River Diversity Under Pressure: Benthic Invertebrates Reveal Urban Stream Syndrome and Guide Mitigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Environmental Parameters
2.2. Laboratory Analyses of Benthic Invertebrates
2.3. Indices and Statistical Analyses
2.4. Identification of River Ecosystem Services and Mitigation
3. Results
3.1. Benthic Invertebrates: Abundance, Diversity and Water Quality Indices
3.2. Benthic Invertebrates: Main Drivers for Distribution and Diversity
3.3. Mitigation Strategies Anchored in Key Findings
4. Discussion
4.1. Urban Stream Syndrome Indicated by Benthic Invertebrates
4.2. Current State of the Someșul Mic River in Cluj-Napoca and Directions for Mitigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGI | Blue–Green Infrastructure |
| EBI | Extensive Biotic Index |
| EPT | Ephemeroptera, Plecoptera, Trichoptera |
| FDis | Functional Dispersion |
| FDiv | Functional Divergence |
| FDR | False Discovery Rate |
| FEve | Functional Evenness |
| FFG | Functional Feeding Groups |
| FRic | Functional Richness |
| ID | Index of Dominance |
| MNWH-AR | Modified New Walley Hawkes, Abundance Related |
| MNWH-PO | Modified New Walley Hawkes, Presence-Only |
| OCH | Oligochaeta, Chironomidae |
| RaoQ | Rao’s Quadratic Entropy |
| SD | Standard deviation |
| USS | Urban Stream Syndrome |
References
- Palmer, M.; Ruhi, A. Linkages between Flow Regime, Biota, and Ecosystem Processes: Implications for River Restoration. Science 2019, 365, eaaw2087. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.R.; Saxena, S. Cities and Rivers: A Symbiotic Relationship. In Managing Urban Rivers; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–24. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Walsh, R.P.D.; Ferreira, A.J.D. Degradation in Urban Areas. Curr. Opin. Environ. Sci. Health 2018, 5, 19–25. [Google Scholar] [CrossRef]
- Barles, S. Urban Metabolism and River Systems: An Historical Perspective—Paris and the Seine, 1790–1970. Hydrol. Earth Syst. Sci. 2007, 11, 1757–1769. [Google Scholar] [CrossRef]
- Steele, M.K.; Heffernan, J.B. Morphological Characteristics of Urban Water Bodies: Mechanisms of Change and Implications for Ecosystem Function. Ecol. Appl. 2014, 24, 1070–1084. [Google Scholar] [CrossRef]
- Gurnell, A.; Lee, M.; Souch, C. Urban Rivers: Hydrology, Geomorphology, Ecology and Opportunities for Change. Geogr. Compass 2007, 1, 1118–1137. [Google Scholar] [CrossRef]
- Bălteanu, D.; Dogaru, D. Geographical Perspectives on Human-Environment Relationships and Anthropic Pressure Indicators. Rom. J. Geogr. 2011, 55, 69–80. [Google Scholar]
- Siegel, F.R. Introduction. In Cities and Mega-Cities; Springer Briefs in Geography; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Cattaneo, A.; Nelson, A.; McMenomy, T. Global Mapping of Urban–Rural Catchment Areas Reveals Unequal Access to Services. Proc. Natl. Acad. Sci. USA 2021, 118, e2011990118. [Google Scholar] [CrossRef] [PubMed]
- Gracias, J.S.; Parnell, G.S.; Specking, E.; Pohl, E.A.; Buchanan, R. Smart Cities—A Structured Literature Review. Smart Cities 2023, 6, 1719–1743. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Choppali, U.; Kougianos, E. Everything You Wanted to Know about Smart Cities: The Internet of Things Is the Backbone. IEEE Consum. Electron. Mag. 2016, 5, 60–70. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.; La Sorte, F.A. Cities as Sanctuaries. Front. Ecol. Environ. 2023, 21, 251–259. [Google Scholar] [CrossRef]
- Kowarik, I.; Fischer, L.K.; Haase, D.; Kabisch, N.; Kleinschroth, F.; Konijnendijk, C.; Straka, T.M.; Von Haaren, C. Promoting Urban Biodiversity for the Benefit of People and Nature. Nat. Rev. Biodivers. 2025, 1, 214–232. [Google Scholar] [CrossRef]
- Bagheri, A. Blue/Green Infrastructures: A Dual Solution for Urban Heat Island and Urban Flooding. Environ. Rev. 2025, 33, 1–14. [Google Scholar] [CrossRef]
- Liao, K.-H.; Deng, S.; Tan, P.Y. Blue-Green Infrastructure: New Frontier for Sustainable Urban Stormwater Management. In Greening Cities; Tan, P.Y., Jim, C.Y., Eds.; Advances in 21st Century Human Settlements; Springer: Singapore, 2017; pp. 203–226. [Google Scholar] [CrossRef]
- Iojă, C.I.; Badiu, D.L.; Haase, D.; Hossu, A.C.; Niță, M.R. How about Water? Urban Blue Infrastructure Management in Romania. Cities 2021, 110, 103084. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zerega, A.; Simões, N.E.; Feio, M.J. How to Improve the Biological Quality of Urban Streams? Reviewing the Effect of Hydromorphological Alterations and Rehabilitation Measures on Benthic Invertebrates. Water 2021, 13, 2087. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The Urban Stream Syndrome: Current Knowledge and the Search for a Cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Hughes, R.M.; Dunham, S.; Maas-Hebner, K.G.; Yeakley, J.A.; Schreck, C.; Harte, M.; Molina, N.; Shock, C.C.; Kaczynski, V.W.; Schaeffer, J. A Review of Urban Water Body Challenges and Approaches: (1) Rehabilitation and Remediation. Fisheries 2014, 39, 18–29. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global Perspectives on the Urban Stream Syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Richardson, M.; Soloviev, M. The Urban River Syndrome: Achieving Sustainability Against a Backdrop of Accelerating Change. Int. J. Environ. Res. Public Health 2021, 18, 6406. [Google Scholar] [CrossRef]
- Ranta, E.; Vidal-Abarca, M.R.; Calapez, A.R.; Feio, M.J. Urban Stream Assessment System (UsAs): An Integrative Tool to Assess Biodiversity, Ecosystem Functions and Services. Ecol. Indic. 2021, 121, 106980. [Google Scholar] [CrossRef]
- Zacharias, I.; Liakou, P.; Biliani, I. A Review of the Status of Surface European Waters Twenty Years after WFD Introduction. Environ. Process. 2020, 7, 1023–1039. [Google Scholar] [CrossRef]
- Vitecek, S.; Johnson, R.; Poikane, S. Assessing the Ecological Status of European Rivers and Lakes Using Benthic Invertebrate Communities: A Practical Catalogue of Metrics and Methods. Water 2021, 13, 346. [Google Scholar] [CrossRef]
- Feio, M.J.; Hughes, R.M.; Serra, S.R.Q.; Nichols, S.J.; Kefford, B.J.; Lintermans, M.; Robinson, W.; Odume, O.N.; Callisto, M.; Macedo, D.R.; et al. Fish and Macroinvertebrate Assemblages Reveal Extensive Degradation of the World’s Rivers. Glob. Change Biol. 2023, 29, 355–374. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Trophic Relationships. In Stream Ecology: Structure and Function of Running Waters; Springer: Cham, Switzerland, 2021; pp. 247–284. [Google Scholar]
- Etemi, F.Z.; Bytyçi, P.; Ismaili, M.; Fetoshi, O.; Ymeri, P.; Shala–Abazi, A.; Muja-Bajraktari, N.; Czikkely, M. The Use of Macroinvertebrate Based Biotic Indices and Diversity Indices to Evaluate the Water Quality of Lepenci River Basin in Kosovo. J. Environ. Sci. Health Part A 2020, 55, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Etriieki, A.M.O.; Küçükbasmacı, İ. Using Macroinvertebrate-based Biotic Indices and Diversity Indices to Assess Water Quality: A Case Study on the Karasu Stream (Kastamonu, Türkiye). Ecohydrology 2024, 17, e2627. [Google Scholar] [CrossRef]
- Calapez, R.A.; Serra, S.R.Q.; Mortágua, A.; Almeida, S.F.P.; João Feio, M. Unveiling Relationships between Ecosystem Services and Aquatic Communities in Urban Streams. Ecol. Indic. 2023, 153, 110433. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. DEVELOPMENTS IN AQUATIC INSECT BIOMONITORING: A Comparative Analysis of Recent Approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef]
- Carrasco-Badajoz, C.; Rayme-Chalco, C.; Arana-Maestre, J.; Álvarez-Tolentino, D.; Ayala-Sulca, Y.; Sanchez-Peña, M. Aquatic Macroinvertebrate Trophic Guilds, Functional Feeding Groups, and Water Quality of an Andean Urban River. Front. Environ. Sci. 2022, 10, 1003207. [Google Scholar] [CrossRef]
- Ofogh, E.A.R.; Dorche, E.E.; Birk, S.; Fathi, P.; Shahraki, Z.M.; Bruder, A. Improving the Performance of Macroinvertebrate Based Multi-Metric Indices by Incorporating Functional Traits and an Index Performance-Driven Approach. Sci. Total Environ. 2024, 931, 172850. [Google Scholar] [CrossRef]
- Földes, I. Demographic Change and Labour Migration in Cluj County, Romania. Rom. J. Popul. Stud. 2020, 13, 67–100. [Google Scholar] [CrossRef]
- Perşoiu, I.; Rădoane, M. Spatial and Temporal Controls on Historical Channel Responses—Study of an Atypical Case: Someşu Mic River, Romania. Earth Surf. Process. Landf. 2011, 36, 1391–1409. [Google Scholar] [CrossRef]
- Perșoiu, I.; Perșoiu, A. Flood Events in Transylvania during the Medieval Warm Period and the Little Ice Age. Holocene 2019, 29, 85–96. [Google Scholar] [CrossRef]
- Charzyński, P.; Markiewicz, M.; Świtoniak, M. Technogenic Soils Atlas; Polish Society of Soil Science: Toruń, Poland, 2013. [Google Scholar]
- Deac, S.O.; Irimuş, I.A.; Păcurar, B.N. The Landscape Morphology of Cluj-Napoca Residential Neighbourhoods. Stud. UBB Geogr. 2013, 58, 99–110. [Google Scholar]
- Dolean, B.-E.; Bilașco, Ș.; Petrea, D.; Moldovan, C.; Vescan, I.; Roșca, S.; Fodorean, I. Evaluation of the Built-Up Area Dynamics in the First Ring of Cluj-Napoca Metropolitan Area, Romania by Semi-Automatic GIS Analysis of Landsat Satellite Images. Appl. Sci. 2020, 10, 7722. [Google Scholar] [CrossRef]
- Suvărășan, O.; Roșian, G.; Martonoș, I.M. Someşul Mic River (Cluj County, Romania) Water Quality Assessment under Anthropogenic Impact. Stud. UBB Ambient. 2020, 65, 87–96. [Google Scholar] [CrossRef]
- Barhoumi, B.; Beldean-Galea, M.S.; Al-Rawabdeh, A.M.; Roba, C.; Martonos, I.M.; Bălc, R.; Kahlaoui, M.; Touil, S.; Tedetti, M.; Driss, M.R.; et al. Occurrence, Distribution and Ecological Risk of Trace Metals and Organic Pollutants in Surface Sediments from a Southeastern European River (Someşu Mic River, Romania). Sci. Total Environ. 2019, 660, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.; Stoica, C.; Harabagiu, A.M.; Neidoni, D.-G.; Mighiu, E.D.; Bumbac, C.; Ionescu, I.A.; Pantazi, A.; Enache, L.-B.; Enachescu, M. Laboratory Assessment for Determining Microplastics in Freshwater Systems—Characterization and Identification along the Somesul Mic River. Water 2024, 16, 233. [Google Scholar] [CrossRef]
- Cîmpean, M. Evaluarea Influenţei Antropice Asupra Calităţii Apei Râului Someşul Mic Şi a Afluenţilor Săi Utilizând Indicele Biotic Extins (I.B.E.). [Assessment of Human Impact on the Water Quality of the Someșul Mic River and Its Tributaries Using the Extended Biotic Index (EBI), in Romanian]. Brukenthal Mus. Stud. Commun. Nat. Sci. 2004, 29, 179–190. [Google Scholar]
- Avram, A.; Cîmpean, M.; Jurcă, A.; Timuş, N. Water Quality Assessment Using Biotic Indices Based on Benthic Macroinvertebrates in the Someşul Mic Catchment Area. Stud. UBB Biol. 2009, 54, 61–70. [Google Scholar]
- Florescu, H.M.; Cîmpean, M.; Momeu, L.; Leonte, L.; Bodea, D.; Battes, K.P. Ecological Analyses on Benthic Diatom and Invertebrate Communities from the Someșul Mic Catchment Area (Transylvania, Romania). Stud. Univ. Babes Bolyai Biol. 2015, 60, 69–87. [Google Scholar]
- Cîmpean, M. Studiul Taxonomic şi Ecologic Asupra Comunităţilor de Acarieni Acvatici (Acari Hydrachnidia)din Bazinul de Drenaj al Râului Someşul Mic şi Rolul lor ca Indicatori ai Calităţii Apei [Taxonomic and Ecological Study of Water Mites (Acari, Hydrachnidia) from the Someșul Mic River Basin and their role As Water Quality Indicators, in Romanian]; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Nagy, A.A.; Erős, N.; Imecs, I.; Bóné, G.; Fülöp, A.; Pap, P.L. Distribution and Diversity of Fishes and Lampreys in Transylvania (Romania): A Complete Survey and Suggestions for New Protected Areas. ZooKeys 2023, 1166, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Lukács, J. Clujul Renascentist. Aspecte Privind Viața Cotidiană în Cluj în Secolul al XVI-lea și al XVII-Lea. Alimentația și Gastronomia [Renaissance Cluj: Aspects of Daily Life in the 16th and 17th Centuries—Food and Gastronomy, in Romanian]. Ph.D. Thesis, Babeș-Bolyai University, Cluj-Napoca, Romania, 2012. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. 2024. Available online: https://qgis.org/ (accessed on 10 September 2025).
- Paisley, M.F.; Trigg, D.J.; Walley, W.J. Revision of the Biological Monitoring Working Party (BMWP) Score System: Derivation of Present-only and Abundance-related Scores from Field Data. River Res. Appl. 2014, 30, 887–904. [Google Scholar] [CrossRef]
- Callisto, M.; Mugnai, R.; Castro, D.M.P.; Linares, M.S. Sampling Methods for Aquatic Insects. In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 523–543. [Google Scholar] [CrossRef]
- Kriska, G. Freshwater Invertebrates in Central Europe: A Field Guide; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Gerecke, R.; Martin, P. The Biology and Ecology of Lotic Water Mites (Hydrachnidia). Freshw. Biol. 2000, 44, 47–62. [Google Scholar] [CrossRef]
- Mihuc, T.B. The Functional Trophic Role of Lotic Primary Consumers: Generalist versus Specialist Strategies. Freshw. Biol. 1997, 37, 455–462. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. Trophic Relationships of Macroinvertebrates. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 413–433. [Google Scholar] [CrossRef]
- Hébert, M.; Beisner, B.E.; Maranger, R. A Meta-analysis of Zooplankton Functional Traits Influencing Ecosystem Function. Ecology 2016, 97, 1069–1080. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Wolf, L.L. Dominance and the Niche in Ecological Systems: Dominance Is an Expression of Ecological Inequalities Arising out of Different Exploitation Strategies. Science 1970, 167, 131–139. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Kraak, M.H.S.; Verdonschot, P.F.M. Complementarity of Community Indices in Characterizing Aquatic Macroinvertebrate Assemblages. Glob. Ecol. Conserv. 2023, 46, e02604. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Bo, T.; Doretto, A.; Laini, A.; Bona, F.; Fenoglio, S. Biomonitoring with Macroinvertebrate Communities in Italy: What Happened to Our Past and What Is the Future? J. Limnol. 2016, 76, 21–28. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857. [Google Scholar] [CrossRef]
- Scrucca, L. GA: A Package for Genetic Algorithms in R. J. Stat. Soft. 2013, 53, 1–37. [Google Scholar] [CrossRef]
- De Bello, F.; Botta-Dukát, Z.; Lepš, J.; Fibich, P. Towards a More Balanced Combination of Multiple Traits When Computing Functional Differences between Species. Methods Ecol. Evol. 2021, 12, 443–448. [Google Scholar] [CrossRef]
- De Bello, F.; Carmona, C.P.; Dias, A.T.C.; Götzenberger, L.; Moretti, M.; Berg, M.P. Handbook of Trait-Based Ecology: From Theory to R Tools, 1st ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Mammola, S.; Carmona, C.P.; Guillerme, T.; Cardoso, P. Concepts and Applications in Functional Diversity. Funct. Ecol. 2021, 35, 1869–1885. [Google Scholar] [CrossRef]
- Cummins, K.W.; Wilzbach, M.; Kolouch, B.; Merritt, R. Estimating Macroinvertebrate Biomass for Stream Ecosystem Assessments. Int. J. Environ. Res. Public Health 2022, 19, 3240. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org/ (accessed on 1 September 2025).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2025. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 20 September 2025).
- Peterson, B.G.; Carl, P. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis. 2024. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 15 September 2025).
- Haines-Young, R. Common Internatonal Classificaton of Ecosystem Services (CICES) V5.2 and Guidance on the Applicaton of the Revised Structure. 2023. Available online: www.cices.eu (accessed on 25 September 2025).
- Yeakley, A.J.; Ervin, D.; Chang, H.; Granek, E.F.; Dujon, V.; Shandas, V.; Brown, D. Ecosystem Services of Streams and Rivers. In River Science; Gilvear, D.J., Greenwood, M.T., Thoms, M.C., Wood, P.J., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 335–352. [Google Scholar] [CrossRef]
- Sousa, M.C.; Martins, R.; Simões, N.E.; Feio, M.J. Ecosystem Services of Urban Rivers: A Systematic Review. Aquat. Sci. 2025, 87, 10. [Google Scholar] [CrossRef]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River Restoration, Habitat Heterogeneity and Biodiversity: A Failure of Theory or Practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Martin, F.-M.; Janssen, P.; Bergès, L.; Dupont, B.; Evette, A. Higher Structural Connectivity and Resistance against Invasions of Soil Bioengineering over Hard-Engineering for Riverbank Stabilisation. Wetl. Ecol. Manag. 2021, 29, 27–39. [Google Scholar] [CrossRef]
- Leblois, S.; Piton, G.; Recking, A.; Jaymond, D.; Buffet, A.; Evette, A. Riverbank Fascines Mostly Fail Due to Scouring: Consistent Evidence from Field and Flume Observations. River Res. Appl. 2025, 41, 108–119. [Google Scholar] [CrossRef]
- Tang, V.T.; Fu, D.; Singh, R.P.; Rene, E.R.; Binh, T.N.; Sharma, A.K. Evaluating the Effectiveness of Ecological Restoration of Hard Bank Rivers: A Case Study from Shedu River Port, China. J. Water Supply Res. Technol.-Aqua 2018, 67, 824–833. [Google Scholar] [CrossRef]
- Francis, R.A.; Hoggart, S.P.G. Waste Not, Want Not: The Need to Utilize Existing Artificial Structures for Habitat Improvement Along Urban Rivers. Restor. Ecol. 2008, 16, 373–381. [Google Scholar] [CrossRef]
- Palt, M.; Hering, D.; Kail, J. Context-specific Positive Effects of Woody Riparian Vegetation on Aquatic Invertebrates in Rural and Urban Landscapes. J. Appl. Ecol. 2023, 60, 1010–1021. [Google Scholar] [CrossRef]
- Weber, A.; Garcia, X.-F.; Wolter, C. Habitat Rehabilitation in Urban Waterways: The Ecological Potential of Bank Protection Structures for Benthic Invertebrates. Urban Ecosyst. 2017, 20, 759–773. [Google Scholar] [CrossRef]
- Wenger, S.J.; Roy, A.H.; Jackson, C.R.; Bernhardt, E.S.; Carter, T.L.; Filoso, S.; Gibson, C.A.; Hession, W.C.; Kaushal, S.S.; Martí, E.; et al. Twenty-Six Key Research Questions in Urban Stream Ecology: An Assessment of the State of the Science. J. N. Am. Benthol. Soc. 2009, 28, 1080–1098. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Tockner, K.; Olden, J.D.; Campos, Z.; Muniz, F.; Svenning, J.-C.; Jähnig, S.C. Hydropower Impacts on Riverine Biodiversity. Nat. Rev. Earth Environ. 2024, 5, 755–772. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. (Eds.) Nature-Based Solutions to Address Global Societal Challenges; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2016; p. 97. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Rippy, M.A.; Fletcher, T.D.; Feldman, D.L.; Peng, J.; Bowler, P.; Mehring, A.S.; Winfrey, B.K.; Vrugt, J.A.; AghaKouchak, A.; et al. From Rain Tanks to Catchments: Use of Low-Impact Development To Address Hydrologic Symptoms of the Urban Stream Syndrome. Environ. Sci. Technol. 2015, 49, 11264–11280. [Google Scholar] [CrossRef]
- Aivazidou, E.; Banias, G.; Lampridi, M.; Vasileiadis, G.; Anagnostis, A.; Papageorgiou, E.; Bochtis, D. Smart Technologies for Sustainable Water Management: An Urban Analysis. Sustainability 2021, 13, 13940. [Google Scholar] [CrossRef]
- Gourbesville, P.; Ma, Q. Smart River Management: What Is Next? River 2022, 1, 37–46. [Google Scholar] [CrossRef]
- Chien, H. Adaptive Management in Urban Stream Governance: A Review and an Urban Commoning Scenario-Building Exercise. Lex Localis J. Local Self-Gov. 2021, 19, 659–688. [Google Scholar] [CrossRef]
- Ahern, J. Urban Landscape Sustainability and Resilience: The Promise and Challenges of Integrating Ecology with Urban Planning and Design. Landsc. Ecol. 2013, 28, 1203–1212. [Google Scholar] [CrossRef]
- Shao, G.P.; Bishop, I.J. Citizen Science in River Monitoring: A Systematic Literature Review of the Whys and Hows. Front. Environ. Sci. 2025, 13, 1609084. [Google Scholar] [CrossRef]
- Dohotaru, A. Soluţii Bazate pe Natură la Someş; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2024. [Google Scholar]
- Costea, G.; Pusch, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A Review of Hydropower Plants in Romania: Distribution, Current Knowledge, and Their Effects on Fish in Headwater Streams. Renew. Sustain. Energy Rev. 2021, 145, 111003. [Google Scholar] [CrossRef]
- Pop, N.; Pop, S.B. Reducing the Effects Caused by Floods, through Hydrological Measurements Made at the Cluj-Napoca Hydrometric Station. Agricultura 2022, 124, 89–96. [Google Scholar]
- Pandi, G.; Horváth, C. Regradation of the Somesul Mic Riverbed. In Proceedings of the 2019 Air and Water—Components of the Environment Conference, Cluj-Napoca, Romania, 22–24 March 2019. [Google Scholar] [CrossRef]
- Serra, S.R.Q.; Calapez, A.R.; Simões, N.E.; Sá Marques, J.A.A.; Laranjo, M.; Feio, M.J. Effects of Variations in Water Quantity and Quality in the Structure and Functions of Invertebrates’ Community of a Mediterranean Urban Stream. Urban Ecosyst. 2019, 22, 1173–1186. [Google Scholar] [CrossRef]
- Medupin, C. Spatial and Temporal Variation of Benthic Macroinvertebrate Communities along an Urban River in Greater Manchester, UK. Environ. Monit. Assess. 2020, 192, 84. [Google Scholar] [CrossRef]
- Ludlam, J.P.; Welsh, D.P.; Clark, E.V.; Downs, E.L.; Gordon, E.S.; Huang, J.X.; Levy, B.; O’Connor, A.M. Assessing Taxonomic and Functional Responses of Stream Macroinvertebrate Communities to Multi-Scale Influences of Urban Land Cover. River Res. Amp. Appl. 2025, rra.70045. [Google Scholar] [CrossRef]
- Barbosa, G.P.; Siqueira, T. Direct and Indirect Relationships of Climate and Land Use Change with Food Webs in Lakes and Streams. Glob. Ecol. Biogeogr. 2023, 32, 2153–2163. [Google Scholar] [CrossRef]
- Czerniawski, R.; Sługocki, Ł.; Krepski, T.; Wilczak, A.; Pietrzak, K. Spatial Changes in Invertebrate Structures as a Factor of Strong Human Activity in the Bed and Catchment Area of a Small Urban Stream. Water 2020, 12, 913. [Google Scholar] [CrossRef]
- Paul, M.J.; Meyer, J.L. Streams in the Urban Landscape. In Urban Ecology; Marzluff, J.M., Shulenberger, E., Endlicher, W., Alberti, M., Bradley, G., Ryan, C., Simon, U., ZumBrunnen, C., Eds.; Springer: Boston, MA, USA, 2008; pp. 207–231. [Google Scholar] [CrossRef]
- Vehkaoja, M.; Niemi, M.; Väänänen, V.-M. Effects of Urban Infrastructure on Aquatic Invertebrate Diversity. Urban Ecosyst. 2020, 23, 831–840. [Google Scholar] [CrossRef]
- Davidson, J.; Gunn, J. Effects of Land Cover Disturbance on Stream Invertebrate Diversity and Metal Concentrations in a Small Urban Industrial Watershed. Hum. Ecol. Risk Assess. Int. J. 2012, 18, 1078–1095. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Williams-Subiza, E.A.; Brand, C.; Horak, C.N.; Assef, Y.A. Macroinvertebrate Functional Traits Differed with Land Use Practices at Patagonian Streams. Aquat. Sci. 2025, 87, 3. [Google Scholar] [CrossRef]
- Lansac-Tôha, F.A.; Velho, L.F.M.; Higuti, J.; Takahashi, E.M. Cyclopidae (Crustacea, Copepoda) from the Upper Paraná River Floodplain, Brazil. Braz. J. Biol. 2002, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Szałkiewicz, E.; Kałuża, T.; Grygoruk, M. Detailed Analysis of Habitat Suitability Curves for Macroinvertebrates and Functional Feeding Groups. Sci. Rep. 2022, 12, 10757. [Google Scholar] [CrossRef]
- Chen, S.; Brokhausen, F.; Wiesner, P.; Hegyi, D.; Citir, M.; Huth, M.; Park, S.; Rabe, J.; Thamsen, L.; Tscheikner-Gratl, F.; et al. Coupled Simulation of Urban Water Networks and Interconnected Critical Urban Infrastructure Systems: A Systematic Review and Multi-Sector Research Agenda. Sustain. Cities Soc. 2024, 104, 105283. [Google Scholar] [CrossRef]
- Rethinking Someș, Cluj-Napoca. Available online: https://oar.archi/concursuri/oar/rethinking-somes-cluj-napoca/ (accessed on 8 October 2025).
- Parcul Feroviar, Cluj-Napoca. Available online: https://oar.archi/concursuri/oar/reabilitarea-peisagera-si-reactivarea-parcului-feroviar-cluj-napoca/ (accessed on 8 October 2025).
- Amenajare Parc Est, Cluj-Napoca. Available online: https://oar.archi/concursuri/oar/amenajare-parc-est-cluj-napoca/ (accessed on 8 October 2025).
- Ilovan, O.-R.; Măgerușan, A.; Boțan, C.N.; Dulamă, M.E.; Ursu, C.-D.; Mutică, P.; Jucu, I.S. Experiencing and Bringing Back the River in the Urban Flow: Someș Delivery. In Education, Reflection, Development—ERD 2019, European Proceedings of Social and Behavioural Sciences; Chis, V., Ed.; European Publisher: Brussels, Belgium, 2020; Volume 85, pp. 262–272. [Google Scholar] [CrossRef]
- Vamos a la Playa. 2025. Available online: https://cluj.com/articole/vamos-a-la-playa-2025-plaja-grigorescu-cluj-napoca/ (accessed on 9 October 2025).
- Lucaciu, M. Territorial Flood Defense: A Romanian Perspective. In Transboundary Floods: Reducing Risks Through Flood Management; Marsalek, J., Stancalie, G., Balint, G., Eds.; Nato Science Series: IV: Earth and Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; Volume 72, pp. 315–333. [Google Scholar] [CrossRef]
- Gurnell, A.M.; England, J.; Shuker, L.; Wharton, G. The Contribution of Citizen Science Volunteers to River Monitoring and Management: International and National Perspectives and the Example of the MoRPh Survey. River Res. Appl. 2019, 35, 1359–1373. [Google Scholar] [CrossRef]
- Violin, C.R.; Cada, P.; Sudduth, E.B.; Hassett, B.A.; Penrose, D.L.; Bernhardt, E.S. Effects of Urbanization and Urban Stream Restoration on the Physical and Biological Structure of Stream Ecosystems. Ecol. Appl. 2011, 21, 1932–1949. [Google Scholar] [CrossRef]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N.; et al. Characteristics, Main Impacts, and Stewardship of Natural and Artificial Freshwater Environments: Consequences for Biodiversity Conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]

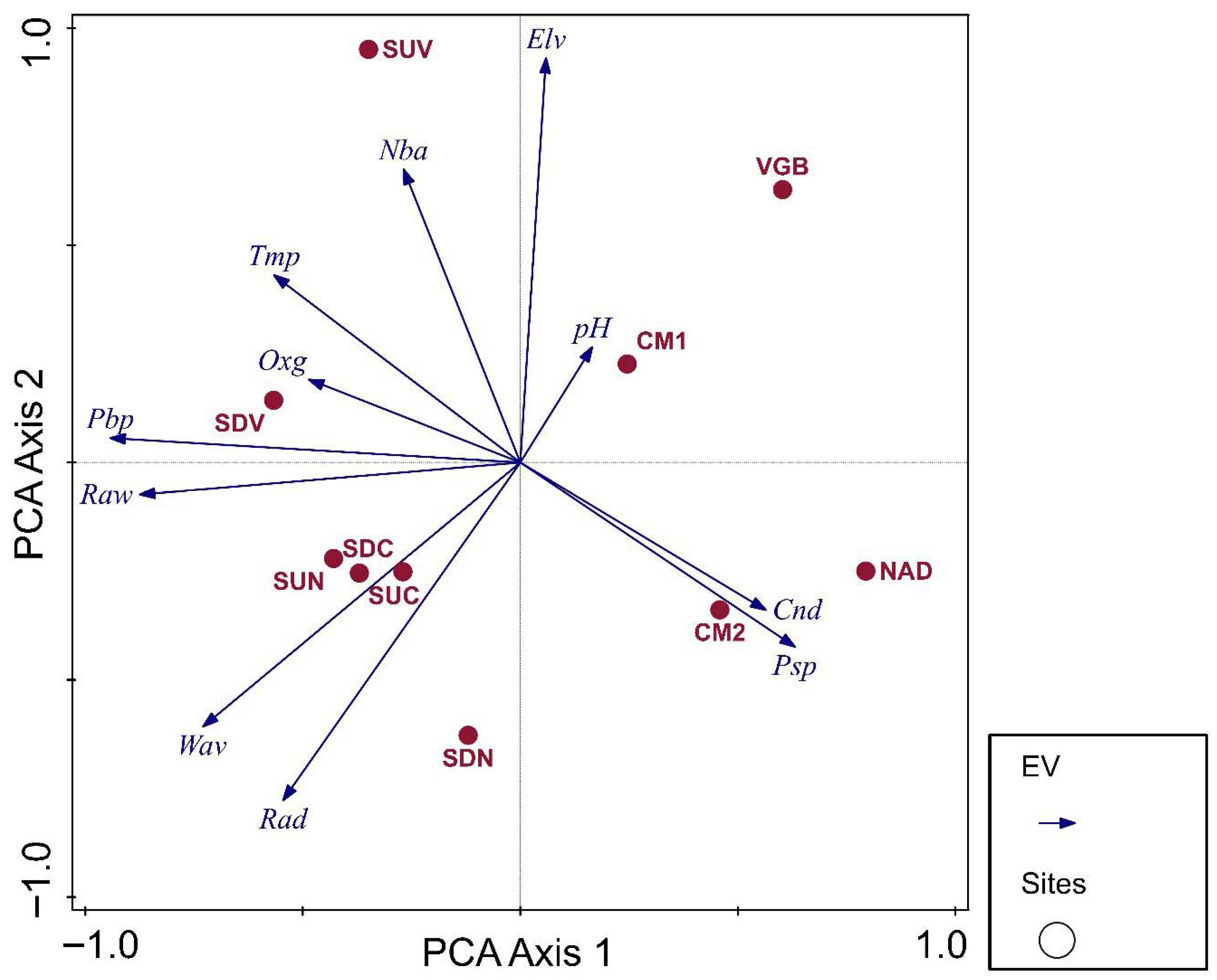
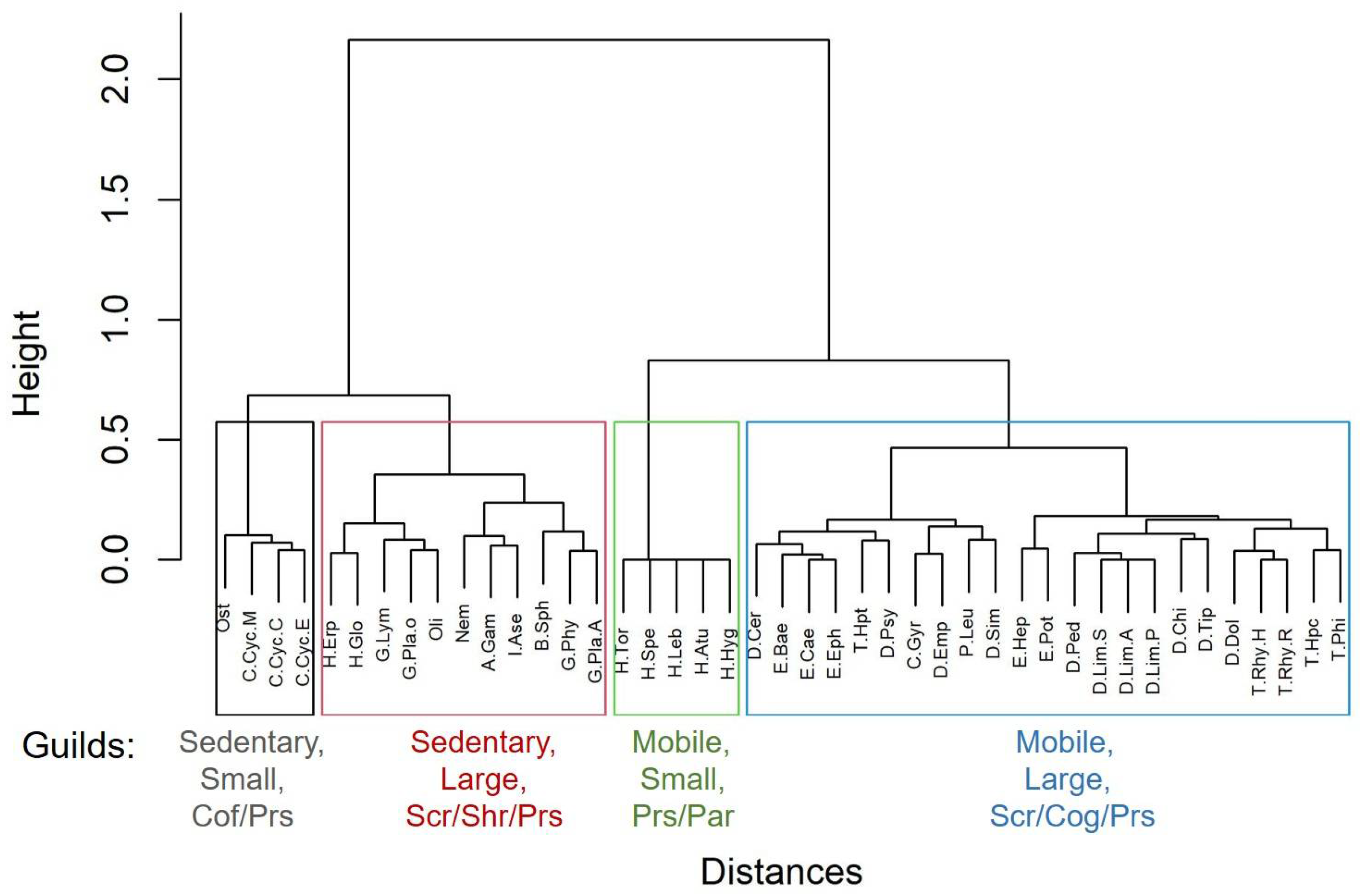
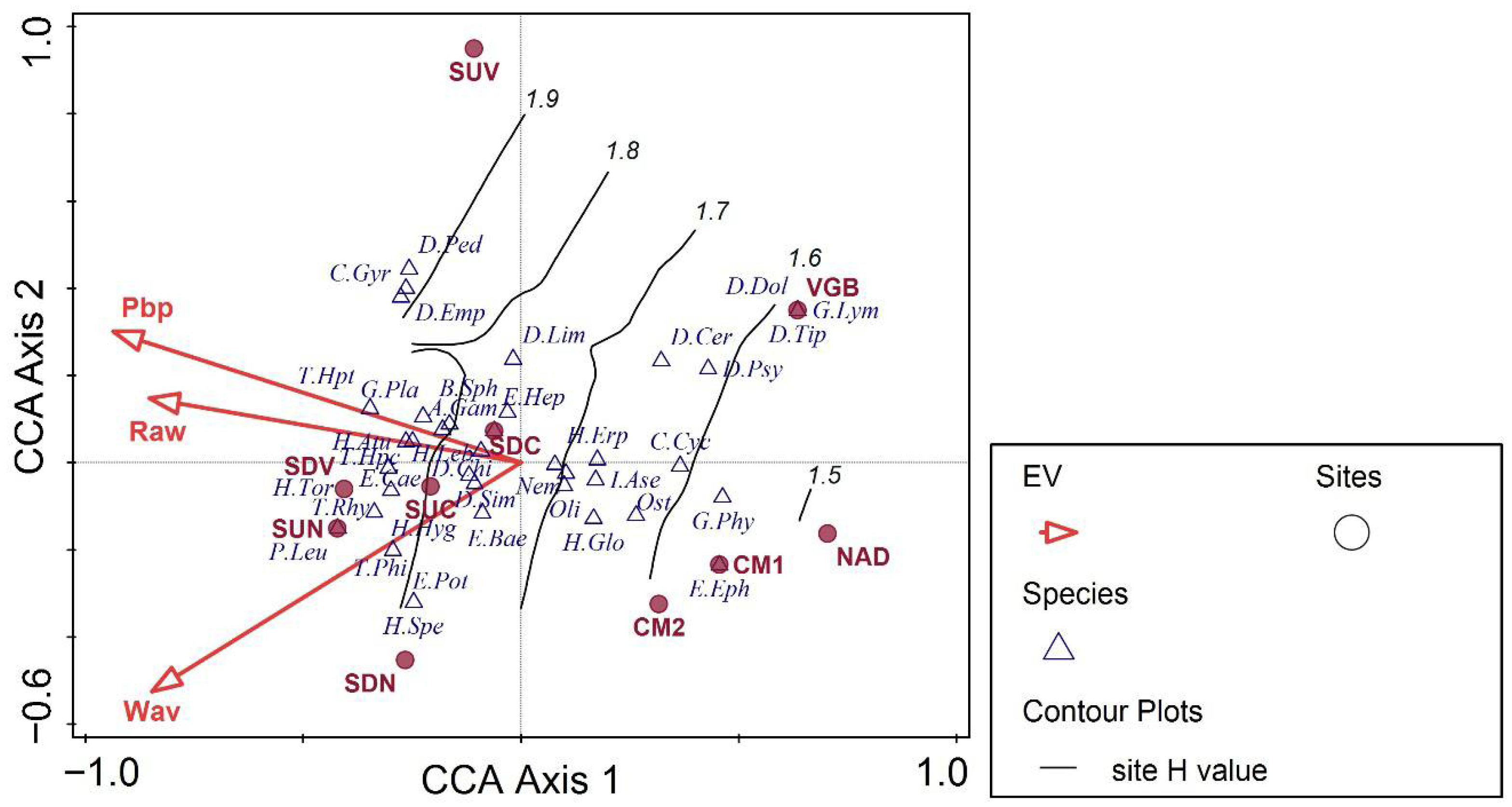
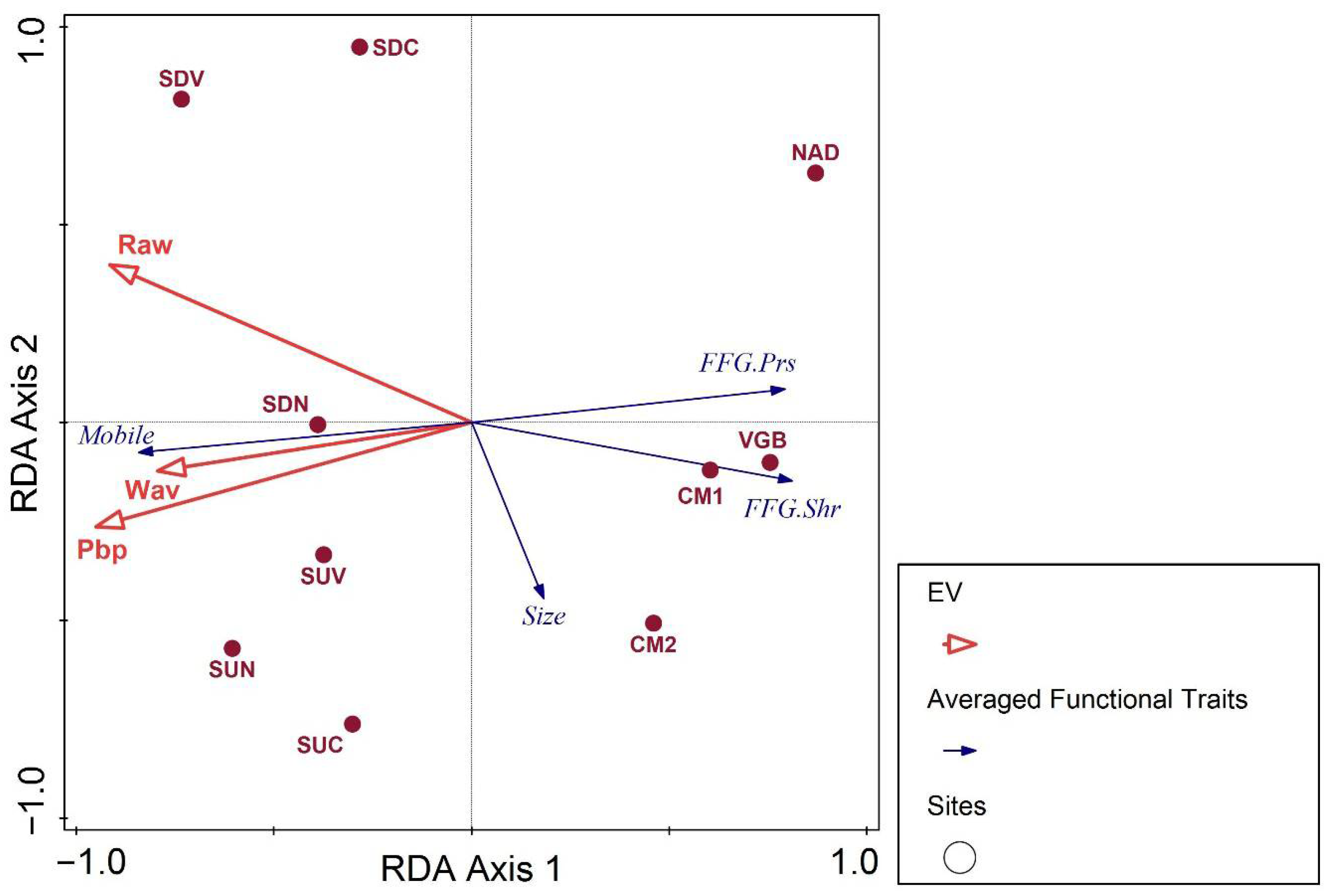
| Predictor | Model 1: Eff.tx | Model 2: No.tx | Model 3: ID | Model 4: EPT% | Model 5: OCH% | Model 6: MNWH-AR |
|---|---|---|---|---|---|---|
| Intercept | 4.61 (p < 0.001) | –2.94 (p = 0.642) | 0.57 (p < 0.001) | –41.90 (p = 0.002) | 529.75 (p = 0.013) | 43.93 (p < 0.001) |
| Wav | 3.31 (p = 0.014) | – | – | 130.16 (p < 0.001) | – | – |
| Psp | –0.32 (p = 0.015) | – | 0.029 (p = 0.016) | –4.46 (p = 0.011) | – | – |
| Nba | 1.84 (p = 0.009) | – | –0.052 (p = 0.289) | 25.92 (p = 0.006) | – | 32.12 (p = 0.066) |
| Pbp | – | 0.32 (p = 0.006) | – | – | – | – |
| Cnd | – | – | – | 0.017 (p = 0.005) | – | – |
| pH | – | – | – | – | –69.23 (p = 0.023) | – |
| Oxg | – | – | – | – | 9.33 (p = 0.076) | – |
| Raw | – | – | – | – | –1.31 (p = 0.002) | 1.22 (p = 0.002) |
| Type | Code | Ecosystem Service |
|---|---|---|
| Provisioning | P1 | Production of energy; |
| P2 | Riverfront restaurants, cafes, shops; | |
| P3 | Changes in riverfront property values; | |
| P4 | Recreational bathing; | |
| P5 | Irrigation of crops (urban gardens); | |
| P6 | Groundwater recharge. | |
| Regulation and Maintenance | RM1 | Bio-remediation made by biota (micro-organisms, algae, plants, animals); |
| RM2 | Filtration of toxic substances by biota (macrophytes); | |
| RM3 | Sequestration of CO2 by primary producers; | |
| RM4 | Sink for organic and inorganic substances (nutrients, metals, organic pollutants); | |
| RM5 | Regulation of water chemistry made by biota (bio-geo-chemical cycles); | |
| RM6 | Support for pollinators; | |
| RM7 | Maintaining viable populations and gene pool; | |
| RM8 | Maintaining habitats (aquatic and riparian); | |
| RM9 | Regulation of temperature and humidity (climate regulation); | |
| RM10 | Control of erosion rates; | |
| RM11 | Sediment transport regulation; | |
| RM12 | Hydrological cycle—including flood regulation. | |
| Cultural | C1 | River traits that allow activities sustaining human health (walks, jogging, water sports, etc.); |
| C2 | River traits that promote mental health (temporary getaway, tranquil location, meditation, feeling of safety, etc.); | |
| C3 | River traits that promote social activities (festivals, artistic events, etc.); | |
| C4 | River traits that allow educational and training activities (environmental awareness, nature laboratory, bird-watching, healthy value systems, etc.); | |
| C5 | River traits that allow recreational activities (recreational fishing, picnics, etc.); | |
| C6 | River traits with historical/heritage importance (water mills, historical bridges, places connected to the history of the city); | |
| C7 | Esthetic experiences (panoramic viewpoint, scenic beauty, natural sounds, charismatic fauna, lack of foul odors, etc.). |
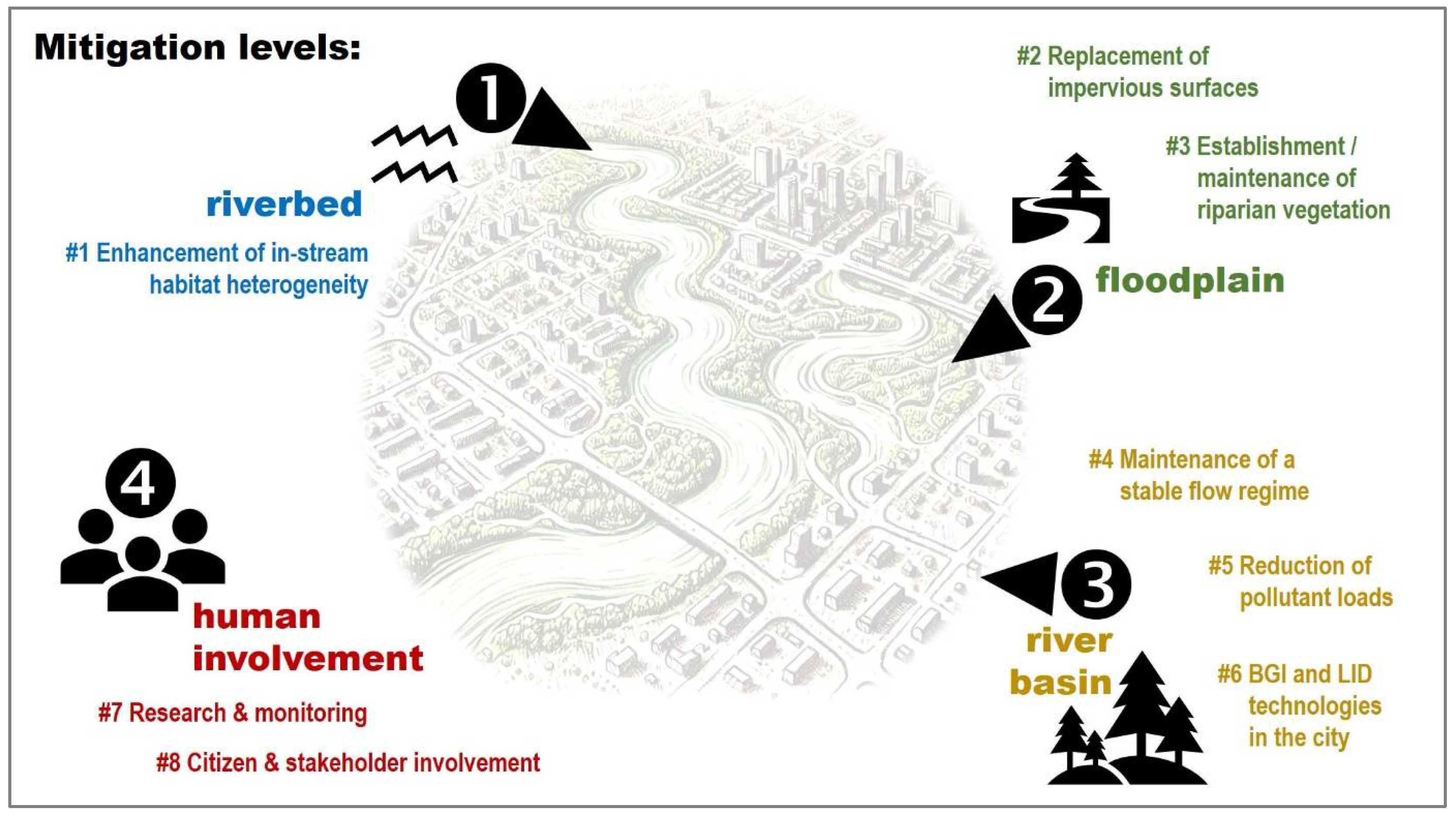
| # | Mitigation Measure | Output/Details | Nbs | Targeted ES | Sources |
|---|---|---|---|---|---|
| 1. | Enhancement of in-stream habitat heterogeneity | Addition of boulders, large cobbles, gravel and/or wooden structures, artificial riffles and meanders; Using combined remediation techniques, addressing pollution sources in the catchment and maintaining refugia within the riverbed are crucial for the successful recolonization of new substrates. | yes | RM1, RM3–8, C2, C4, C5 | [18,76] |
| 2. | Replacement of impervious surfaces, in/near the river with more natural substrates | Impervious surfaces (e.g., concrete riverbed/banks) disrupt the connection between river and groundwater through the hyporheic zone (which acts as a refuge and a regulator of water quality and floods); There is a direct connection between the flow of the Someșul Mic River and the groundwater level beneath Cluj-Napoca; until the late 19th century, the river served as the primary water source in the city, with its discharge directly determining the water supply; More natural substrates should be used (e.g., gravel, fascines and geotextiles, porous concrete, ripraps, mixed-techniques like lower-bank ripraps with upper-bank plantings); Positive effects: urban stormwater runoff reduction; riparian habitat heterogeneity increase; in-stream habitat heterogeneity increase; unlimited connections between riverbed and riparian communities (lateral connectivity). | yes | P6, RM1–9, RM12 C1, C2, C4, C5 | [18,48,77,78,79] |
| 3. | Establishment and/or maintenance of riparian vegetation | Herbs, shrub or trees, native species, living directly on the river banks or on artificial substrates (like walls), acting as riparian buffers; Positive effects: urban stormwater runoff reduction and filtration, floodplain habitat increase; constant food source in the river coming from terrestrial vegetation; increased shading leading to less extreme temperatures; decreased heat stress. | yes | P3, P6, RM1–3, RM5–12, C1–5 | [18,80,81,82,83] |
| 4. | Maintenance of a stable flow regime, downstream Florești II Dam | The Florești II Dam regulates the flow on the Someșul Mic, in Cluj-Napoca, to its confluence with the Someșul Mare. Intermittent water releases often generate hydropeaking effects in downstream sections; high flows lead to organism removal, reduction in habitat, increases in turbidity and disturbed sediments; low flows lead to reduction of habitat and food, increased concentrations of nutrients or pollutants and decreased oxygenation. | no | P1, RM4–5, RM7–9, C2–5 | [1,18,84,85] |
| 5. | Reduction in pollutant loads, at the catchment scale | Addressing pollution problems upstream of the city and within the tributary catchments is essential to secure good physico-chemical quality along the urban river reach. | yes | P2–6, RM2, RM4–5, C7 | [18] |
| 6. | Blue–Green Infrastructure BGI and Low-Impact Development LID technologies in the city | Examples of BGI and LID: permeable pavements, urban tree planting, green roofs, bioswales, rain gardens, infiltration trenches, biofilters, rain tanks, etc. Positive effects: reduction in runoff, filtering pollutants, and enhancing natural infiltration processes. | yes | P5, RM6, RM9, RM12, C1–2, C4–5 | [20,86,87] |
| 7. | Continuous research and monitoring; adaptative and ecosystem-based management and design | Smart water management: the use of sensors, Internet of Things, machine learning, big data analyses, and digital twins concepts in order to monitor, detect changes, predict, support decision-makers, etc.; Adaptive design in urban planning, together with focus of biodiversity, multifunctionality, redundancy and modularization are strategies for increasing urban resilience capacity. | no | RM7–8, C4 | [86,88,89,90,91] |
| 8. | Citizen and stakeholder involvement | Active involvement of the general public (including citizen science) and of all stakeholders, such as: authorities (the Romanian Waters National Administration—Someș-Tisa Water Basin Administration, the Someș Water Company, Hidroelectrica S.A., Cluj-Napoca Municipality); the County Association of Anglers, various NGOs, civic activists, riparian residents, etc. | no | P2–5, C1–7 | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battes, K.P.; Goia, B.-I.; Clinci, S.D.; Cîmpean, M. River Diversity Under Pressure: Benthic Invertebrates Reveal Urban Stream Syndrome and Guide Mitigation. Urban Sci. 2025, 9, 496. https://doi.org/10.3390/urbansci9120496
Battes KP, Goia B-I, Clinci SD, Cîmpean M. River Diversity Under Pressure: Benthic Invertebrates Reveal Urban Stream Syndrome and Guide Mitigation. Urban Science. 2025; 9(12):496. https://doi.org/10.3390/urbansci9120496
Chicago/Turabian StyleBattes, Karina P., Bogdan-Iosif Goia, Sorin Dan Clinci, and Mirela Cîmpean. 2025. "River Diversity Under Pressure: Benthic Invertebrates Reveal Urban Stream Syndrome and Guide Mitigation" Urban Science 9, no. 12: 496. https://doi.org/10.3390/urbansci9120496
APA StyleBattes, K. P., Goia, B.-I., Clinci, S. D., & Cîmpean, M. (2025). River Diversity Under Pressure: Benthic Invertebrates Reveal Urban Stream Syndrome and Guide Mitigation. Urban Science, 9(12), 496. https://doi.org/10.3390/urbansci9120496









