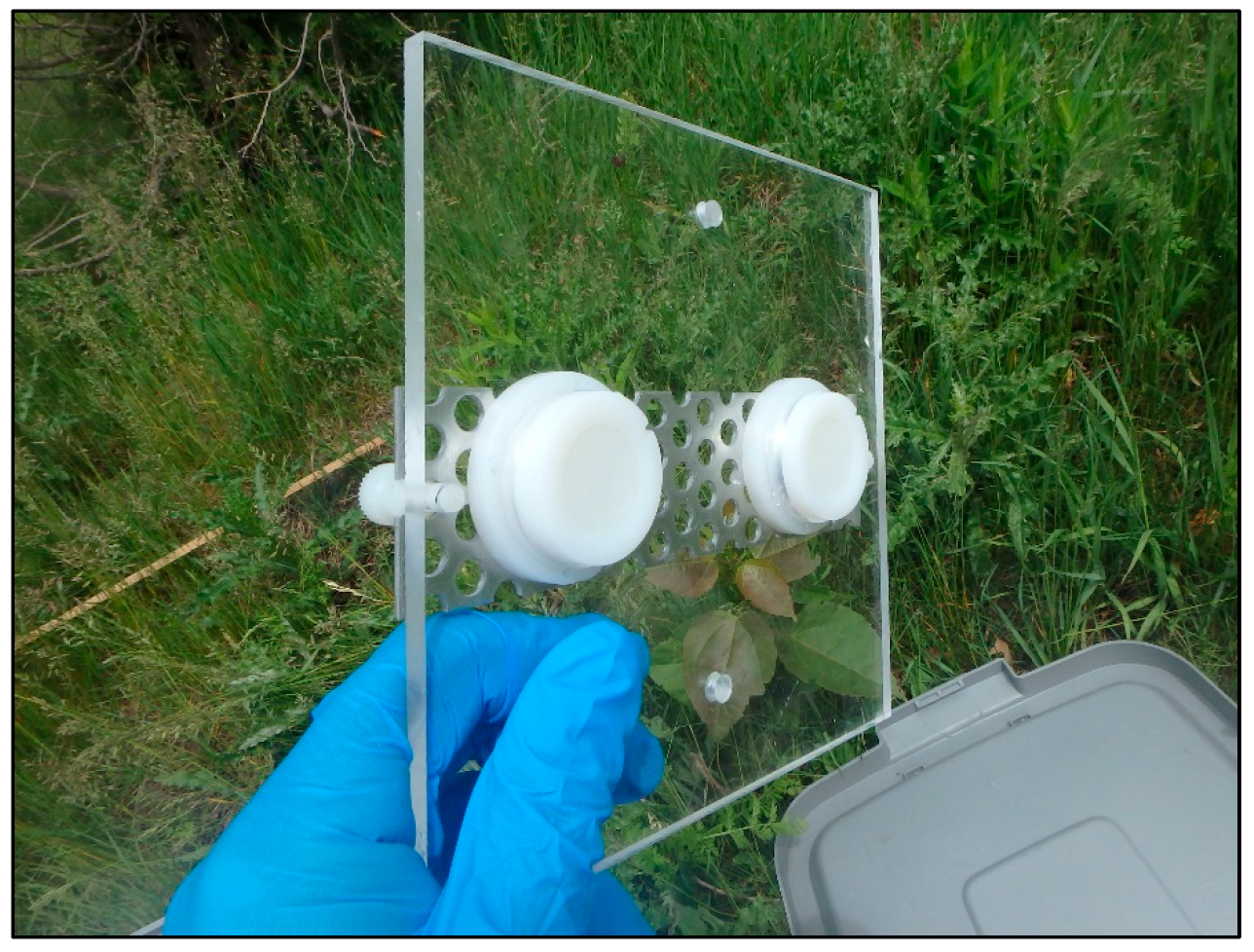
2.3.3. o-DGT Samplers
2.4. Pesticide Analysis
2.5. Calculation of Detection Frequencies on Common Set of Analytes
2.6. Calculation of Time-Weighted Average Concentrations in o-DGT Samplers
2.6.1. Modeling of Diffusion Coefficients
2.6.2. Sampling Rates
2.6.3. Time-Weighted Average Concentrations
2.6.4. Method Detection Limits
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pesticide Detections
3.1.1. The Influence of Analytical Sensitivity on Pesticide Detections
3.1.2. Sampling Pesticides with Three Different Environmental Matrices
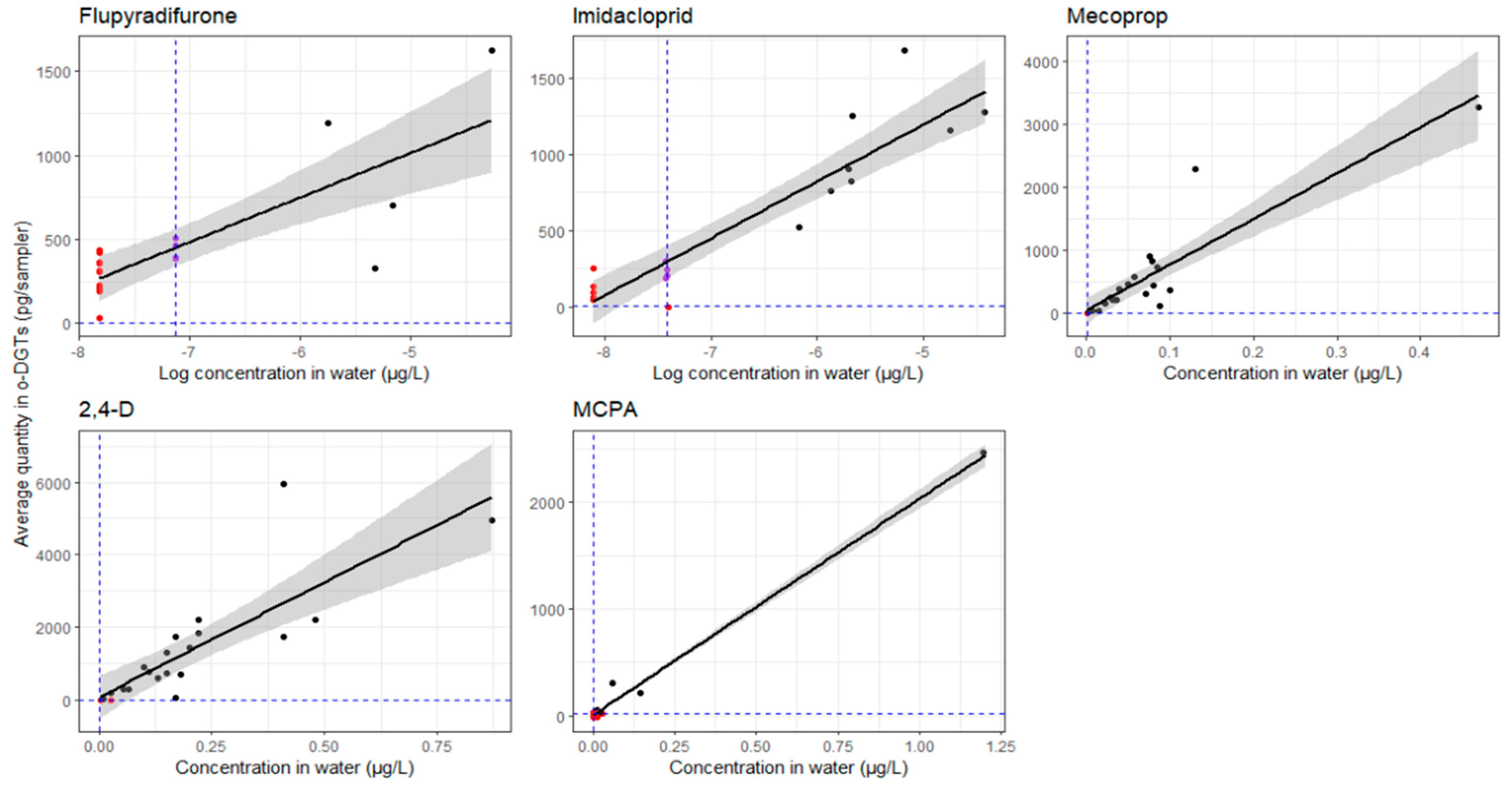
3.2. Pesticide Concentrations
Pesticide Concentrations in Water Samples vs. o-DGTs
3.3. Practicality and Reproducibility
3.3.1. Variability in o-DGT Duplicates
3.3.2. Limitations and Recommendations for Pesticide Monitoring in Urban Areas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAFC | Agriculture and Agri-Food Canada |
| AFL | Agriculture and Food Laboratory |
| ALB | Aquatic Life Benchmark |
| CCME | Canadian Council of Ministers of the Environment |
| COA | Canada-Ontario Agreement |
| COV | coefficient of variance |
| EPA | Environmental Protection Agency (United States) |
| EPSs | extracellular polymeric substances |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MDL | method detection limit |
| MQL | method quantification limit |
| MECP | Ministry of Environment, Conservation, and Parks (Ontario) |
| o-DGT | organic-Diffusive Gradients in Thin films |
| OMAFRA | Ontario Ministry of Agriculture, Food, and Rural Affairs |
| PMRA | Pest Management Regulatory Agency (Canada) |
| POCIS | polar organic compound sampling device |
| TWA | time-weighted average |
References
- Behnisch, M.; Krüger, T.; Jaeger, J.A. Rapid rise in urban sprawl: Global hotspots and trends since 1990. PLoS Sustain. Transform. 2022, 1, e0000034. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Goonetilleke, A.; Thomas, E.; Ginn, S.; Gilbert, D. Understanding the role of land use in urban stormwater quality management. J. Environ. Manag. 2005, 74, 31–42. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Bradley, P.M. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H. Pesticides in stormwater runoff—A mini review. Front. Environ. Sci. Eng. 2019, 13, 72. [Google Scholar] [CrossRef]
- Izma, G.; Raby, M.; Prosser, R.; Rooney, R. Urban-use pesticides in stormwater ponds and their accumulation in biofilms. Sci. Total Environ. 2024, 918, 170534. [Google Scholar] [CrossRef]
- Challis, J.K.; Popick, H.; Prajapati, S.; Harder, P.; Giesy, J.P.; McPhedran, K.; Brinkmann, M. Occurrences of tire rubber-derived contaminants in cold-climate urban runoff. Environ. Sci. Technol. Lett. 2021, 8, 961–967. [Google Scholar] [CrossRef]
- Hwang, H.M.; Fiala, M.J.; Park, D.; Wade, T.L. Review of pollutants in urban road dust and stormwater runoff: Part 1. Heavy metals released from vehicles. Int. J. Urban Sci. 2016, 20, 334–360. [Google Scholar] [CrossRef]
- Stenstrom, M.K.; Silverman, G.S.; Bursztynsky, T.A. Oil and grease in urban stormwaters. J. Environ. Eng. 1984, 110, 58–72. [Google Scholar] [CrossRef]
- Marsalek, J. Road salts in urban stormwater: An emerging issue in stormwater management in cold climates. Water Sci. Technol. 2003, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.D.; Kuivila, K.M.; Hladik, M.L.; Haluska, T.; Cole, M.B. Storm-event-transport of urban-use pesticides to streams likely impairs invertebrate assemblages. Environ. Monit. Assess. 2016, 188, 345. [Google Scholar] [CrossRef] [PubMed]
- Gołdyn, R.; Szpakowska, B.; Świerk, D.; Domek, P.; Buxakowski, J.; Dondajewska, R.; Barałkiewicz, D.; Sajnóg, A. Influence of stormwater runoff on macroinvertebrates in a small urban river and a reservoir. Sci. Total Environ. 2018, 625, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Wolfand, J.M.; Seller, C.; Bell, C.D.; Cho, Y.M.; Oetjen, K.; Hogue, T.S.; Luthy, R.G. Occurrence of urban-use pesticides and management with enhanced stormwater control measures at the watershed scale. Environ. Sci. Technol. 2019, 53, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
- Nowell, L.H.; Moran, P.W.; Bexfield, L.M.; Mahler, B.J.; Van Metre, P.C.; Bradley, P.M.; Qi, S.L. Is there an urban pesticide signature? Urban streams in five US regions share common dissolved-phase pesticides but differ in predicted aquatic toxicity. Sci. Total Environ. 2021, 793, 148453. [Google Scholar] [CrossRef]
- Raina, R.; Etter, M.L.; Buehler, K.; Starks, K.; Yowin, Y. Phenoxyacid herbicides in stormwater retention ponds: Urban inputs. Am. J. Anal. Chem. 2011, 2, 962. [Google Scholar] [CrossRef][Green Version]
- Weston, D.P.; Holmes, R.W.; Lydy, M.J. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ. Pollut. 2009, 157, 287–294. [Google Scholar] [CrossRef]
- Weston, D.P.; Chen, D.; Lydy, M.J. Stormwater-related transport of the insecticides bifenthrin, fipronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay, California. Sci. Total Environ. 2015, 527, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, U.E.; Tang, C.; Eriksson, E.; Jönsson, K.; Vollertsen, J.; Bester, K. Biocides in urban wastewater treatment plant influent at dry and wet weather: Concentrations, mass flows and possible sources. Water Res. 2014, 60, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, Y.; Conkle, J.L.; Li, J.; Gan, J. Pesticides on residential outdoor surfaces: Environmental impacts and aquatic toxicity. Pest Manag. Sci. 2016, 72, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Schiff, K.; Bay, S.; Stransky, C. Characterization of stormwater toxicants from an urban watershed to freshwater and marine organisms. Urban Water 2002, 4, 215–227. [Google Scholar] [CrossRef]
- Seiber, J.N. Environmental fate of pesticides. In Pesticides in Agriculture and the Environment; CRC Press: Boca Raton, FL, USA, 2002; pp. 141–176. [Google Scholar]
- Ijzerman, M.M.; Raby, M.; Letwin, N.V.; Kudla, Y.M.; Anderson, J.D.; Atkinson, B.J.; Prosser, R.S. New insights into pesticide occurrence and multicompartmental monitoring strategies in stream ecosystems using periphyton and suspended sediment. Sci. Total Environ. 2024, 915, 170144. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of the Environment, Conservation, and Parks (MECP) and Environment and Climate Change Canada (ECCC). Canada-Ontario Agreement on Great Lakes Water Quality and Ecosystem Health. 2021. Available online: https://www.ontario.ca/document/canada-ontario-great-lakes-agreement (accessed on 15 January 2024).
- Raby, M.; Lissemore, L.; Kaltenecker, G.; Beaton, D.; Prosser, R.S. Characterizing the exposure of streams in southern Ontario to agricultural pesticides. Chemosphere 2022, 294, 133769. [Google Scholar] [CrossRef] [PubMed]
- United States Geological Survey (USGS). Chapter A4: Collection of water samples. In National Field Manual for the Collection of Water-Quality Data; United States Geological Survey: Reston, VA, USA, 2006. Available online: https://pubs.usgs.gov/twri/twri9a4/twri9a4_Chap4_v2.pdf (accessed on 15 January 2024).
- Health Canada. Water Monitoring Pilot Program for Pesticides. The Pest Management Regulatory Agency: Programs and Initiatives. 2024. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/protecting-your-health-environment/programs-initiatives/water-monitoring-pesticides/pilot-program.html (accessed on 15 January 2024).
- Xing, Z.; Chow, L.; Rees, H.; Meng, F.; Li, S.; Ernst, B.; Hewitt, L.M. Influences of sampling methodologies on pesticide-residue detection in stream water. Arch. Environ. Contam. Toxicol. 2013, 64, 208–218. [Google Scholar] [CrossRef] [PubMed]
- la Cecilia, D.; Dax, A.; Ehmann, H.; Koster, M.; Singer, H.; Stamm, C. Continuous high-frequency pesticide monitoring to observe the unexpected and the overlooked. Water Res. X 2021, 13, 100125. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodriguez-Mozaz, S.; Nannou, C.; Nakis, L.; Ruhí, A.; Acuña, V.; Barceló, D. Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in biofilm from a waste water treatment plant-impacted river. Sci. Total Environ. 2016, 540, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Bastos, M.C.; de Vargas JP, R.; Le Guet, T.; Clasen, B.; Dos Santos, D.R. The use of epilithic biofilms as bioaccumulators of pesticides and pharmaceuticals in aquatic environments. Ecotoxicology 2020, 29, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Rheinheimer dos Santos, D.; Monteiro de Castro Lima, J.A.; Paranhos Rosa de Vargas, J.; Camotti Bastos, M.; Santanna dos Santos, M.A.; Mondamert, L.; Labanowski, J. Pesticide bioaccumulation in epilithic biofilms as a biomarker of agricultural activities in a representative watershed. Environ. Monit. Assess. 2020, 192, 232. [Google Scholar] [CrossRef]
- Rooney, R.C.; Davy, C.; Gilbert, J.; Prosser, R.; Robichaud, C.; Sheedy, C. Periphyton bioconcentrates pesticides downstream of catchment dominated by agricultural land use. Sci. Total Environ. 2020, 702, 134472. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Bonnineau, C.; Artigas, J.; Chaumet, B.; Dabrin, A.; Faburé, J.; Ferrari, B.J.D.; Lebrun, J.D.; Margoum, C.; Mazzella, N.; Miège, C.; et al. Role of biofilms in contaminant bioaccumulation and trophic transfer in aquatic ecosystems: Current state of knowledge and future challenges. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 253, pp. 115–153. [Google Scholar]
- Rheinheimer Dos Santos, D.; Camotti Bastos, M.; Monteiro De Castro Lima, J.A.; Le Guet, T.; Vargas Brunet, J.; Fernandes, G.; Labanowski, J. Epilithic biofilms, POCIS, and water samples as complementary sources of information for a more comprehensive view of aquatic contamination by pesticides and pharmaceuticals in southern Brazil. J. Environ. Sci. Health Part B 2023, 58, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Aparicio, V.C.; De Gerónimo, E.; Prestes, O.D.; Zanella, R.; Ebling, E.; Dos Santos, D.R. Epilithic biofilms as a discriminating matrix for long-term and growing season pesticide contamination in the aquatic environment: Emphasis on glyphosate and metabolite AMPA. Sci. Total Environ. 2023, 900, 166315. [Google Scholar] [CrossRef] [PubMed]
- Guasch, H.; Ricart, M.; López-Doval, J.; Bonnineau, C.; Proia, L.; Morin, S.; Sabater, S. Influence of grazing on triclosan toxicity to stream periphyton. Freshw. Biol. 2016, 61, 2002–2012. [Google Scholar] [CrossRef]
- Izma, G.; Ijzerman, M.M.; McIsaac, D.; Raby, M.; Prosser, R.S.; Rooney, R.C. Dietary exposure of stormwater contaminants in biofilm to two freshwater macroinvertebrates. Sci. Total Environ. 2024, 957, 177390. [Google Scholar] [CrossRef] [PubMed]
- Huckins, J.N.; Tubergen, M.W.; Manuweera, G.K. Semipermeable membrane devices containing model lipid: A new approach to monitoring the bioavailability of lipophilic contaminants and estimating their bioconcentration potential. Chemosphere 1990, 20, 533–552. [Google Scholar] [CrossRef]
- Chen, C.E.; Zhang, H.; Ying, G.G.; Jones, K.C. Evidence and recommendations to support the use of a novel passive water sampler to quantify antibiotics in wastewaters. Environ. Sci. Technol. 2013, 47, 13587–13593. [Google Scholar] [CrossRef]
- Challis, J.K.; Hanson, M.L.; Wong, C.S. Development and calibration of an organic-diffusive gradients in thin films aquatic passive sampler for a diverse suite of polar organic contaminants. Anal. Chem. 2016, 88, 10583–10591. [Google Scholar] [CrossRef]
- Challis, J.K.; Stroski, K.M.; Luong, K.H.; Hanson, M.L.; Wong, C.S. Field evaluation and in situ stress testing of the organic-diffusive gradients in thin-films passive sampler. Environ. Sci. Technol. 2018, 52, 12573–12582. [Google Scholar] [CrossRef]
- Vrana, B.; Allan, I.J.; Greenwood, R.; Mills, G.A.; Dominiak, E.; Svensson, K.; Morrison, G. Passive sampling techniques for monitoring pollutants in water. TrAC Trends Anal. Chem. 2005, 24, 845–868. [Google Scholar] [CrossRef]
- Guibal, R.; Buzier, R.; Charriau, A.; Lissalde, S.; Guibaud, G. Passive sampling of anionic pesticides using the Diffusive Gradients in Thin films technique (DGT). Anal. Chim. Acta 2017, 966, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Chen CE, L.; Chen, W.; Chen, J.; Cai, X.; Jones, K.C.; Zhang, H. Development of a passive sampling technique for measuring pesticides in waters and soils. J. Agric. Food Chem. 2019, 67, 6397–6406. [Google Scholar] [CrossRef]
- Hageman, K.J.; Aebig, C.H.; Luong, K.H.; Kaserzon, S.L.; Wong, C.S.; Reeks, T.; Matthaei, C.D. Current-use pesticides in New Zealand streams: Comparing results from grab samples and three types of passive samplers. Environ. Pollut. 2019, 254, 112973. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Canada’s Fasting Growing and Decreasing Municipalities from 2016 to 2021. Analytical Products, Census, 2022. 2021. Available online: https://www12.statcan.gc.ca/census-recensement/2021/as-sa/98-200-x/2021001/98-200-x2021001-eng.cfm (accessed on 15 January 2024).
- Albaseer, S.S.; Mukkanti, K.; Rao, R.N.; Swamy, Y.V. Analytical artifacts, sample handling and preservation methods of environmental samples of synthetic pyrethroids. TrAC Trends Anal. Chem. 2011, 30, 1771–1780. [Google Scholar] [CrossRef]
- Renaud, J.B.; Sabourin, L.; Hoogstra, S.; Helm, P.; Lapen, D.R.; Sumarah, M.W. Monitoring of environmental contaminants in mixed-use watersheds combining targeted and nontargeted analysis with passive sampling. Environ. Toxicol. Chem. 2022, 41, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Hayduk, W.; Laudie, H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChE J. 1974, 20, 611–615. [Google Scholar] [CrossRef]
- Posit Team RStudio. Integrated Development Environment for, R. Posit Software, PBC, Boston, MA. 2023. Available online: http://www.posit.co/ (accessed on 15 January 2024).
- Metcalfe, C.D.; Sultana, T.; Li, H.; Helm, P.A. Current-use pesticides in urban watersheds and receiving waters of western Lake Ontario measured using polar organic chemical integrative samplers (POCIS). J. Great Lakes Res. 2016, 42, 1432–1442. [Google Scholar] [CrossRef]
- Burant, A.; Selbig, W.; Furlong, E.T.; Higgins, C.P. Trace organic contaminants in urban runoff: Associations with urban land-use. Environ. Pollut. 2018, 242, 2068–2077. [Google Scholar] [CrossRef]
- Fairbairn, D.J.; Elliott, S.M.; Kiesling, R.L.; Schoenfuss, H.L.; Ferrey, M.L.; Westerhoff, B.M. Contaminants of emerging concern in urban stormwater: Spatiotemporal patterns and removal by iron-enhanced sand filters (IESFs). Water Res. 2018, 145, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; Marvin, C. Occurrence and distribution of carbamate pesticides and metalaxyl in southern Ontario surface waters 2007–2010. Bull. Environ. Contam. Toxicol. 2016, 96, 423–431. [Google Scholar] [CrossRef]
- Santos, E.; Correia, N.; Silva, J.; Velini, E.; Durigan, J.; Passos, A.B.R.J.; Teixeira, M. Occurrence of waste herbicides in surface water from North of São Paulo (Brazil). J. Exp. Agric. Int. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Barizon RR, M.; Kummrow, F.; de Albuquerque, A.F.; Assalin, M.R.; Rosa, M.A.; de Souza Dutra, D.R.C.; Pazianotto, R.A.A. Surface water contamination from pesticide mixtures and risks to aquatic life in a high-input agricultural region of Brazil. Chemosphere 2022, 308, 136400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, Y.; Li, S.; He, Z.; Xu, S.; Xia, W. Occurrence, spatial variation, seasonal difference, and risk assessment of neonicotinoid insecticides, selected agriculture fungicides, and their transformation products in the Yangtze River, China: From the upper to lower reaches. Water Res. 2023, 247, 120724. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E.; Mahler, B.J.; Nowell, L.H.; Van Metre, P.C.; Sandstrom, M.W.; Corbin, M.A.; McWhirter, K.J. Daily stream samples reveal highly complex pesticide occurrence and potential toxicity to aquatic life. Sci. Total Environ. 2020, 715, 136795. [Google Scholar] [CrossRef]
- Ponsatí, L.; Corcoll, N.; Petrović, M.; Picó, Y.; Ginebreda, A.; Tornés, E.; Sabater, S. Multiple-stressor effects on river biofilms under different hydrological conditions. Freshw. Biol. 2016, 61, 2102–2115. [Google Scholar] [CrossRef]
- Tien, C.J.; Lin, M.C.; Chiu, W.H.; Chen, C.S. Biodegradation of carbamate pesticides by natural river biofilms in different seasons and their effects on biofilm community structure. Environ. Pollut. 2013, 179, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jones, K.C.; Zhang, H. Monitoring organic pollutants in waters using the diffusive gradients in the thin films technique: Investigations on the effects of biofouling and degradation. Environ. Sci. Technol. 2020, 54, 7961–7969. [Google Scholar] [CrossRef]
- Wang, P.; Challis, J.K.; He, Z.X.; Wong, C.S.; Zeng, E.Y. Effects of biofouling on the uptake of perfluorinated alkyl acids by organic-diffusive gradients in thin films passive samplers. Environ. Sci. Process. Impacts 2022, 24, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. Int. J. 2004, 23, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Helm, P.A.; Paterson, G.; Metcalfe, C.D. The effects of dissolved organic matter and pH on sampling rates for polar organic chemical integrative samplers (POCIS). Chemosphere 2011, 83, 271–280. [Google Scholar] [CrossRef]
- Morin, N.; Camilleri, J.; Cren-Olivé, C.; Coquery, M.; Miège, C. Determination of uptake kinetics and sampling rates for 56 organic micropollutants using “pharmaceutical” POCIS. Talanta 2013, 109, 61–73. [Google Scholar] [CrossRef]
- University of Hertfordshire. Pesticide Properties Database. Hatfield, Hertfordshire, UK. 2020. Available online: https://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 10 April 2020).
- Pest Management Regulatory Agency (PMRA). Registration Decision RD2021-03, Imazapyr, Habitat Aqua. Consumer Product Safety Report. 2021. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/registration-decision/2021/imazapyr-habitat-aqua.html (accessed on 15 January 2024).
- Selling, H.A.; Vonk, J.W.; Sijpesteijn, A.K. Transformation of the systematic fungicide methyl thiophanate into 2-benzimidazole carbamic acid methyl ester. Chem Ind. 1970, 19, 1625–1626. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides. 2024. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk (accessed on 15 January 2024).
- Hatt, B.E.; Fletcher, T.D.; Walsh, C.J.; Taylor, S.L. The influence of urban density and drainage infrastructure on the concentrations and loads of pollutants in small streams. Environ. Manag. 2004, 34, 112–124. [Google Scholar] [CrossRef]
- Marsalek, J.; Anderson, B.C.; Watt, W.E. Suspended particulate in urban stormwater ponds: Physical, chemical and toxicological characteristics. In Proceedings of the Global Solutions for Urban Drainage, Portland, OR, USA, 8–13 September 2002; pp. 1–12. [Google Scholar]
- Paul, M.J.; Meyer, J.L. Streams in the urban landscape. Annu. Rev. Ecol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Harmel, R.D.; Slade, R.M., Jr.; Haney, R.L. Impact of sampling techniques on measured stormwater quality data for small streams. J. Environ. Qual. 2010, 39, 1734–1742. [Google Scholar] [CrossRef]
- la Cecilia, D.; Dax, A.; Stravs, M.; Ort, C.; Singer, H.; Stamm, C. Continuous high-frequency pesticides monitoring reveals underestimated environmental threats and unique insights into transport patterns. In Proceedings of the EGU General Assembly 2020, online, 4–8 May 2020; p. 9601. [Google Scholar]
- Tang, J.Y.; Aryal, R.; Deletic, A.; Gernjak, W.; Glenn, E.; McCarthy, D.; Escher, B.I. Toxicity characterization of urban stormwater with bioanalytical tools. Water Res. 2013, 47, 5594–5606. [Google Scholar] [CrossRef]
- Pamuru, S.T.; Forgione, E.; Croft, K.; Kjellerup, B.V.; Davis, A.P. Chemical characterization of urban stormwater: Traditional and emerging contaminants. Sci. Total Environ. 2022, 813, 151887. [Google Scholar] [CrossRef]
- Roeselers, G.; Zippel, B.; Staal, M.; Van Loosdrecht, M.; Muyzer, G. On the reproducibility of microcosm experiments–different community composition in parallel phototrophic biofilm microcosms. FEMS Microbiol. Ecol. 2006, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Guibal, R.; Buzier, R.; Lissalde, S.; Guibaud, G. Adaptation of diffusive gradients in thin films technique to sample organic pollutants in the environment: An overview of o-DGT passive samplers. Sci. Total Environ. 2019, 693, 133537. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]

| Grab Water Sampling | Biofilm Sampling | Passive Sampling with o-DGTs | |
|---|---|---|---|
| Description of method | Instantaneous sampling of a water body by filling a sampling bottle with water directly from the study site | Harvesting biofilm grown in situ at a study site from either artificial or natural substrates | Deployment of passive sampling device (e.g., o-DGT) in situ at a study site |
| Benefits of this method | Concentrations in water are comparable with other studies and with ecotoxicological benchmarks or thresholds | Accumulation of pesticides in biofilms from the surrounding water can help detect pesticides at relatively low levels; time-integration allows capture of fluctuations in concentrations; biologically relevant as many aquatic organisms consume biofilm directly | Accumulation of pesticides from the water can help detect pesticides at relatively low levels; time-integration allows capture of fluctuations in concentrations; well supported in the literature |
| Limitations of this method | Snapshot of concentrations in time may not represent all conditions or exposures; bias toward hydrophilic compounds | No dietary ecotoxicological thresholds or benchmarks exist for comparison | Artificial media is not as biologically relevant; assumptions in the calculation of TWA concentrations complicate informed risk assessments |
| Water | Biofilm | o-DGT | All 3 Matrices | |
|---|---|---|---|---|
| Water | 3 | 5 | ||
| Biofilm | 0 | 1 | ||
| o-DGT | 6 | 3 | 64 |
| Detection Frequency (%) | |||
|---|---|---|---|
| Pesticide | Water | Biofilm | o-DGT |
| 2,4-D | 100 | 14 | 90 |
| atrazine | n.d. | n.d. | 100 |
| azoxystrobin | n.d. | 43 | 100 |
| carbendazim | 5 | 10 | 100 |
| chlorantraniliprole | n.d. | n.d. | 100 |
| clomazone | n.d. | n.d. | 100 |
| fluopyram | n.d. | n.d. | 100 |
| MCPA | 100 | 5 | 43 |
| mecoprop | 100 | n.d. | 95 |
| metalaxyl | n.d. | n.d. | 100 |
| metolachlor | n.d. | n.d. | 100 |
| propazine | n.d. | n.d. | 100 |
| propiconazole | n.d. | n.d. | 100 |
| simazine | n.d. | n.d. | 100 |
| tebuconazole | n.d. | 19 | 100 |
| tebufenozide | n.d. | n.d. | 100 |
| thiabendazole | n.d. | 5 | 100 |
| diuron | 5 | 19 | 95 |
| imazethapyr | n.d. | n.d. | 95 |
| prometon | n.d. | n.d. | 95 |
| triclopyr | 95 | n.d. | n.d. |
| clothianidin | 33 | n.d. | 90 |
| flupyradifurone | 38 | n.d. | 90 |
| paclobutrazol | n.d. | n.d. | 90 |
| imidacloprid | 62 | n.d. | 86 |
| dimethenamid | n.d. | n.d. | 81 |
| ametryn | n.d. | n.d. | 71 |
| tebuthiuron | n.d. | n.d. | 71 |
| difenoconazole | n.d. | n.d. | 67 |
| carbaryl | n.d. | n.d. | 52 |
| epoxiconazole | n.d. | n.d. | 52 |
| hexazinone | n.d. | n.d. | 48 |
| myclobutanil | n.d. | n.d. | 48 |
| cyantraniliprole | n.d. | n.d. | 38 |
| sulfentrazone | n.d. | n.d. | 38 |
| acetamiprid | n.d. | n.d. | 33 |
| diazinon | n.d. | n.d. | 33 |
| ethiofencarb | n.d. | n.d. | 33 |
| benalaxyl | n.d. | n.d. | 29 |
| metribuzin | n.d. | n.d. | 29 |
| pyrimethanil | n.d. | n.d. | 29 |
| bentazon | 24 | n.d. | 14 |
| bromoxynil | n.d. | n.d. | 24 |
| indaziflam | n.d. | n.d. | 24 |
| dichlorprop | 19 | n.d. | 5 |
| dithiopyr | n.d. | n.d. | 19 |
| flonicamid | 19 | n.d. | n.d. |
| flufenoxuron | n.d. | n.d. | 19 |
| flutriafol | n.d. | n.d. | 19 |
| prometryn | n.d. | n.d. | 19 |
| pyridate | n.d. | n.d. | 19 |
| spiroxamine | n.d. | n.d. | 19 |
| dimethomorph | n.d. | n.d. | 14 |
| fenpropimorph | n.d. | n.d. | 14 |
| pyridaben | n.d. | n.d. | 14 |
| terbuthylazine | n.d. | n.d. | 14 |
| terbutryn | n.d. | n.d. | 14 |
| trifloxystrobin | n.d. | n.d. | 14 |
| picolinafen | n.d. | n.d. | 10 |
| pyraclostrobin | n.d. | n.d. | 10 |
| pyriproxyfen | n.d. | n.d. | 10 |
| spiromesifen | n.d. | n.d. | 10 |
| alanycarb | n.d. | n.d. | 5 |
| benfuracarb | n.d. | n.d. | 5 |
| benzoximate | n.d. | n.d. | 5 |
| bifenazate | n.d. | n.d. | 5 |
| bromacil | n.d. | n.d. | 5 |
| bromuconazole | n.d. | n.d. | 5 |
| bupirimate | n.d. | n.d. | 5 |
| butafenacil | n.d. | n.d. | 5 |
| chlorpropham | 5 | 5 | 5 |
| etoxazole | n.d. | n.d. | 5 |
| fenazaquin | n.d. | n.d. | 5 |
| fenobucarb | n.d. | n.d. | 5 |
| fluazifop-p-butyl | n.d. | n.d. | 5 |
| fludioxonil | n.d. | n.d. | 5 |
| hexaconazole | n.d. | n.d. | 5 |
| imazapyr | 5 | n.d. | n.d. |
| indoxacarb | n.d. | n.d. | 5 |
| methiocarb | n.d. | n.d. | 5 |
| ofurace | n.d. | n.d. | 5 |
| picoxystrobin | n.d. | n.d. | 5 |
| propoxur | n.d. | n.d. | 5 |
| quizalofop-ethyl | n.d. | n.d. | 5 |
| simetryn | n.d. | n.d. | 5 |
| tetraconazole | n.d. | n.d. | 5 |
| thiacloprid | n.d. | n.d. | 5 |
| thiophanate-methyl | n.d. | 5 | n.d. |
| Water (µg L−1) | Biofilm (µg kg−1) | o-DGTs (µg L−1) | ||||
|---|---|---|---|---|---|---|
| Pesticide: | Min | Max | Min | Max | Min | Max |
| 2,4-D | 0.0036 | 0.87 | <MQL | <MQL | 0.0002 | 0.019 |
| acetamiprid | <MQL | 0.00016 | ||||
| ametryn | <MQL | 0.0011 | ||||
| atrazine | 0.0056 | 0.087 | ||||
| azoxystrobin | <MQL | 35 | 0.000016 | 0.022 | ||
| benalaxyl | <MQL | 0.000019 | ||||
| bentazon | <MQL | <MQL | 0.000022 | 0.000031 | ||
| bromacil | <MQL | 0.0021 | ||||
| bromoxynil | 0.00031 | 0.00089 | ||||
| bromuconazole | 0.00024 | 0.00024 | ||||
| bupirimate | 0.000075 | 0.000075 | ||||
| carbaryl | 0.00029 | 0.025 | ||||
| carbendazim | 0.52 | 0.52 | <MQL | <MQL | 0.000026 | 0.019 |
| chlorantraniliprole | 0.000036 | 0.01 | ||||
| chlorpropham | <MQL | <MQL | 33 | 33 | 0.44 | 1.36 |
| clomazone | 0.00011 | 0.00074 | ||||
| clothianidin | <MQL | <MQL | 0.00024 | 0.0011 | ||
| cyantraniliprole | 0.0004 | 0.0011 | ||||
| diazinon | 0.00042 | 0.00044 | ||||
| dichlorprop | 0.0022 | 0.028 | 0.00097 | 0.00098 | ||
| difenoconazole | 0.000039 | 0.043 | ||||
| dimethenamid | 0.00017 | 0.00038 | ||||
| dimethomorph | <MQL | 0.00023 | ||||
| dithiopyr | <MQL | 0.00035 | ||||
| diuron | <MQL | <MQL | <MQL | 23 | 0.00014 | 0.423 |
| epoxiconazole | <MQL | 0.00063 | ||||
| ethiofencarb | 0.00017 | 0.0009 | ||||
| etoxazole | 0.000056 | 0.000056 | ||||
| fenazaquin | 0.00029 | 0.00029 | ||||
| fenobucarb | <MQL | 0.000072 | ||||
| fenpropimorph | 0.00031 | 0.00046 | ||||
| flonicamid | <MQL | 0.014 | ||||
| fluazifop-p-butyl | <MQL | <MQL | ||||
| fludioxonil | 0.0013 | 0.004 | ||||
| flufenoxuron | 0.0021 | 0.0035 | ||||
| fluopyram | 0.00095 | 0.0043 | ||||
| flupyradifurone | <MQL | 0.014 | 0.00026 | 0.01 | ||
| flutriafol | 0.00067 | 0.0017 | ||||
| hexaconazole | 0.00016 | 0.00016 | ||||
| hexazinone | 0.000032 | 0.00016 | ||||
| imazapyr | 0.0089 | 0.0089 | ||||
| imazethapyr | <MQL | 0.000054 | ||||
| imidacloprid | <MQL | 0.012 | 0.00031 | 0.0084 | ||
| indaziflam | 0.00018 | 0.00025 | ||||
| indoxacarb | <MQL | <MQL | ||||
| MCPA | <MQL | 1.2 | <MQL | <MQL | <MQL | 0.0077 |
| mecoprop | <MQL | 0.47 | 0.000054 | 0.01 | ||
| metalaxyl | 0.000038 | 0.0023 | ||||
| methiocarb | 0.000086 | 0.000086 | ||||
| metolachlor | 0.00073 | 0.032 | ||||
| metribuzin | 0.00062 | 0.0024 | ||||
| myclobutanil | 0.00038 | 0.0011 | ||||
| ofurace | 0.0007 | 0.0007 | ||||
| paclobutrazol | 0.000068 | 0.0026 | ||||
| picolinafen | 0.00065 | 0.0011 | ||||
| picoxystrobin | 0.000043 | 0.000043 | ||||
| prometon | <MQL | 0.00026 | ||||
| prometryn | 0.000033 | 0.00009 | ||||
| propazine | 0.000035 | 0.00044 | ||||
| propiconazole | 0.00042 | 0.049 | ||||
| propoxur | 0.00037 | 0.00062 | ||||
| pyraclostrobin | 0.000053 | 0.00014 | ||||
| pyridaben | 0.0005 | 0.007 | ||||
| pyridate | 0.00094 | 0.01 | ||||
| pyrimethanil | 0.00015 | 0.0028 | ||||
| pyriproxyfen | 0.000023 | 0.00025 | ||||
| quizalofop-ethyl | 0.0012 | 0.0012 | ||||
| simazine | 0.00014 | 0.0015 | ||||
| Simetryn | <MQL | 0.000042 | ||||
| spiromesifen | 0.00074 | 0.003113124 | ||||
| spiroxamine | 0.000016 | 0.000039 | ||||
| sulfentrazone | 0.00014 | 0.0096 | ||||
| tebuconazole | <MQL | 29 | 0.0051 | 1.723 | ||
| tebufenozide | 0.00064 | 0.0039 | ||||
| tebuthiuron | <MQL | 0.00059 | ||||
| terbuthylazine | 0.00039 | 0.00074 | ||||
| terbutryn | <MQL | 0.0002 | ||||
| tetraconazole | 0.00064 | 0.00064 | ||||
| thiabendazole | <MQL | <MQL | 0.000032 | 0.02 | ||
| thiacloprid | 0.000055 | 0.00013 | ||||
| thiophanate-methyl | <MQL | <MQL | ||||
| triclopyr | <MQL | 0.065 | ||||
| trifloxystrobin | <MQL | 0.000082 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izma, G.; Raby, M.; Renaud, J.B.; Sumarah, M.; Helm, P.; McIsaac, D.; Prosser, R.; Rooney, R. Three Complementary Sampling Approaches Provide Comprehensive Characterization of Pesticide Contamination in Urban Stormwater. Urban Sci. 2025, 9, 43. https://doi.org/10.3390/urbansci9020043
Izma G, Raby M, Renaud JB, Sumarah M, Helm P, McIsaac D, Prosser R, Rooney R. Three Complementary Sampling Approaches Provide Comprehensive Characterization of Pesticide Contamination in Urban Stormwater. Urban Science. 2025; 9(2):43. https://doi.org/10.3390/urbansci9020043
Chicago/Turabian StyleIzma, Gab, Melanie Raby, Justin B. Renaud, Mark Sumarah, Paul Helm, Daniel McIsaac, Ryan Prosser, and Rebecca Rooney. 2025. "Three Complementary Sampling Approaches Provide Comprehensive Characterization of Pesticide Contamination in Urban Stormwater" Urban Science 9, no. 2: 43. https://doi.org/10.3390/urbansci9020043
APA StyleIzma, G., Raby, M., Renaud, J. B., Sumarah, M., Helm, P., McIsaac, D., Prosser, R., & Rooney, R. (2025). Three Complementary Sampling Approaches Provide Comprehensive Characterization of Pesticide Contamination in Urban Stormwater. Urban Science, 9(2), 43. https://doi.org/10.3390/urbansci9020043






