Abstract
Monitoring nitrate levels in water is critical to protect public health and ensure compliance with regulatory standards. This study provides a comprehensive evaluation of four analytical techniques—test strips, ion-selective electrodes (ISE), colorimetric methods, and titration—to assess nitrate levels in a variety of water sources, including standard solutions, rainwater, bottled water, and groundwater from both shallow and deep wells located in semi-urban regions of Saudi Arabia. Each method was assessed for sensitivity, accuracy, detection limits, reproducibility, and operational practicality. Test strips offer rapid, low-cost screening but consistently underestimate nitrate concentrations, particularly at low levels. The ISE demonstrated broad applicability and reliable performance across a wide concentration range when properly calibrated, making it suitable for both field and laboratory applications. Colorimetric methods provide excellent sensitivity for trace-level detection, whereas titration delivers the highest accuracy for high-nitrate samples despite its time-intensive nature. By calibrating and validating the methods against certified standards, we quantitatively demonstrated their reliability through statistical measures such as precision and accuracy rates. Moreover, the application of Geographic Information System (GIS) techniques in spatial analysis has revealed significant differences in the distribution of nitrates. Notably, shallow wells located in the northern regions surpass the 50 mg/L limit set by the World Health Organization (WHO), thereby indicating the presence of localized contamination hotspots. This study is among the first to systematically compare nitrate detection methods across a wide range of water types in a semi-urban area of Saudi Arabia. Building on a detailed analysis of each method, we underline the crucial need for the strategic selection of nitrate analysis techniques. This selection should be tailored to specific operational contexts, accuracy requirements, and concentration ranges to guide stakeholders towards more informed decision-making. These findings provide actionable guidance for public health officials and water managers to prioritize monitoring, safeguard drinking-water sources, and mitigate nitrate-related health risks in semi-urban communities.
1. Introduction
Groundwater contamination with excessive nitrate (NO3−) poses a significant global environmental and health challenge, with adverse effects on public health, particularly in newborns [1,2,3,4]. The World Health Organization (WHO) sets a threshold of 50 mg/L for NO3−; exceeding this poses risks such as methemoglobinemia and thyroid dysfunction. This relationship is particularly concerning in areas with intensive agricultural practices, where nitrate contamination of the groundwater is more prevalent. The International Agency for Research on Cancer classifies nitrate as a group 2A substance, indicating that it is a potential threat to human health [5,6]. In Saudi Arabia, the national water quality guidelines align closely with the WHO recommendations and also set the same limit [4,7]. These regulatory thresholds highlight the importance of accurately detecting nitrate concentrations, particularly in groundwater sources that serve as primary water sources [8]. Northeastern Saudi Arabia faces unique challenges related to water quality and availability, exacerbated by its semi-arid climate and rapid urbanization. Understanding the nitrate levels in this context is crucial for sustainable water management and public health. Regular nitrate testing can provide early detection of increasing contamination trends. The importance of monitoring nitrate levels in water, industry, food products, and environmental settings cannot be overstated. However, the accurate estimation of nitrate in the presence of similar ions poses a significant challenge. Therefore, detection methods are crucial to avoid interference in diverse settings. To overcome these challenges, various analytical and sensing methods have been developed. These methods employ different principles such as ion selectivity, spectrometry, electrochemical methods, and colorimetry [9,10]. These methods are not only limited to detecting nitrate ions but also accurately quantify their concentration levels in various media. The sensitivity, selectivity, and accuracy of these methods continually improve when detecting and quantifying nitrate ions in complex samples [11,12].
Nitrate measurement techniques have evolved significantly over the years, offering various methods for detecting and quantifying nitrate in environmental samples [7]. Spectrophotometric methods are widely used because of their high sensitivity, low detection limit, and cost-effectiveness [11]. These methods often employ the Griess reaction, which entails converting nitrate into nitrite followed by the creation of a colored azo dye [13]. Although many techniques have focused on laboratory analysis, there have been advancements in on-site and real-time nitrate monitoring systems. For example, an automated nitrate monitoring system using an ion-selective electrode was developed for in-field measurements [14]. A smartphone-based device has similarly been employed for the on-site evaluation of nitrate levels in water samples [15]. Collectively, these device-level advances underscore an operational need for field-deployable nitrate measurements that deliver timely, decision-grade data without sacrificing analytical quality. Although spectrophotometric methods remain popular owing to their reliability and sensitivity, emerging technologies enable real-time in situ measurements. This diversity of approaches allows for more comprehensive nitrate monitoring in various environmental contexts, from soil and water quality assessments to precise agricultural applications [12,16,17].
Building upon the capabilities of the ISE method, this study further investigates the photometric approach to understand its complementary application in nitrate detection [18]. The nitrate concentration in water is directly related to the color intensity. This approach offers advantages, such as high sensitivity, rapid analysis, and reasonable cost. However, it also has limitations, including the requirement for specialized equipment, possible interference from other sample components, and the need for precise calibration and quality control [19,20]. Another technique, the ISE method, measures the electrical potential difference between a reference electrode and nitrate-sensitive electrode in the presence of nitrate ions. This method offers several benefits, including high specificity, rapid analysis times, and the capability to analyze samples on-site. It also offers rapid results and excellent efficiency. Nevertheless, the ISE method has drawbacks, such as the need for specialized equipment, a dependable reference electrode, and potential interference from other compounds in the sample [21,22,23].
The ultraviolet spectrophotometric approach estimates nitrate by measuring absorbance in the UV range where NO3− exhibits characteristic bands; appropriate baseline correction and derivative processing can mitigate interferences from co-absorbing species [11,19,24]. The advantages of this method include its high sensitivity and specificity, rapid analysis, and its ability to measure a wide range of nitrate concentrations. Potential interferences include dissolved organic carbon and nitrite, which absorb in the UV range; these were minimized by baseline correction and reagent blanks [24,25]. Similarly to the colorimetric method, the photometric approach employs a different reagent, resulting in a distinctly colored product. Colorimetric techniques offer several advantages including high sensitivity, rapid analysis, and cost-effectiveness. Its limitations include potential interference from other sample components, and the necessity for accurate calibration and quality control procedures [19].
Titration is a commonly employed analytical method for determining nitrate levels in water samples [25,26]. In the classical titrimetric workflow, nitrate is chemically reduced (commonly using Devarda’s alloy), the liberated ammonia is distilled and trapped in boric acid, and the collected ammonium is titrated with standardized acid to an indicator endpoint; the consumed acid equivalents are stoichiometrically related to the original nitrate content [25,26]. Careful control of reduction efficiency, distillation, and endpoint detection is required to achieve the method’s typical high accuracy.
Various analytical techniques offer varying levels of precision, sensitivity, and specificity. Choosing the most suitable technique for a specific application ensures that the collected data are dependable and serves its intended purpose. Despite the availability of various nitrate detection methods, few studies have systematically compared their performance under identical environmental conditions. This study aimed to fill this gap by evaluating four analytical techniques across diverse water sources, focusing on accuracy, sensitivity, and field applicability. A comparative analysis was performed to determine the optimal method. Furthermore, the evaluation and comparison of methods have driven advancements and enhancements in the analytical procedures. Recognizing the limitations or deficiencies of the current methods can stimulate the creation of novel, precise, and efficient techniques for measuring nitrate. Few studies have systematically compared field-deployable and laboratory nitrate methods under identical conditions in semi-urban areas. Previously, we reported the nitrate conductivity relationship in which nitrate was measured using the ISE method. This study compared the sensitivity, specificity, analysis time, and cost of different approaches including photometric measurements, UV spectrophotometry, and colorimetric methods. We compared nitrate quantification techniques to identify the most suitable method for water analysis, and reviewed these techniques, considering their advantages and disadvantages under various circumstances and specific analytical requirements. This study aims not only to compare analytical techniques under identical semi-arid conditions but also to integrate these findings with GIS-based spatial mapping of nitrate in groundwater. This dual approach provides actionable insights for selecting cost-effective monitoring strategies and identifying contamination hotspots in resource-limited semi-urban settings. A wide range of water samples, including standard solutions, rainwater, bottled water, and groundwater from both shallow and deep aquifers, were collected across a spectrum of nitrate concentrations. This study highlighted the strengths, limitations, and field applicability of each method. Furthermore, this study presents a time-resolved analysis of reagent degradation and examines spatial nitrate variability using GIS techniques, which represents a methodological innovation not commonly addressed in the existing literature.
From a societal perspective, selecting nitrate methods that balance sensitivity, speed, and practicality directly support timely risk screening, cost-effective surveillance of private wells, and prioritization of remediation in vulnerable neighborhoods. In semi-urban settings with constrained laboratory access, clear method guidance can accelerate protective actions for infants and other sensitive populations. Although APHA (Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA) was considered, adaptations were necessary to accommodate field constraints and limited laboratory infrastructure while maintaining traceability and QA/QC integrity.
2. Materials and Methods
2.1. Samples
The present study utilized a selection of water samples meticulously chosen to encompass a broad range of nitrate concentration levels, as listed in Table 1, corresponding to the classifications presented in Table S1 in the Supplementary Materials. Sampling sites were strategically chosen to encompass a range of land-use types and depths of groundwater sources, with sampling conducted during both the wet and dry seasons to capture temporal variability. The inclusion of diverse water types ensured that the methods were tested across a realistic range of nitrate concentrations and environmental conditions.

Table 1.
The different water samples utilized in this study, along with their predicted nitrate levels.
The first category consisted of three standards provided by Horiba (Kyoto, Japan). These standards, with exact nitrate concentrations of 150, 500, and 2000 mg/L were used to calibrate and validate the analytical methods applied in this study. The 150, 500, and 2000 mg/L standards were manufacturer-provided certified reference materials (CRMs). These standards include manufacturer-specified traceability and uncertainty of 150 ± 2, 500 ± 5 and 2000 ± 10 mg/L, ensuring reliability for calibration and comparison. The use of CRMs helps to minimize measurement bias and supports cross-validation accuracy. Integrating these standards serves a dual purpose within the experimental setup. They played a pivotal role in adjusting the analytical tools and techniques used throughout the study, ensuring that the measurements remained accurate and dependable. Second, CRMs were used as benchmarks to validate the performance of the analytical method, allowing cross-comparison of the results obtained using different techniques. This ensured consistency across the methods, minimized measurement bias, and confirmed the traceability of the measured nitrate concentrations to certified values. Use of CRMs for calibration and cross-validation is consistent with best practice for traceability and bias control [27]. The second category included seven reference samples that were designed to cover a range of nitrate concentrations from high to low. The reference samples were carefully prepared by diluting the standard solution with nanopore water under controlled conditions to ensure both precision and consistency. The nitrate concentration in each reference sample was accurately determined using a specific dilution factor. The third group comprised rainwater samples collected from two distinct locations in the Eastern Province. The first location, near Wadi Al Batin, is predominantly agricultural (Rains 1 and 2). This area is known for its vegetable and local crop cultivation, as well as livestock farming, where pesticide use is a common practice. The second set of rainwater samples (Rains 3 and 4) was collected from the rooftop of a government building in Hafr Al Batin City, approximately 11 m above ground level. Unlike the Wadi Al-Batin region, this location does not engage in agricultural activities.
The fourth and fifth sets consisted of groundwater samples collected from both shallow and deep wells located in various parts of Hafr Al Batin. It was assumed that these wells contained a mixture of high and low nitrate levels. To reduce the risk of contamination, samples were taken directly from the wells, carefully stored in glass containers, and immediately sealed. The selected areas can be characterized by their distinct geographical and climatic attributes. This region, identified by the coordinates 28°26′3″ N and 45°57′49″ E, lies within the vast arid valley of Wadi Al Batin. This valley is part of the larger Wadi Al Rummah, which holds the distinction of being the longest and most extensive dry river on the Arabian Peninsula, stretching an impressive 2000 km across the landscape [8,28,29]. The name “Hafr Al Batin” itself is derived from this prominent geological feature, literally translating to “Al Batin Valley.” The Saq Aquifer serves as a crucial freshwater resource in Hafr Al-Batin; however, excessive extraction has resulted in declining water levels and potential concerns regarding the water quality. The aquifer is composed of sedimentary layers that predominantly consist of sandstone, limestone, and porous formations. In this region, the aquifer is recharged primarily by infrequent and sporadic rainfall. Owing to its hydrogeological characteristics, the Saq Aquifer can store substantial quantities of groundwater. Extreme temperature variations and minimal precipitation characterize the climate of the Hafr Al Basin. Winters in the region are relatively mild, with evening temperatures ranging from 2 to 8 °C. In contrast, summers are exceptionally hot and arid, with daytime temperatures ranging between 40 °C and 50 °C. Rainfall is scarce throughout the year, with the winter months experiencing occasional precipitation, whereas summers remain virtually rainless. Harsh climate has a significant impact on the ecological and agricultural potential of the region. Despite these challenging conditions, the nearby Al Qassim oasis, through which a substantial portion of Wadi Al Rummah flows, has emerged as one of the leading agricultural regions in the area [8]. This oasis serves as a vital source of cultivation and sustenance in an otherwise arid landscape, highlighting the importance of water resources in shaping the human settlements and economic activities in this region.
The sixth group includes bottled water purchased from local markets in Saudi Arabia. This could involve assessing various brands, types, and sources of bottled water available to consumers in the country. The last category comprised one nanopore and distilled water, with an almost negligible nitrate concentration. Water served as a crucial control, providing a reference sample for evaluating nitrate levels that were nearly zero.
2.2. Hydrogeological and Ion Origin Background
The study area, Hafr Al Batin, situated in the Eastern Province of Saudi Arabia, is encompassed within the arid expanse of the Wadi Al Batin dry valley system, which forms part of the extensive Wadi Al Rummah watershed [8]. Hafr Al Batin was selected because of its hydrogeological diversity and known risk of nitrate contamination, making it an ideal location for comparative analysis. The local geology is dominated by sedimentary sequences of sandstone, limestone, and interbedded silt and clay layers, which form the primary hydrostratigraphic framework of groundwater. The main aquifer, known as the SAQ aquifer, consists of porous sandstones with high permeability, and is capable of storing substantial volumes of groundwater. However, over-extraction and limited recharge due to infrequent rainfall have raised concerns regarding declining water quality and quantity [30].
From a hydrogeological perspective, shallow aquifers in this region are more susceptible to surface-derived contamination, owing to minimal protective layering. In contrast, deep aquifers, which are better protected, are still vulnerable to leaching and the long-term accumulation of contaminants if over-pumped or improperly sealed. The lithostratigraphy of the region facilitates both the lateral and vertical movement of dissolved ions, which is crucial when considering the transport of contaminants.
The presence of nitrate and related ions in the studied water samples can be attributed to several factors. Agricultural activities in the surrounding areas, including the use of nitrogen-rich fertilizers, are a primary source of nitrate infiltration into shallow groundwater. Septic system leakage and livestock waste may also contribute to elevated nitrate levels. In urban areas, stormwater runoff and domestic wastewater may enhance nitrate loading into the rainwater and shallow groundwater. Atmospheric deposition, although less dominant, may explain the low measurable nitrate levels in rainwater samples taken from elevated urban locations. This integrated understanding of geological formations and aquifer vulnerability may support the interpretation of spatial and compositional variations observed in the nitrate data presented in this study.
2.3. Nitrate Measurement Methods
This research employed four different analytical techniques to assess nitrate levels: test strips, ISE, colorimetric methods, and titration. The selection of these four analytical techniques was based on their widespread use in similar studies, feasibility for implementation in semi-urban settings in Saudi Arabia, and varying levels of sensitivity and specificity, which allowed for a comprehensive evaluation across a range of conditions.
2.3.1. Nitrate Measurement Using Test Strips
A quick and practical method to determine the nitrate level in water involves measuring the nitrate levels using commercially available test strips. SJ wave test strips (SJ wave, Shanghai, China) were used. Although the test strip could estimate 16 parameters (Table S2 in the Supplementary Materials), including pH, hardness, chlorine, lead, iron, copper, nitrate, and nitrite, we were only concerned with nitrate measurements for simplicity. The procedure for using nitrate test strips was as follows.
Water samples were collected and tested to ensure that they were clean, well-mixed, and noticeably debris-free. To prevent contamination, the nitrate test strip was entirely immersed into the water sample. The reaction proceeded for approximately one minute after the test strip was removed from water. The amount of nitrate in the water changed the color of the nitrate test strips. The estimated nitrate content was determined by comparing the color of the test strip with that provided by the manufacturer. Test strip readings were validated against Certified Reference Material standards (150 and 500 mg/L) to assess bias. Given the visual endpoint and discrete color scale, test strips are best suited for screening rather than quantification, particularly near decision thresholds [7,11].
2.3.2. Ion-Selective Electrode Method
Ion-selective electrodes are commonly used devices for measuring nitrate levels in water-based solutions. This technique uses an electrode that is specifically responsive to nitrate ions. Calibration was required before each measurement using standard nitrate solutions of known concentrations [31]. ISE calibration includes ionic strength adjustment using an ionic strength adjustment solution to minimize activity coefficient variability [21,22,23]. The ion-selective electrode was dipped into the water sample to measure nitrate levels after the electrode had stabilized. The electrode provides a signal that is inversely proportional to the number of nitrate ions in the water. The nitrate concentration was calculated based on the signal generated by the electrode and calibration curve generated during the calibration stage. To ensure the accuracy and precision of the results, it is crucial to perform proper quality control steps such as blank testing and repeated measurements, as in any analytical process.
The nitrate levels in the water samples were determined using LAQUAtwin ion-selective electrode pocket meters from Horiba, Kyoto, Japan. A sample of 0.3–2.0 mL is poured into the small measurement well of the ISE system using a pipette without the addition of any reagents or sample preparation. The process of taking measurements lasted about one minute. The detection limit is 0.8 mg/L; linearity confirmed up to 90 mg/L (R2 > 0.995); interference from nitrite minimized by zinc reduction step. According to the manufacturer, the device is capable of determining nitrate levels with a precision of ±10% when properly calibrated. However, the performance of the device relies on the precise correlation of its readings with established nitrate concentrations. ISE performance can be affected by chloride and bicarbonate; ionic strength adjustment and calibration checks were applied to reduce this effect [21,22]. Calibration is essential in this process, as it aligns the readings of the instrument with the actual nitrate concentrations found in the standard solutions. Routine two-point calibration with ionic-strength adjustment, drift checks, and replicate readings were performed to manage activity-coefficient effects and electrode drift [23]. Before each measurement session, a two-point calibration was performed using standards of 150 and 2000 mg/L, as provided by the manufacturer.
The default calibration settings for the Horiba nitrate meter were 150 mg/L for the initial point and 2000 mg/L for the second point. These concentrations were chosen to match the expected range of the sample concentrations. In the calibration procedure, multiple drops of the standard solution were used to completely cover the sensor′s flat surface. It should be noted that washing the sensor with the standard solution prior to its application can enhance the accuracy of the calibration. This initial rinse ensured comprehensive contact between the sensor and the standard solution, reducing the risk of contamination from previous samples.
2.3.3. Photometric Measurement with the Nitratest Method
YSI 9500 photometers (YSI Inc., Yellow Springs, OH, USA) were employed for photometric analysis. This instrument can be adapted to both laboratory and field applications. It is a portable device capable of measuring over 100 parameters by choosing a suitable program; nonetheless, for the sake of simplicity, we focus on measuring nitrate. For general analytical purposes, the transmittance (test program 0) and absorbance (test program 1) were evaluated. A specialized test tube containing the sample solution and a colored filter was used to transmit light onto a photodetector. Filters were automatically selected to ensure the passage of light at a specific wavelength. In instances where the solution was completely colorless, all the light passed through without absorption. In contrast, when light is absorbed by the colored samples, there is a corresponding decrease in the amount of light that passes through. The photometer quantifies the color produced when chemical reagents react with a water sample. The photometer was pre-programmed with calibrations for each test parameter. The YSI 9500 photometer was factory calibrated and its programmed calibration curve was validated using certified reference materials (CRMs) for nitrate standards. The manufacturer reported a full-scale calibration accuracy of  ± 2%. To assess its contribution to the total measurement uncertainty, the internal calibration error of the instrument, reproducibility of replicate measurements, and variation in the optical response were considered. Different test procedures can be performed at different wavelengths to optimize the sensitivity of each test [27]. The instrument was designed to select the required wavelength autonomously. Calibration was performed using a specialized program unique to each test procedure. This feature allows the device to automatically select the appropriate wavelength filter and convert the photodiode′s response into a concentration measurement [11,13]. Consequently, the instrument displays the test outcomes directly. Figure 1 shows a schematic representation of the process for measuring nitrate concentration in a groundwater sample using the photometric method.
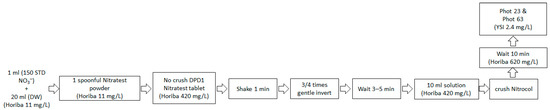
Figure 1.
A schematic diagram was constructed using a photometric analysis technique to measure nitrate concentrations in the water samples.
To determine the nitrate concentration in the water samples, nitrate was initially converted into nitrite, which was subsequently identified through a diazonium reaction that produced a reddish dye. This conversion was facilitated by a zinc-based nitrate-based powder and tablet, which enabled rapid flocculation within one minute of contact. A specialized Nitratest Tube, featuring a graded sample container with a hopper bottom, was employed to streamline sample preparation and decanting. In the presence of N-(1-naphthyl)-ethylenediamine, sulfanilic acid (SAA) reacts with the residual nitrite from the reduction process, resulting in a crimson coloration. A Nitricol tablet containing the requisite reagents was then introduced into the test solution. After diluting 10 mL of the water sample, the absorbance of the resulting red dye was measured at 493 nm using a YSI 9500 photometer. This measurement was then compared to a blank sample that contained distilled water. A calibration graph was also created.
2.3.4. Titration Method
The measurement of nitrate in water via titration involves a series of precise steps as shown in Figure 2. First, a known volume of water sample was placed in a flask. A reducing agent, typically Devarda′s alloy, was added to convert the nitrate to ammonia. The solution was then distilled to collect the ammonia in a receiving flask containing boric acid. This ammonia–boric acid solution is titrated using a suitable indicator with a standardized acid, usually hydrochloric or sulfuric acid. Hydrochloric acid was used for routine titrations due to its sharp endpoint with methyl red, whereas sulfuric acid was applied when chloride interference was suspected, as it minimizes volatilization losses during distillation [26]. The endpoint of titration was reached when the indicator changed color. The original nitrate concentration in the water sample was determined by calculating the amount of acid used in titration [32]. Each titration was performed in triplicate, and the RSD was calculated to assess the precision. Although time-consuming, this method provides accurate results, and is particularly useful for samples with high nitrate concentrations.

Figure 2.
A step-by-step systematic diagram for nitrate measurement following the titration method.
2.4. Data Analysis
Data analysis involved both descriptive and inferential statistical approaches to evaluate the performance of nitrate measurement techniques and interpret spatial variability. Initially, descriptive statistics (mean, median, range, and standard deviation) were computed for the nitrate concentrations across sample types and analytical methods. Accuracy and precision were assessed by comparing the measured values against certified reference materials, with relative standard deviations (RSD) calculated from replicate measurements. Precision was summarized as RSD, accuracy was established against CRMs, and inter-method agreement was examined graphically and by paired comparisons, consistent with standard analytical validation practice [25,27].
Calibration curves were generated for the photometric and ISE methods using linear regression, and their performances were evaluated based on the correlation coefficients (R2) and limits of detection (LOD). Method bias was quantified as the percentage deviation from the true values across concentration ranges. Inter-method agreement was examined using paired comparisons and graphical analysis.
To assess differences in nitrate concentrations among water source categories (rainwater, shallow wells, deep wells, and bottled water), an analysis of variance (ANOVA) was conducted in one direction, and Tukey′s post hoc test was subsequently used for pairwise comparisons with a confidence level of 95%. Sensitivity analysis using Pearson’s correlation coefficients identified the influence of key parameters (nitrate concentration, ingestion rate, and body weight) on health risk indices.
Geographic Information System (GIS) tools were employed to examine how nitrate is distributed spatially in groundwater. Inverse Distance Weighting (IDW) interpolation was applied to visualize the concentration gradients and identify contamination hotspots. All statistical analyses were performed using Microsoft Excel and were validated using standard statistical procedures.
3. Results and Discussion
3.1. Calibration and Validation with a Standard Sample
Starting with a standard solution of 2000 mg/L provided by Horiba, a series of dilutions were made using nanopore water to create reference solutions with known nitrate concentrations. The process involved careful measurement and mixing to ensure precision and minimize errors. The resulting concentrations were 1000, 500, 250, 125, 62.5, 31.25, and 15.75 mg/L. These concentrations provided a wide dynamic range for validation, enabling the detection of both high and low nitrate levels in the samples. The inclusion of lower concentrations, such as 15.75 mg/L, ensures sensitivity for detecting trace amounts of nitrates, whereas higher concentrations, such as 1000 mg/L, allow for quantification of heavily contaminated samples. The dilution uncertainty was assessed to ensure the reliability of the calibration process. Each dilution step introduces volumetric variability, primarily from the pipette calibration tolerance and operator handling. Based on the standard deviation of repeated pipetted volumes and the uncertainty associated with stock solution concentrations, the cumulative dilution uncertainty was estimated and found to contribute approximately 4.5% of the relative uncertainty to the final concentration values. This comprehensive range also facilitated the assessment of the linearity and detection limits of the analytical method employed. For UV-VIS measurements, samples exceeding the linear absorbance range were diluted after color development to maintain compliance with Beer–Lambert law and instrument specifications.
An analysis of the differences between the predicted and observed nitrate levels in the standard samples, as shown in Figure 3a,b, reveals an identical relationship. The calculated values, derived through a mathematical approach incorporating dilution factors, were closely aligned with the concentrations measured directly using four different methods. This high degree of similarity between the two methodologies indicates the reliability and accuracy of both calculation and experimental methods. The near-identical results obtained from these distinct approaches not only validate the precision of the measurement procedures, but also emphasize the critical importance of meticulous calibration in ensuring dependable and accurate nitrate concentration determinations. This finding provides a solid foundation for confidence in nitrate concentration data obtained using various methods with negligible errors.

Figure 3.
The calculated and measured nitrate concentration using different methods: (a) test strips (green) and colorimetric method (blue) and (b) ISE method (black) and titration method (red) for reference samples.
To validate the photometric method, we determined the limit of detection (LOD) determination, linearity, and interference control. The LOD was calculated by analyzing blank samples and determining the standard deviation of the response, which was then multiplied by three to estimate the minimum detectable concentration in accordance with the established guidelines. Linearity was evaluated by preparing calibration standards across a concentration range from 0 to 100 mg/L, followed by measuring absorbance at a wavelength of 493 nm. Linear regression analysis of the resulting data produced an equation with a correlation coefficient of R2 = 0.99, confirming excellent linearity throughout the tested range (Figure 4). To assess potential interference, known interfering substances were introduced in separate trials. The absence of significant deviations in the absorbance values compared to the control samples indicated that the method was robust and free from matrix effects.

Figure 4.
The absorbance as a function of nitrate distribution using the photometric method, which involved preparing calibration standards across a concentration range of 0–100 mg/L and measuring absorbance at 493 nm.
To further illustrate the comparative performance of the analytical methods used in this study, Figure 5 shows the nitrate concentrations measured across five representative water samples using four analytical techniques: test strips, ion-selective electrodes (ISE), colorimetric analysis, and titration. This figure highlights the distinct performance characteristics of each method in terms of the sensitivity, accuracy, and suitability for different concentration ranges.
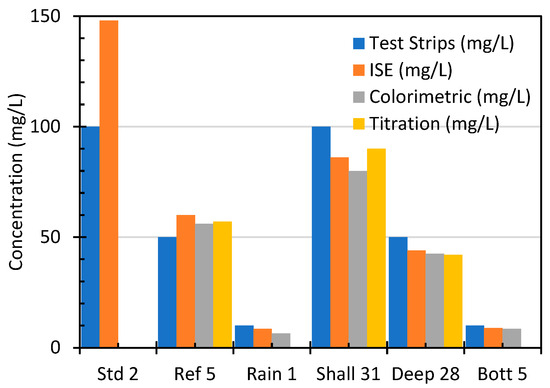
Figure 5.
Comparison of nitrate concentrations across analytical methods (Test Strips, ISE, Colorimetric, Titration) for representative samples.
Test strips, which are convenient for rapid screening, consistently underestimate nitrate levels, particularly in low-concentration samples such as Bott 5, reflecting their limited precision and susceptibility to color interpretation errors. The standard deviation across methods for Bott 5 was 1.2 mg/L, indicating high inter-method agreement. In contrast, the ISE method demonstrated superior sensitivity and produced measurements closely aligned with the expected values, especially for medium-to-high-nitrate samples, such as Shall 31 and Deep 28, confirming its reliability for both field and laboratory applications. The colorimetric method exhibited a strong performance in detecting low nitrate concentrations, making it particularly effective for rainwater and bottled water samples, where trace-level detection is critical. Although titration is time intensive, its high accuracy for samples with elevated nitrate concentrations, as demonstrated by comparative analysis with certified standards, supports its continued use as a reference method. Collectively, these findings underscore the importance of selecting an analytical technique based on target concentration range, required precision, and operational context, thereby supporting the robustness and validity of the methodological framework employed in this study. Our cross-method patterns align with reported strengths of each technique: UV/visible spectrophotometry and Griess-based colorimetry exhibit low LODs and strong linearity for trace to moderate ranges, but require careful interference control [20,24]; ISEs provide rapid, field-deployable measurements with broad linear ranges yet can show calibration-dependent bias at the upper end or in the presence of interfering ions if not compensated [23,31]; classical Devarda-based titration remains a high-accuracy option for elevated concentrations, albeit with greater time and operator demands [25,26]. The observed underestimation by test strips at low concentrations is consistent with their role as screening tools rather than quantitative methods [7,33].
3.2. Nitrate Measurements Using Various Methods
Table 2 presents the nitrate concentration levels in various water samples estimated using four analytical methods: test strips, ISE method, colorimetric methods, and titration methods. To compare the obtained nitrate concentrations, these methods were applied to different sample types, including rainwater, groundwater (shallow and deep), bottled water, and distilled water. The results showed significant variations in nitrate concentration readings across methods, reflecting their sensitivity, specificity, and detection ranges.

Table 2.
The nitrate concentration (mg/L) in various types of water samples estimated using various methods.
As shown in the table, for the 2000 mg/L standard (Type 1, Std 1), ISE provided a close approximation (1963 mg/L), whereas for the Std 2 sample, the 150 mg/L standard was accurately detected by ISE (148 mg/L) and the test strips (100 mg/L), demonstrating their low effectiveness at high nitrate levels. For the reference water (Type 2), while the ISE method successfully detected elevated nitrate concentrations, such as 880 mg/L and 424 mg/L for references 1 and 2, respectively, the associated calibration bias at these points was the highest among the tested ranges, recorded at 12% and 15%. This suggests that, although ISE is effective in identifying high-concentration nitrate levels, its quantitative precision at these concentrations is limited. In contrast, at lower concentrations (for example Ref. Seven at 15 mg/L), all methods showed comparable performances for trace nitrate levels (test strips: 10 mg/L, ISE: 17 mg/L, colorimetric: 12 mg/L, titration: 12 mg/L). For rain water (Type 3), test strips provided consistent but generalized results (10 mg/L for all samples), while ISE and colorimetric methods revealed variability, with ISE detecting higher concentrations (e.g., 8.6–9.4 mg/L) compared to the colorimetric method (4.21–6.48 mg/L). In the case of shallow well water, where only five samples were classified as Type 4, the ISE values varied between 36 mg/L and 342 mg/L, indicating a high level of sensitivity among the samples. The colorimetric method was closely aligned with ISE for samples for which data were available (e.g., Shall 36:35 mg/L vs. 36 mg/L). Titration demonstrated a high accuracy for Shall 36 and Shall 31, validating its precision at high nitrate concentrations. In contrast, for deep well water (only five samples are tabulated in Type 5), ISE detected a range of 14.8 to 44 mg/L, which was closely matched by colorimetric methods. Titration provided consistent results for the samples to which it was applied, confirming its reliability at moderate nitrate levels. Low nitrate levels (<10 mg/L) were detected across all methods, with the colorimetric method distinguishing between lower concentrations (Bott 1:1.4 mg/L and Bott 3:0.8 mg/L). Test strips generalized nitrate detection to 10 mg/L for higher concentrations (e.g., Bott 4 and Bott 5) for bottled water (Type 6). As expected, nitrate levels were minimal across methods, with only the colorimetric method detecting trace amounts (0.8 mg/L) for distilled water (Type 7).
A careful examination of the numerical values in Table 3 shows the variability in accuracy among the methods, particularly for the standard samples with known concentrations. For example, in the standard diluted to 15 mg/L, the measured values deviated as follows: the test strips reported 10 mg/L (−33% bias), ISE yielded 17 mg/L (+11%), the colorimetric method showed 12 mg/L (−20%), and titration measured 12 mg/L (−20%). These biases suggest that, although some methods yield visually similar results, they may significantly diverge from the true value in either direction. This variability persisted in the higher concentration calibration range (15–2000 mg/L). Test strip measurements at 150 mg/L (Std 2), 125 mg/L (Ref 4), 62.5 mg/L (Ref 5), and 31.25 mg/L (Ref 6) showed consistent underestimation, with biases ranging from −20% to −33%. The ISE method demonstrated improved performance at elevated concentrations, with comparatively lower bias (approximately 2–15%), making it a more suitable technique for detecting high nitrate levels. It is noteworthy that, among all the tested samples, Shall 31, Shall 36, Deep 3, Deep 19, Deep 28, and Deep 32 yielded the most comparable results across the ion-selective electrode, colorimetric, and titration methods. These samples displayed low variance between techniques, suggesting a consistent nitrate concentration profile. As such, they were used as benchmarks to assess the inter-method reliability.

Table 3.
The detection range in this study, along with the relative standard deviation (RSD) for the method used.
The above results for nitrate measurements in water samples demonstrate distinct strengths and limitations across different concentration ranges and water types. Test strips, which are useful for initial assessments, lack precision for low and medium nitrate levels, particularly for rainwater and bottled water. Although convenient, test strips are prone to subjective interpretation and limited sensitivity, particularly at low concentrations. The ISE method showed high accuracy and versatility, particularly at higher concentrations, making it suitable for both field and laboratory applications, with regular calibration. The colorimetric method excelled in detecting low nitrate concentrations, proving to be ideal for analyzing trace amounts in bottled water and rainwater. Titration is the most accurate method to obtain high nitrate concentrations, particularly in shallow- and deep-well water samples. However, its time-intensive nature limits its practicality in routine monitoring. Compared to photometric and titration methods, ISE offers faster results, but may suffer from ion interference without proper calibration.
The distribution of nitrate concentrations across different well categories is shown in Figure 6. The nitrate levels in the treated commercial wells were consistently lower, with median values significantly under the WHO threshold of 50 mg/L. In contrast, untreated private domestic wells exhibited the widest variability, including extreme outliers exceeding 350 mg/L, indicating localized hotspots of severe contamination. Agricultural wells displayed persistently elevated values, with interquartile ranges above the WHO threshold. These findings underscore the differential vulnerability of water sources, highlighting the effectiveness of treatment systems and risks associated with shallow untreated private wells.

Figure 6.
Plot of nitrate concentrations by water types. The red circles represent the mean nitrate value of the corresponding wells, and the dotted line represents the limit based on the WHO standard.
These elevated levels are attributable to diffuse and point-source pollution, including nitrogen-based fertilizers, animal manure, and infiltration from poorly maintained septic tanks. Geological and hydrological factors, such as soil porosity, aquifer type, and groundwater flow velocity, also influence nitrate distribution. In contrast, deep wells generally access older and less contaminated water because of the natural filtration and longer residence times in the subsurface.
To assess whether the differences in nitrate concentrations between the treated and untreated wells were statistically significant, one-way ANOVA was performed across well categories (treated commercial, untreated commercial, private domestic, and agricultural). The analysis demonstrated that the treatment status significantly influenced nitrate levels, with results showing F = 18.6 and p < 0.001. Post hoc Tukey tests indicated that treated commercial wells had significantly lower nitrate concentrations than all untreated wells (p < 0.01), whereas differences between untreated private and agricultural wells were not statistically significant (p > 0.05). These results confirm that the treatment substantially reduced nitrate contamination, reinforcing the importance of the water treatment infrastructure.
3.3. Dilution Effect of Reducing the Higher Nitrate Concentration
The dilution process employed for water samples with high nitrate concentrations is an important step in this study, particularly for samples that exceed the detection limits of the measurement instrument. This technique allows for the accurate quantification of nitrate levels, which would otherwise be too high to be measured directly. However, it is important to note that dilution introduces additional considerations and potential sources of error into the analysis. When diluting a sample, the relationship between the original concentration and the diluted concentration follows a linear pattern, provided that proper mixing and accurate measurements of both the sample and diluent are used. This linearity allows the calculation of the original concentration by multiplying the measured concentration in the diluted sample by the dilution factor. However, the dilution process introduces uncertainties and errors in various stages. These may include volumetric errors during pipetting or measurement of the diluent, incomplete homogenization of the diluted sample, or contamination from the diluent or equipment used. Furthermore, as the sample becomes more diluted, the analyte concentration approaches the detection limit of the analytical method, potentially reducing measurement precision and accuracy. Therefore, it was necessary to validate the dilution procedure. Distilled water was used to dilute standard nitrate solutions of 150 mg/L and 2000 mg/L. The concentrations were measured using the ISE method. Figure 7 illustrates the correlation between concentration and dilution ratio.

Figure 7.
Relationship between nitrate concentration and dilution ratios for both standard and diluted samples. The diluted samples were prepared from two standard nitrate solutions: one with a concentration of 150 mg/L (open symbol) and the other with a concentration of 2000 mg/L (solid symbol). The arrow indicates the secondary axis.
The trends for both sets of samples appeared parallel, suggesting that the dilution process was independent of the initial concentration within this range. This demonstrates the accuracy and reproducibility of the method used to prepare dilutions. As the dilution ratio increased, nitrate concentration decreased proportionally. For example, doubling the dilution ratio approximately halves the nitrate concentration, which aligns with expectations for linear dilution. The graph confirms that the method for preparing the diluted samples is reliable and follows a predictable dilution law. Additionally, the results suggested that this procedure can be consistently applied across a range of initial nitrate concentrations.
3.4. Nitrate Concentration as a Function of Reaction Time
The concentration of nitrate in a sample may fluctuate temporally, depending on the specific experimental conditions and reaction kinetics. In the colorimetric method, the nitrate concentration in a sample may decrease over time owing to various processes, such as degradation, oxidation, or reduction. For instance, in a nitrate solution undergoing nitrite reduction, the nitrate concentration diminishes as the reaction progresses and nitrate is converted to nitrite. The rate of this reduction process is influenced by factors such as the concentration of the reducing agent, temperature, and pH. Conversely, if a sample undergoes reduction to nitrite, the nitrate concentration decreases over time as nitrate is converted to nitrite. Similarly, the rate of this oxidation reaction is affected by factors such as the concentration, temperature, and pH. Figure 8 shows the reaction time-dependent nitrate concentrations during the nitridation method immediately after adding and crushing the nitrocol tablet at various times.

Figure 8.
The concentration of nitrate (measured using the YSI colorimetry) varied over time during the reaction. The experiment utilized a reference sample of a standard solution with a nitrate level of 15 mg/L diluted by a factor of 0.3.
The graph demonstrates a power–law relationship, showing a gradual decrease in nitrate concentration over time. This power–law decay exhibited a nonlinear pattern of concentration reduction. In just 60 min, the standard nitrate concentration significantly decreased, eventually dropping to about 20% of its original level. This indicates a significant reduction in the nitrate ion levels over a short period. Additionally, the graph indicates a point in the colorimetric reaction where the color change stabilizes, forming a plateau. This plateau signifies the endpoint of the reaction, beyond which additional time does not significantly alter color. The color intensity at this endpoint was directly correlated with the nitrate concentration in the sample. A plateau is estimated to occur approximately 10 min after the reaction. In contrast to exponential decay, where the rate of change is directly linked to the current concentration, power–law decay features a more complex connection between time and concentration. These findings suggest that the process governing this reduction is not a simple exponential decay but rather involves more complex kinetics, such as the degradation of color over time. Generally, the longer the reaction time, the more intense the color development. However, there is a time limit for the reaction in which the color intensity may begin to plateau or even decrease because of other chemical reactions or degradation of reagents.
Figure 9 shows the absorbance readings of the corresponding nitrate concentration after the addition of the (N, N-diethyl-p-phenylenediamine) DPD No. 1 tablet, with a 20 min reaction time completed, as recommended by the manufacturer. The nitrate concentration over time for a representative Shall 36 sample demonstrated a progressive color change that occurred as the nitrate in the water specimen interacted with the test reagents. The photometric device began to detect the nitrate concentrations at approximately 20 min. Initially, it exhibited a high concentration that decreased exponentially over time. Finally, the concentration stabilized after a reaction time of approximately 40 min. Therefore, following the recommended reaction times specified by test manufacturers, accurate and reliable results are essential. Deviations from the recommended reaction time may lead to inaccurate or inconsistent results. Additionally, it is essential to ensure that the reaction times are the same for all the samples to be compared to obtain meaningful and comparable results.

Figure 9.
The reaction time after DPD No. 1 was added as a function of the nitrate concentration for a typical sample of Shall 36.
The time-dependent deterioration of the reagents in the Shall 36 sample, as shown in Figure 10, revealed notable alterations in both the visual characteristics and chemical makeup of the sample as time progresses. Initially, a typical sample exhibited deep coloration, indicating a strong initial reaction between the reagents and sample components. This intense initial color suggests a high concentration of nitrate or rapid chemical interactions occurring immediately upon mixing. As time progressed, the color changed from red to orange/brown after 30 min. This color shift likely resulted from the degradation of the initial reaction products or the formation of secondary compounds. The change in the hue can be attributed to various factors, as previously mentioned. Furthermore, the instability of reagents in the Shall 36 sample environment highlights the need to develop improved stabilization techniques or alternative reagent formulations that can maintain their integrity over longer periods, ensuring consistent and reliable analytical results across different time points and sample conditions.

Figure 10.
Time-dependent deterioration of reagents for a representative Shall 36 sample: (a) reference sample (left, noncolored) and typical sample (right, colored) immediately prior to photometric measurement and (b) reference sample (left, noncolored) and typical sample (right, colored) 30 min after initial photometer reading.
3.5. Data Accuracy and Precision for the Used Method
The measurement of nitrate using the various methods exhibited different levels of accuracy, precision, and reproducibility (Table 3). These methods exhibit variable statistical performance. To evaluate accuracy, the measured values were compared with certified reference materials, while precision was determined by calculating the relative standard deviation (RSD) from multiple measurements. For example, the test strips showed a precision range of 5–19% RSD and lower accuracy for concentrations near the detection limits. In comparison, titration provided a precision of 1–2% RSD and excellent agreement with known values, highlighting its suitability for high-nitrate samples. Ion-selective electrodes offer improved performance, with detection limits as low as 6 mg/L and a linear range of up to 9900 mg/L. These electrodes generally provide good accuracy and precision of 2–11% RSD) when properly calibrated. Colorimetric methods achieve much lower detection limits (0–90 mg/L) and offer excellent linearity at concentrations of up to 90 mg/L. Their accuracy was typically within ±5%, with a precision of 1–3% RSD.
Several factors can significantly influence the reproducibility of nitrate analysis methods. The sample matrix, which refers to the composition of the sample being analyzed, can affect the accuracy and precision of measurements owing to potential interference from other compounds. Operator skill also plays a crucial role in reproducibility because the proper execution of analytical procedures, including sample handling, reagent preparation, and instrument operation, can significantly affect the consistency of the results. Experienced operators are more likely to identify and mitigate potential sources of errors, leading to more reliable and reproducible measurements.
The reproducibility of the nitrate measurement techniques was validated using selected representative samples (Shall 31, Shall 36, Deep 3, Deep 19, Deep 28, and Deep 32), where measurements from the ISE, colorimetric, and titration methods produced closely matched results, indicating high inter-method reliability. As shown in Table 3, the concentration values from these three methods varied by less than 5% for these samples. This consistency highlights the reproducibility of the results across analytical platforms when standard procedures and calibration protocols are followed.
Instrument calibration is another critical factor affecting the reproducibility of nitrate analysis. Regular and accurate calibration of the analytical instruments ensured that the measurements were consistent with and traceable to the known standards. Variations in the calibration procedures or the use of different calibration standards between laboratories can lead to discrepancies in the results. To address these challenges and improve reproducibility, it is essential to implement standardized protocols and robust quality control measures. These might involve utilizing certified reference materials and engaging in inter-laboratory comparison studies, and the implementation of rigorous training programs for operators.
3.6. Nitrate Concentration in Groundwater
In this study, the Ion-Selective Electrode technique was applied to assess the nitrate levels in both deep and shallow groundwater in Hafr Al Batin. Geographic Information System (GIS) methodologies, particularly the inverse distance weighting (IDW) interpolation technique, were employed to map and analyze the spatial distribution of nitrate concentrations in groundwater wells. Utilizing GIS-based methods improves data visualization and allows for a more accurate and thorough assessment of nitrate concentrations in various geographic areas. The IDW method is generally more straightforward to implement and requires less computational power than the other interpolation techniques, rendering it a practical choice for spatial analysis. This method is particularly effective when sample points are relatively evenly distributed because it assumes that points in close proximity are more closely related than those further apart. The results of this method are depicted in Figure 11 for both deep and shallow wells. Although the number of groundwater samples (n = 45; 32 shallow and 13 deep) may appear modest, sampling was conducted at geospatially representative sites across the Hafr Al Batin region to reflect the hydrogeological diversity and land-use variability. In this study, shallow wells were ~400 m in depth, whereas deep wells were 800 m or deeper. Spatial mapping using Inverse Distance Weighting (IDW) was performed for exploratory visualization of nitrate distribution. As IDW is suitable for interpolating sparse but evenly distributed data, it provides a preliminary insight into spatial nitrate trends. While the number of groundwater samples used in this study (n = 45) may appear modest relative to studies such as Ohlert et al. [34], who employed over 5000 data points, our use of IDW was limited to exploratory visualization rather than statistical modeling or predictive accuracy assessment. The geospatial distribution of our wells ensured representative coverage of different hydrogeological zones in Hafr Al Batin. We acknowledge the limitations posed by a smaller dataset and emphasize the importance of caution when interpreting the interpolated surfaces. Future research should focus on expanding the monitoring network to increase the spatial resolution and facilitate the application of more advanced interpolation techniques, such as kriging.

Figure 11.
The nitrate distribution, determined through the ISE method using a Horiba meter, is depicted for the regions studied, highlighting (a) deep wells and (b) shallow wells.
The IDW method revealed differences in nitrate levels in the deep wells across the studied regions. Notably, the central areas, particularly the river valley, exhibited higher nitrate levels, whereas the northern hilly regions had lower nitrate concentrations. These disparities can be linked to factors such as geology, hydrology, and human activity. Nitrate levels are significantly influenced by natural geological formations, groundwater movement, and proximity to contamination sources including agricultural practices, septic systems, and industrial sites. Moreover, factors such as permeability, porosity, and hydraulic conductivity of aquifers can also influence nitrate concentrations in deep wells.
The spatial distribution of nitrate concentrations in shallow wells presents a markedly different pattern than that in deep wells. Through the application of IDW interpolation, a pronounced north–south gradient was discerned with elevated nitrate levels detected in the northern regions. This spatial discrepancy implies that local environmental factors may play a role in influencing groundwater quality in shallow wells. Notably, the nitrate concentrations in the northern areas exceeded the WHO guidelines, prompting concerns regarding the potential health risks for individuals consuming water from these wells.
In summary, the disparity in nitrate concentrations between deep and shallow wells reveal the intricate relationship among geological, hydrological, and anthropogenic factors that influence groundwater quality. In deep wells, the higher nitrate levels found in central areas, particularly within the river valley, suggest the impact of geological formations that promote nitrate accumulation, groundwater flow patterns that concentrate nitrates in specific locations, and proximity to pollution sources such as agricultural or industrial activities. In contrast, the lower nitrate levels in the hilly northern regions may be attributed to their unique geological composition, reduced human activity, and natural filtration processes. For shallow wells, the north–south gradient, with increased nitrate levels in the northern regions, suggests that localized factors significantly impact groundwater quality, including potential differences in land-use practices and variations in soil characteristics and hydrogeological conditions. Spatial heterogeneity comparable to shallow-well pattern has been documented where land-use intensity and shallow protective cover co-vary, producing localized hotspots that exceed drinking-water limits [35,36]. In semi-arid systems, intermittent recharge and long residence times can further modulate nitrate storage and transport, explaining the lower dispersion typically seen in deeper wells relative to shallow private wells tapping more vulnerable horizons [8,37]. Moreover, the sedimentary rock formations in this region are characterized by a diverse composition, primarily consisting of sandstone, limestone, and interbedded silt layers. This geological arrangement plays a crucial role in determining the movement and distribution of nitrates within the subsurface. Sandstone, known for its high porosity, allows for significant fluid flow and storage capacity. Similarly, limestone, susceptible to carbonate dissolution, can develop secondary porosity through the formation of cavities and channels. These characteristics of both sandstone and limestone contribute to enhanced nitrate mobility, enabling the contaminant to travel more freely through the rock matrix. The exceedance of WHO guidelines in northern shallow wells raises concerns about potential health risks, necessitating immediate mitigation measures, and highlighting the importance of regular monitoring and testing. To address these challenges, it is vital to conduct thorough hydrogeological surveys, enforce stricter regulations on fertilizer use and waste management in high-risk areas, develop targeted remediation strategies, and enhance public awareness of water quality issues and safe water practices. Further research should focus on identifying the specific sources of nitrate contamination in the affected areas and evaluating their impact.
3.7. Advantages and Limitations of Various Methods
Testing kits, ISE methods, colorimetric methods, and titration techniques offer distinct advantages and limitations for nitrate analysis, making them suitable for various applications, based on specific requirements. A condensed overview of method advantages and limitations is presented here; a detailed discussion is provided in Supplementary Materials (Section S3). Each method serves distinct operational needs. Test strips are best for quick field screening, ISE balances speed and sensitivity, colorimetric methods excel in trace detection, and titration remains the gold standard for precision in high-concentration samples. Method selection should align with the specific context, accuracy needs, and resource availability.
3.8. Simple Summary
The choice of the nitrate analysis method depends on the specific requirements of the application, including accuracy, speed, cost, and practicality, as summarized in Table 4. Testing kits are ideal for rapid, low-cost field monitoring, whereas ISEs provide a good balance between speed and sensitivity for on-site application. Colorimetric methods are versatile and precise for moderate concentrations, and titration methods remain the gold standard for high-accuracy laboratory analysis. Tailoring the method to environmental and operational contexts is essential for achieving reliable and meaningful results.

Table 4.
Comparative overview of nitrate measurement methods used in this study, highlighting detection range, accuracy, field suitability, cost, and time efficiency. This summary supports method selection based on operational context and analytical requirements.
4. Conclusions
This study assessed various methods for estimating nitrate concentration in water, including testing kits, ion-selective electrodes, colorimetric techniques, and titration methods. This study demonstrates that no single nitrate measurement method is universally optimal, and each method has distinct advantages and limitations. Testing kits are convenient and provide rapid results, but exhibit limited accuracy and precision. When properly calibrated, ion-selective electrodes offer enhanced accuracy and precision. Colorimetric methods have low detection limits, excellent linearity, and high accuracy, making them ideal for numerous applications. Although less commonly used, titration methods deliver high accuracy and precision, particularly for samples with elevated nitrate concentrations. Therefore, the selection of an appropriate method should be guided by specific requirements and balancing factors, such as accuracy, speed, cost, and practicality in the intended application. Photometric techniques are particularly effective in detecting low nitrate concentrations, whereas colorimetric methods are better suited for routine monitoring in resource-constrained settings.
These findings emphasize the need to select nitrate analysis methods based on environmental conditions, technical requirements, and practical constraints. Continued research is essential to improve the accuracy, sensitivity, and cost-effectiveness of existing techniques while enhancing reproducibility and reliability. By incorporating diverse water types—standard solutions, rainwater, groundwater (shallow and deep), and bottled water—this study provides a comprehensive performance assessment across a wide concentration range. The results offer practical guidance for choosing analytical methods based on sensitivity, cost, and field applicability, while informing the development of simplified and robust testing solutions. Additionally, integrating GIS mapping with analytical data revealed spatial variability in nitrate contamination, underscoring the importance of combining chemical analyses with spatial tools for effective water quality management. Future studies should incorporate nitrite analysis, given its lower permissible limit (0.5 mg/L) and higher toxicity, to provide a more comprehensive health risk assessment. Additionally, explore the integration of machine-learning algorithms with sensor data to enhance real-time nitrate monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/urbansci9110444/s1, Table S1. The categorization of nitrate concentrations in drinking water was based on international guideline thresholds (WHO, US EPA, and the EU). Values above 50 mg/L exceed the maximum permissible limits set by major health agencies, and are considered unsafe for human consumption, particularly for infants. Table S2. lists the parameters and measurement ranges for the test strips. A detailed discussion of method advantages and limitations is provided in Section S3).
Author Contributions
Conceptualization, A.M.; methodology, A.M.; formal analysis, A.M.; investigation, A.M.; data curation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, H.O.S. and S.B.; visualization, A.S.A.; supervision, A.M.; project administration, M.A.; funding acquisition, A.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Institutional Funding Project (IFP-0062-1446-S) of the Ministry of Education, of Saudi Arabia.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFP-0062-1446-S.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study design, data collection, analysis, interpretation, manuscript writing, or decision to publish results.
References
- Brender, J.D.; Weyer, P.J.; Romitti, P.A.; Mohanty, B.P.; Shinde, M.U.; Vuong, A.M.; Sharkey, J.R.; Dwivedi, D.; Horel, S.A.; Kantamneni, J. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- Lin, L.; St Clair, S.; Gamble, G.D.; Crowther, C.A.; Dixon, L.; Bloomfield, F.H.; Harding, J.E. Nitrate contamination in drinking water and adverse reproductive and birth outcomes: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 563. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef] [PubMed]
- Kubisiak-Banaszkiewicz, L.; Żukiewicz-Sobczak, W.; Starek-Wójcicka, A.; Mazur, J.; Sobczak, P. Methods of Assessing Water Quality in Terms of Public Health. Water 2025, 17, 70. [Google Scholar] [CrossRef]
- Aly, A.A.; Al-Omran, A.M.; Alharby, M.M. The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin, Saudi Arabia. Arab. J. Geosci. 2015, 8, 4177–4190. [Google Scholar] [CrossRef]
- Cuartero, M.; Ruiz, A.; Galián, M.; Ortuño, J.A. Potentiometric Electronic Tongue for Quantitative Ion Analysis in Natural Mineral Waters. Sensors 2022, 22, 6204. [Google Scholar] [CrossRef]
- da Ascenção, W.D.; Augusto, C.C.; de Melo, V.H.; Batista, B.L. A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry. Toxics 2024, 12, 383. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2018, 191, 364–381. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Fang, T.; Li, H.; Bo, G.; Lin, K.; Yuan, D.; Ma, J. On-site detection of nitrate plus nitrite in natural water samples using smartphone-based detection. Microchem. J. 2021, 165, 106117. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, H.J.; Kim, J.Y.; Park, S.H.; Cho, W.J. Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes. Sensors 2021, 21, 2703. [Google Scholar] [CrossRef]
- Di Nonno, S.; Ulber, R. Smartphone-based optical analysis systems. Analyst 2021, 146, 2749–2768. [Google Scholar] [CrossRef]
- Yeshno, E.; Dahan, O.; Arnon, S. Real-time monitoring of nitrate in soils as a key for optimization of agricultural productivity and prevention of groundwater pollution. Hydrol. Earth Syst. Sci. 2019, 23, 3997–4010. [Google Scholar] [CrossRef]
- Stoewer, M.M.; Knöller, K.; Stumpp, C. Tracing freshwater nitrate sources in pre-alpine groundwater catchments using environmental tracers. J. Hydrol. 2015, 524, 753–767. [Google Scholar] [CrossRef]
- Mamun, A.; Sharif, H.O. Quantification of Nitrate Level in Shallow and Deep Groundwater Wells for Drinking, Domestic and Agricultural Uses in Northeastern Arid Regions of Saudi Arabia. Limnol. Rev. 2024, 24, 178–191. [Google Scholar] [CrossRef]
- Gentle, B.S.; Ellis, P.S.; Grace, M.R.; McKelvie, I.D. Flow analysis methods for the direct ultra-violet spectrophotometric measurement of nitrate and total nitrogen in freshwaters. Anal. Chim. Acta 2011, 704, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; da S Petruci, J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry–A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Bomar, E.; Owens, G.; Murray, G. Nitrate Ion Selective Electrode Based on Ion Imprinted Poly(N-methylpyrrole). Chemosensors 2017, 5, 2. [Google Scholar] [CrossRef]
- Tang, W.; Ping, J.; Fan, K.; Wang, Y.; Luo, X.; Ying, Y.; Wu, J.; Zhou, Q. All-solid-state nitrate-selective electrode and its application in drinking water. Electrochim. Acta 2012, 81, 186–190. [Google Scholar] [CrossRef]
- Park, S.; Maier, C.S.; Koley, D. Anodic stripping voltammetry on a carbon-based ion-selective electrode. Electrochim. Acta 2021, 390, 138855. [Google Scholar] [CrossRef]
- Causse, J.; Thomas, O.; Jung, A.-V.; Thomas, M.-F. Direct DOC and nitrate determination in water using dual pathlength and second derivative UV spectrophotometry. Water Res. 2016, 108, 312–319. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-g.; Zhu, J.-p.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Sandu, M.; Lupascu, T.; Tarita, A.; Goreacioc, T.; Turcan, S.; Mosanu, E. Method for Nitrate Determination in Water in the Presence of Nitrite. Chem. J. Mold. 2014, 9, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, E.; Nadeau, K.; Mihai, O.; Pihillagawa Gedara, I.; Mester, Z. From sea salt to seawater: A novel approach for the production of water CRMs. Anal. Bioanal. Chem. 2022, 414, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- El-Taher, A.; Al-Turki, A. Radon activity measurements in irrigation water from Qassim Province by RAD7. J. Environ. Biol. 2016, 37, 1299–1302. [Google Scholar]
- El-Taher, A.M.; Abojassim, A.A.; Najam, L.A.; Mraity, H.A.A.B. Assessment of Annual effective Dose for Different Age Groups based on Radon Concentrations in the Groundwater of Qassim, Saudi Arabia. Iran. J. Med. Phys. 2020, 17, 15–20. [Google Scholar] [CrossRef]
- Mamun, A.; Alazmi, A.S. Investigation of Radon in Groundwater and the Corresponding Human-Health Risk Assessment in Northeastern Saudi Arabia. Sustainability 2022, 14, 14515. [Google Scholar] [CrossRef]
- Mishra, P.; Bhawna, J.; Ratnakar, D.S.; Dagdag, O.; Ebenso, E.E.; Kumar Tyagi, M.; Berdimurodov, E.; Verma, D.K.; Patel, R.; Berdimuradov, K.; et al. A review on the determination methods of nitrate and the routes for its removal from environmental samples. Int. J. Environ. Anal. Chem. 2025, 105, 33–79. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Ikwuka, C.F.; Agbasi, J.C.; Nweke, N.D.; Refadah, S.S.; Nwazelibe, V.E.; Uwajingba, H.C.; Abba, S.I.; Khan, M.Y.A. Contamination levels of water sources and the associated nitrate health risks to six age groups. Toxin Rev. 2025, 44, 48–61. [Google Scholar] [CrossRef]
- Jemison, J.M.; Fox, R.H. A quick-test procedure for soil and plant tissue nitrates using test strips and a hand-held reflectometer. Commun. Soil Sci. Plant Anal. 1988, 19, 1569–1582. [Google Scholar] [CrossRef]
- Ohlert, P.L.; Bach, M.; Breuer, L. Accuracy assessment of inverse distance weighting interpolation of groundwater nitrate concentrations in Bavaria (Germany). Environ. Sci. Pollut. Res. 2023, 30, 9445–9455. [Google Scholar] [CrossRef]
- Jia, H.; Qian, H. Groundwater nitrate response to hydrogeological conditions and socioeconomic load in an agriculture dominated area. Sci. Rep. 2025, 15, 1315. [Google Scholar] [CrossRef] [PubMed]
- Nolan, B.T.; Hitt, K.J. Vulnerability of Shallow Groundwater and Drinking-Water Wells to Nitrate in the United States. Environ. Sci. Amp. Technol. 2006, 40, 7834–7840. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 11–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).