Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability
Abstract
1. Introduction
2. Materials and Methods
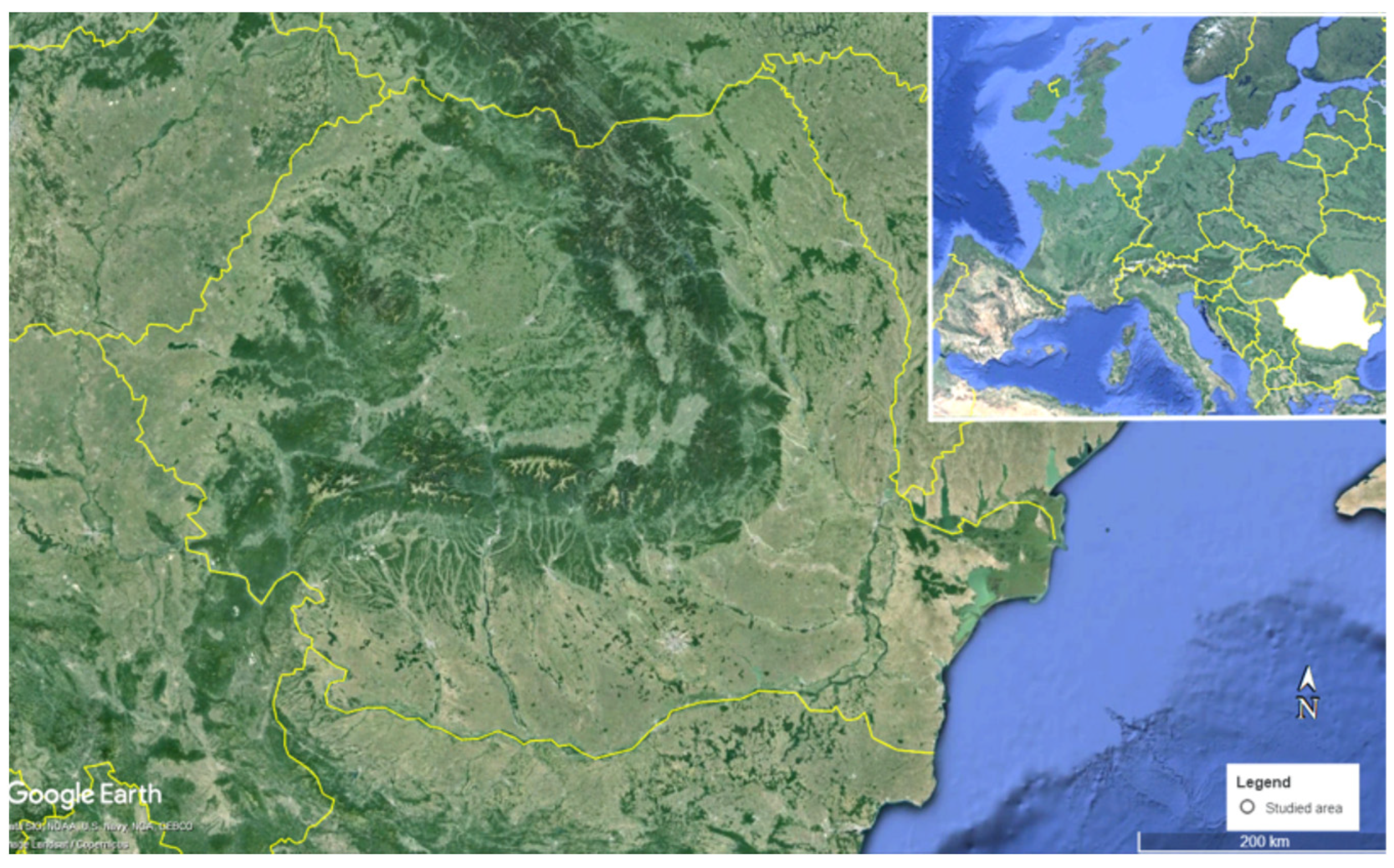
2.1. Area of Study and Sampling Sites
2.2. Field Methods
2.3. Data Analysis
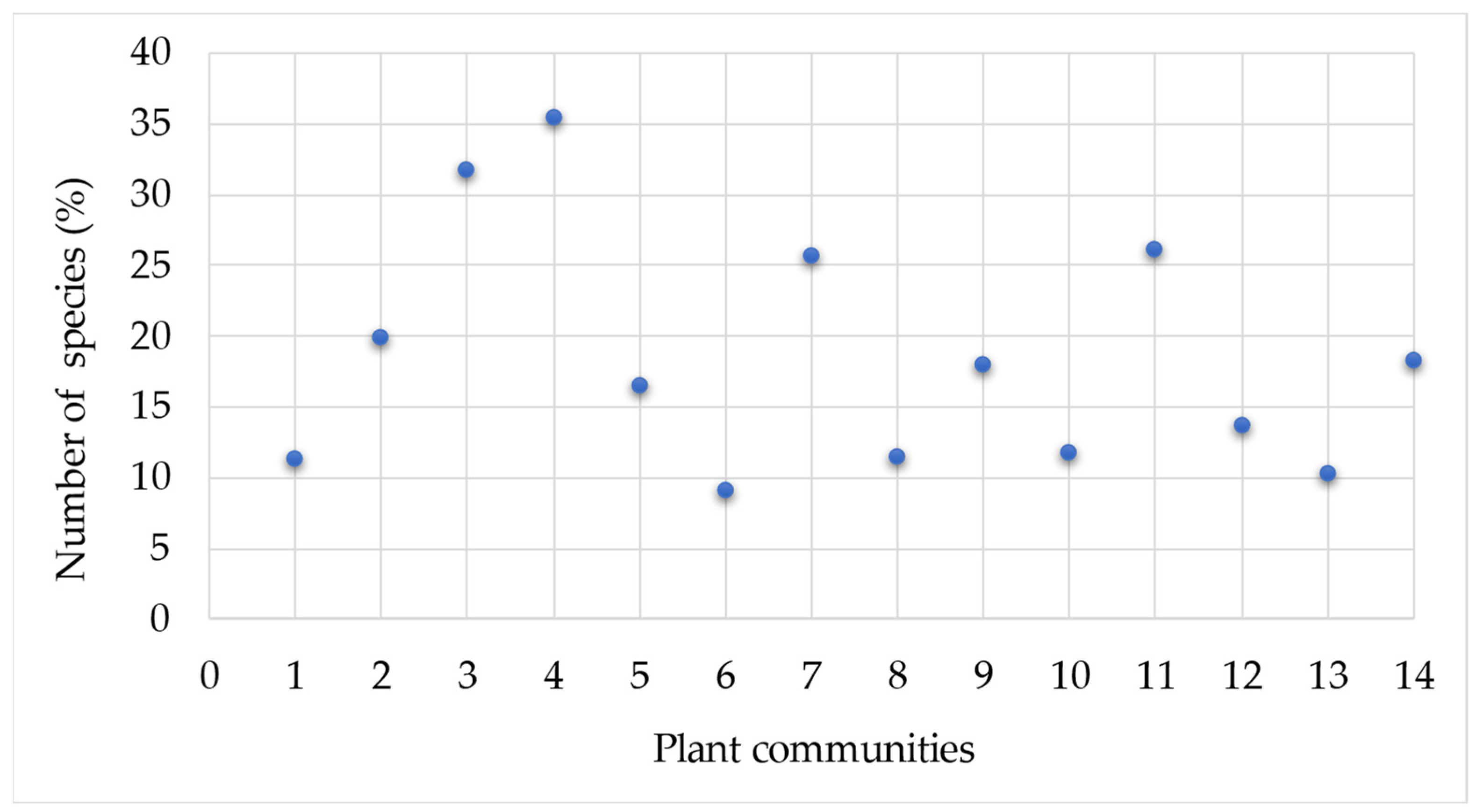
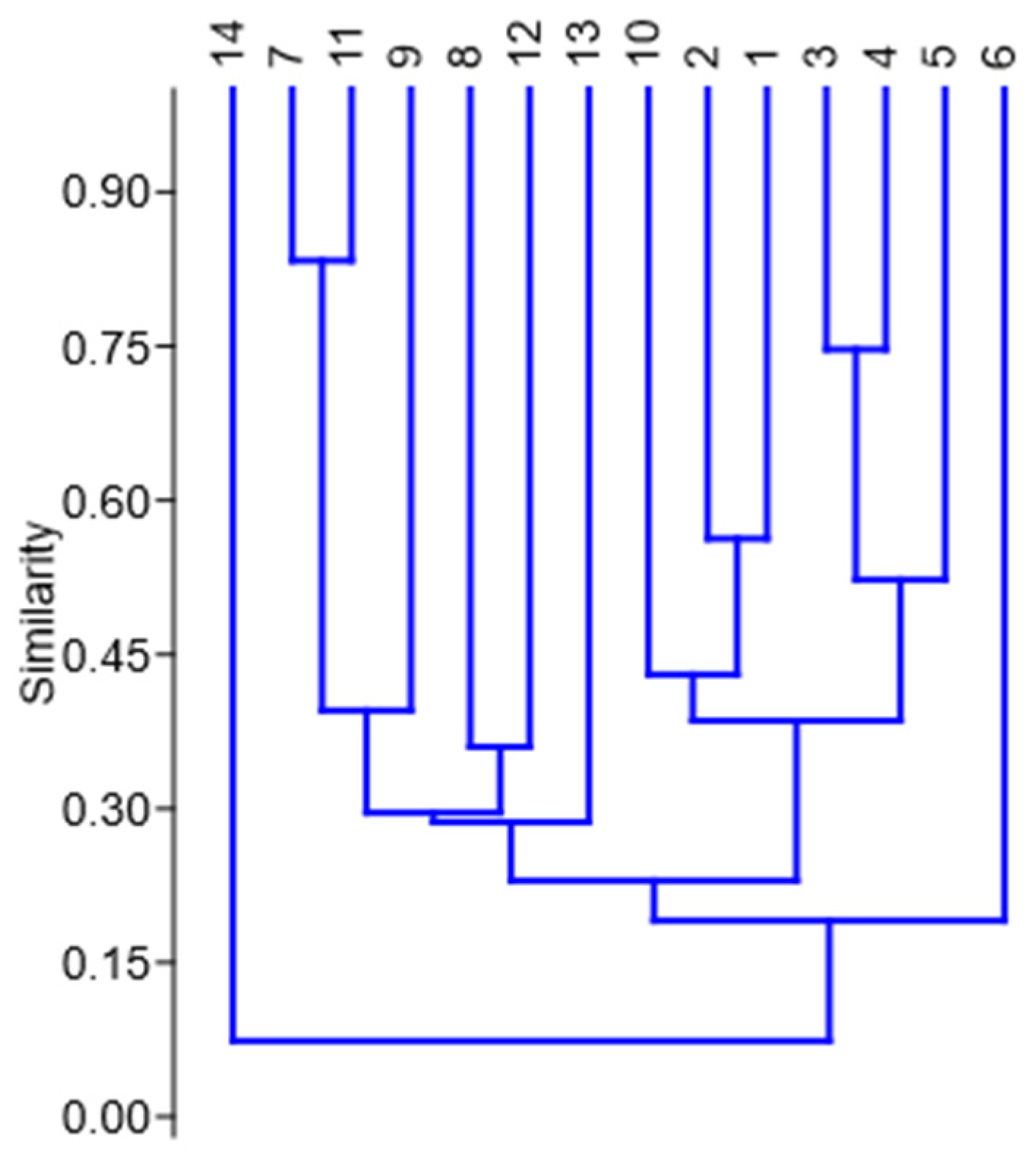
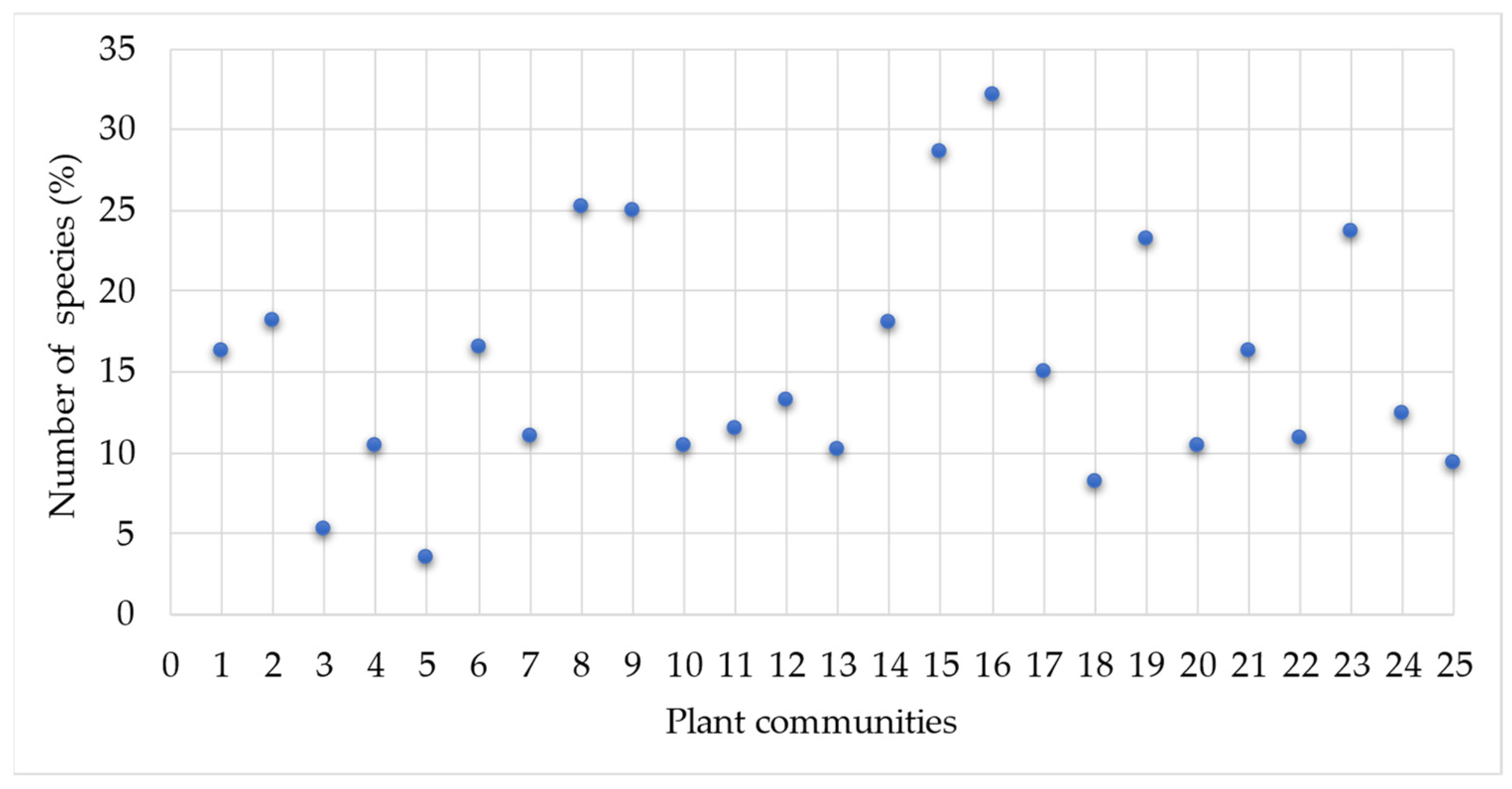
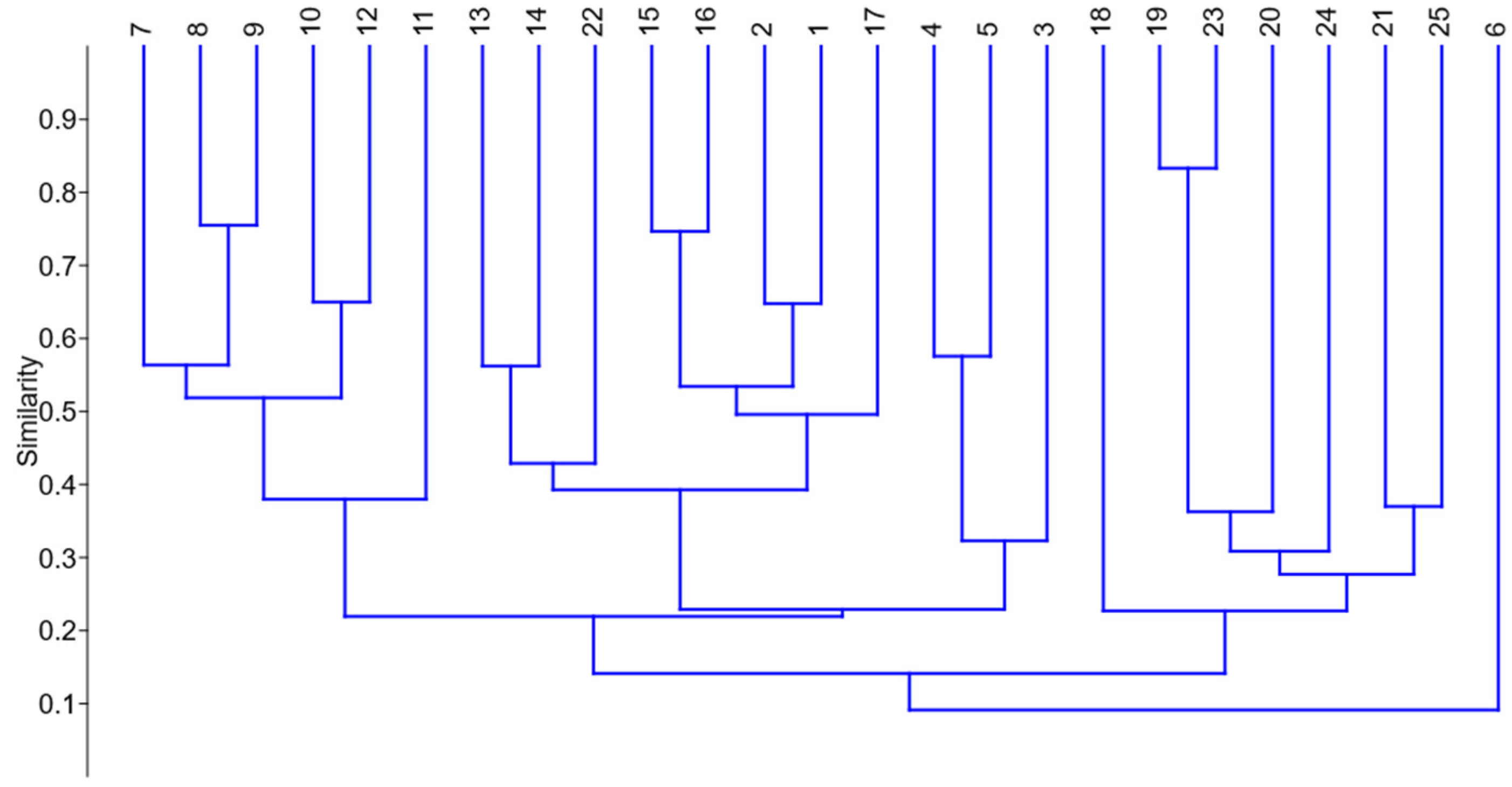
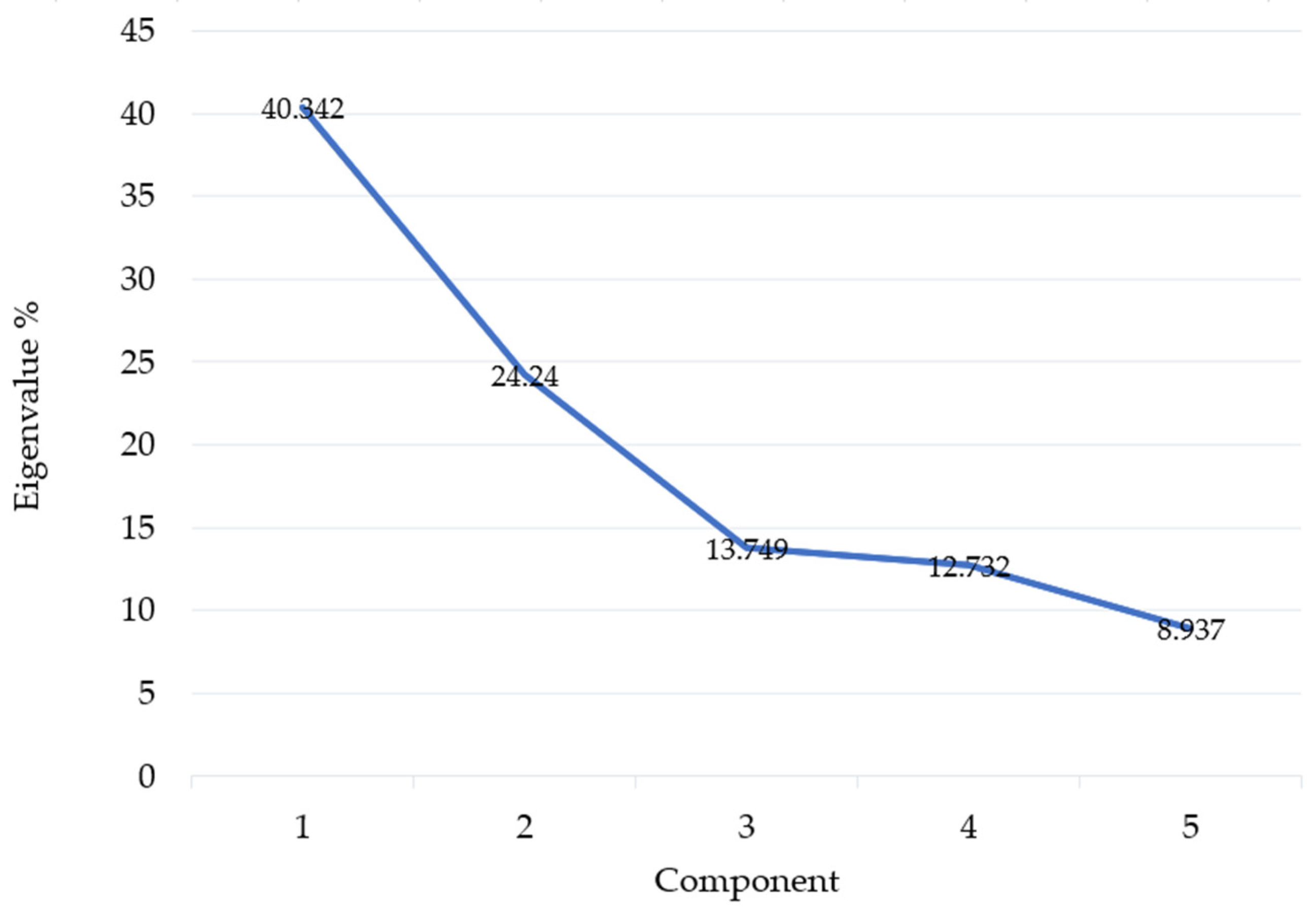
3. Results
Statistical Analysis of Studied Ruderal Communities
4. Discussion
4.1. Statistical Results
4.2. Communities of Studied Ruderal Species
4.3. Assessing Multifunctional Benefits of Community Species
4.4. Limitations and Broader Implications
4.5. Conservation and Urban Biodiversity
5. Conclusions
Conservation Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knapp, S.; Kühn, I.; Mosbrugger, V.; Klotz, S. Do protected areas in urban and rural landscapes differ in species diversity? Biodivers. Conserv. 2008, 17, 1595–1612. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P.; Bastl, M. Spontaneous vegetation succession in human-disturbed habitats: A pattern across seres. Appl. Veg. Sci. 2001, 4, 83–88. [Google Scholar] [CrossRef]
- Medvecka, J.; Jarolimek, I.; Zaliberova, M. Ruderal vegetation of the Horná Orava Region 2. Galio-Urticetea, Epilobietea angustifolii. Thaiszia J. Bot. 2010, 20, 17–52. [Google Scholar]
- Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Protopopova, V.V.; Shevera, M.V. Alien species in the pioneer and ruderal vegetation of Ukraine. Diversity 2022, 14, 1085. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Kalisz, S.; Sujka, K. Effect of the addition of dried dandelion roots (Taraxacum officinale FH Wigg.) on wheat dough and bread properties. Molecules 2021, 26, 7564. [Google Scholar] [CrossRef] [PubMed]
- Radanovic, D.; Antic-Mladenovic, S.; Jakovljevic, M. Influence of some soil characteristics on heavy metal content in Hypericum perforatum L. and Achillea millefolium L. In Proceedings of the International Conference on Medicinal and Aromatic Plants. Possibilities and Limitations of Medicinal and Aromatic Plant, Budapest, Hungary, 8–11 July 2001; pp. 295–301. [Google Scholar]
- Eichelmann, H.; Oja, V.; Rasulov, B.; Padu, E.; Bichele, I.; Pettai, H.; Kasparova, M.I.; Laisk, A. Photosynthetic parameters of birch (Betula pendula Roth) leaves growing in normal and in CO2-and O3-enriched atmospheres. Plant Cell Environ. 2004, 27, 479–495. [Google Scholar] [CrossRef]
- Gebauer, A.D.; Brown, R.; Schwab, S.; Nezat, C.; McNeely, C. Effects of an invasive grass (Phalaris arundinacea) on water availability in semi-arid riparian zones. Wetlands 2016, 36, 59–72. [Google Scholar] [CrossRef]
- Jovanović, S.; Jakovljević, K.; Djordjević, V.; Vukojičić, S. Ruderal flora and vegetation of the town of Žabljak (Montenegro)—An overview for the period 1990–1998. Bot. Serb. 2013, 37, 55–69. [Google Scholar]
- Panitsa, M.; Iliadou, E.; Kokkoris, I.; Kallimanis, A.; Patelodimou, C.; Strid, A.; Raus, T.; Bergmeier, E.; Dimopoulos, P. Distribution patterns of ruderal plant diversity in Greece. Biodivers. Conserv. 2020, 29, 869–891. [Google Scholar] [CrossRef]
- Shem-Tov, S.; Fennimore, S.A. Seasonal changes in annual bluegrass (Poa annua) germinability and emergence. Weed Sci. 2003, 51, 690–695. [Google Scholar] [CrossRef]
- Hurley, P.T.; Emery, M.R. Locating provisioning ecosystem services in urban forests: Forageable woody species in New York City, USA. Landsc. Urb. Plann. 2018, 170, 266–275. [Google Scholar] [CrossRef]
- Pyšek, P.; Chocholousková, Z.; Pyšek, A.; Jarošík, V.; Chytrý, M.; Tichý, L. Trends in species diversity and composition of urban vegetation over three decades. J. Veg. Sci. 2004, 15, 781–788. [Google Scholar] [CrossRef]
- Chiuffo, M.C.; Cock, M.C.; Prina, A.O.; Hierro, J.L. Response of native and non-native ruderals to natural and human disturbance. Biol. Invasions 2018, 20, 2915–2925. [Google Scholar] [CrossRef]
- Yalçinalp, E.; Dihkan, A.; Meral, A.; Akbulut, S. The effects of habitat on the distribution of urban ruderal vegetation. Braz. J. Bot. 2023, 46, 1141–1151. [Google Scholar] [CrossRef]
- Čepelová, B.; Münzbergová, Z. Factors determining the plant species diversity and species composition in a suburban landscape. Landsc. Urb. Plan. 2012, 106, 336–346. [Google Scholar] [CrossRef]
- Jha, R.K.; Nölke, N.; Diwakara, B.N.; Tewari, V.P.; Kleinn, C. Differences in tree species diversity along the rural-urban gradient in Bengaluru, India. Urb. For. Urb. Green. 2019, 46, 126464. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Kuang, S.; He, Y.; Chen, G.; Huang, Y.; Song, C.; Anderson, P.; Łowicki, D. Plant diversity along the urban–rural gradient and its relationship with urbanization degree in Shanghai, China. Forests 2020, 11, 171. [Google Scholar] [CrossRef]
- Kitazawa, T.; Ohsawa, M. Patterns of species diversity in rural herbaceous communities under different management regimes, Chiba, central Japan. Biol. Conserv. 2002, 104, 239–249. [Google Scholar] [CrossRef]
- Güler, B. Plant species diversity and vegetation in urban grasslands depending on disturbance levels. Biologia 2020, 75, 1231–1240. [Google Scholar] [CrossRef]
- Rega-Brodsky, C.C.; Aronson, M.F.J.; Piana, M.R.; Carpenter, E.-S.; Hahs, A.K.; Herrera-Montes, A.; Knapp, S.; Kotze, D.J.; Lepczyk, C.A.; Moretti, M.; et al. Urban biodiversity: State of the science and future directions. Urb. Ecosyst. 2022, 25, 1083–1096. [Google Scholar] [CrossRef]
- Paltineanu, C.; Mihailescu, I.F.; Seceleanu, I.; Dragota, C.; Vasenciuc, F. Using aridity indices to describe some climate and soil features in Eastern Europe: A Romanian case study. Theor. Appl. Climatol. 2007, 90, 263–274. [Google Scholar] [CrossRef]
- Mitrică, B.; Şerban, P.; Mocanu, I.; Grigorescu, I.; Damian, N.; Dumitraşcu, M. Social development and regional disparities in the rural areas of Romania: Focus on the social disadvantaged areas. Soc. Indic. Res. 2020, 152, 67–89. [Google Scholar] [CrossRef]
- Google Earth. 2022. Available online: https://earth.google.com (accessed on 15 May 2024).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Vienna, Austria; New York, NY, USA, 1964; 631p. [Google Scholar]
- World Flora Online. Available online: http://wfoplantlist.org/ (accessed on 20 February 2023).
- Biță-Nicolae, C.; Sanda, V. Cormophlora of Romania: Spontaneous and cultivated Cormophytes in Romania; Lambert Academic Publishing: Saarbrucken, Germany, 2011. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Sanda, V.; Öllerer, K.; Burescu, P. Fitocenozele din România. Sintaxonomie, Structură, Dinamică Şi Evoluţie [Plant Associations of Romania. Syntaxonomy, Structure, Dynamics and Evolution]; Ars Docendi Publishing House, Universitatea din București: București, Romania, 2008. [Google Scholar]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 30 March 2023).
- Sanda, V. Vademecum Ceno-Structural Privind Covorul Vegetal al României (Ceno-Structural Vademecum on the Romanian Vegetation, in Romanian); Vergiliu Press: București, Romania, 2002. [Google Scholar]
- Antoniadis, V.; Shaheen, S.; Stärk, H.-J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.; Baker, A. Metal-Accumulating Plants. In Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment; John Wiley: New York, NY, USA, 2000; p. 6. [Google Scholar]
- Kumar, S.; Singh, R.; Kumar, V.; Rani, A.; Jain, R. Cannabis sativa: A Plant Suitable for Phytoremediation and Bioenergy Production. In Phytoremediation Potential and Bioenergy Production; Kumar, V., Ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Bini, C.; Maleci, L.; Wahsha, M. The impact of former mining activity in the vicinity of an old mercury mine (Vallalta, Northern Italy). In Proceedings of the International Conference on Environment and Renewable Energy, Venice, Italy, 6–8 June 2018. [Google Scholar]
- González, H.; Fernández-Fuego, D.; Bertrand, A.; Sánchez-López, M. Effect of pH and citric acid on the growth, arsenic accumulation, and phytochelatin synthesis in Eupatorium cannabinum L., a promising plant for phytostabilization. Environ. Sci. Pollut. Res. 2019, 26, 26242–26253. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Yu, C.; Peng, X.; Yan, H.; Li, X.; Zhou, Z.; Yan, T. Phytoremediation ability of Solanum nigrum L. to Cd-contaminated soils with high levels of Cu, Zn, and Pb. Water Air Soil Pollut. 2015, 226, 2424. [Google Scholar] [CrossRef]
- Kano, N.; Hori, T.; Zhang, H.; Miyamoto, N.; Anak, D.E.V.; Mishima, K. Study on the behavior and removal of cadmium and zinc using Taraxacum officinale and Gazania under the application of biodegradable chelating agents. Appl. Sci. 2021, 11, 1557. [Google Scholar] [CrossRef]
- D’Arco, M.; Ferroni, L.; Velli, A.; Speranza, M. Growth and spread of native perennial herbaceous species on a green roof. Acta Hortic. 2018, 1215, 237–246. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Eicker, U.; Karimi, S. Plant selection for green roofs and their impact on carbon sequestration and the building carbon footprint. Environ. Chall. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Erwin, J.; Hensley, J. Plants with horticultural and ecological attributes for green roofs in a cool, dry climate. HortScience 2019, 54, 1703–1711. [Google Scholar] [CrossRef]
- Hawke, R. An evaluation study of plants for use on green roofs. Plant Eval. Notes 2015, 38, 1–22. [Google Scholar]
- Jacobs, J.; Beenaerts, N.; Artois, T. Green roofs and pollinators, useful green spots for some wild bee species (Hymenoptera: Anthophila), but not so much for hoverflies (Diptera: Syrphidae). Sci. Rep. 2023, 13, 1449. [Google Scholar] [CrossRef]
- Walters, S.A.; Stoelzle Midden, K. Sustainability of urban agriculture: Vegetable production on green roofs. Agriculture 2018, 8, 168. [Google Scholar] [CrossRef]
- Piana, M.; Carlisle, S. Green Roof Plant Assemblage and Dynamics. In Green Roof Ecosystems; Sutton, R.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 285–310. [Google Scholar]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Hitchmough, J. Naturalistic herbaceous vegetation for urban landscapes. In The Dynamic Landscape; Taylor & Francis: Abingdon, UK, 2004; pp. 172–245. [Google Scholar]
- Frouz, J.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Gail, L.; Andony, M.; Isabella, M.; Aaron, A.; Nathan, M.; Lucas, C. Garden Pollinators and the potential for ecosystem service flow to urban and peri-urban agriculture. Sustainability 2018, 10, 2047. [Google Scholar] [CrossRef]
- Chiuffo, M.C.; MacDougall, A.S.; Hierro, J.L. Native and non-native ruderals experience similar plant–soil feedbacks and neighbor effects in a system where they coexist. Oecologia 2015, 179, 843–852. [Google Scholar] [CrossRef]
- Sukopp, H.; Wurzel, A. Changing climate and the effects on vegetation in central European cities. Arboric. J. 2000, 24, 257–281. [Google Scholar] [CrossRef]
- Hipps, N.A.; Davies, M.J.; Dodds, P.; Buckley, G.P. The effects of phosphorus nutrition and soil pH on the growth of some ancient woodland indicator plants and their interaction with competitor species. Plant Soil 2005, 271, 131–141. [Google Scholar] [CrossRef]
- Rebele, F. Vegetation development on deposit soils starting at different seasons. Plant Ecol. 2008, 195, 1–12. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia, S.; Pesaresi, S. Nitrophilous and ruderal species as indicators of climate change. Case study from the Italian Adriatic coast. Plant Biosyst. 2012, 146, 134–142. [Google Scholar] [CrossRef]
- Crawley, M.J. Timing of disturbance and coexistence in a species-rich ruderal plant community. Ecology 2004, 85, 3277–3288. [Google Scholar] [CrossRef]
- Roem, W.J.; Berendse, F. Soil acidity and nutrient supply ratio as possible factors determining changes in plant species diversity in grassland and heathland communities. Biol. Conserv. 2000, 92, 151–161. [Google Scholar] [CrossRef]
- Morecroft, M.D.; Paterson, J.S. Effects of Temperature and Precipitation Changes on Plant Communities. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Wiley: Hoboken, NJ, USA, 2006; pp. 146–164. [Google Scholar] [CrossRef]
- Tabašević, M.; Jovanović, S.; Lakušić, D.; Vukojičić, S.; Kuzmanović, N. Diversity of ruderal communities in urban environments—A case study from Serbia (SE Europe). Diversity. 2021, 13, 638. [Google Scholar] [CrossRef]
- Myśliwy, M.; Pešić, V. Tall herb fringe vegetation on banks of Montenegrin Rivers as a habitat type of European importance. Water 2023, 15, 3684. [Google Scholar] [CrossRef]
- Biţă-Nicolae, C.; Indreica, A. Artemisietea vulgaris in Romania—An Overview. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 512–518. [Google Scholar] [CrossRef]
- Molina, J.A.; Martín-Sanz, J.P.; Casermeiro, M.Á.; Quintana, J.R. Spontaneous urban vegetation as an indicator of soil functionality and ecosystem services. Appl. Veg. Sci. 2023, 26, e12827. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.; Pywell, R.; Goulson, D.; Nowakowski, M. Comparing the efficacy of agri-environment schemes to enhance bumblebee abundance and diversity on arable farmland. J. Appl. Ecol. 2006, 44, 29–40. [Google Scholar] [CrossRef]
- Pille, L.; Säume, I. The water-sensitive city meets biodiversity: Habitat services of rain water management measures in highly urbanized landscapes. Ecol. Soc. 2021, 26, 23. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, A. The Encyclopedia of Medicinal Plants: An Excellent Guide to Over 500 of the More Well-Known Medicinal Herbs from around the World; Dorling Kindersley: London, UK, 1996. [Google Scholar]
- Tozyo, T.; Yoshimura, Y.; Sakurai, K.; Uchida, N.; Takeda, Y.; Nakai, H.; Ishii, H. Novel antitumor sesquiterpenoids in Achillea millefolium. Chem. Pharm. Bull. 1994, 42, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Marinas, I.; Oprea, E.; Gaboreanu, D.; Pircalabioru, G.; Buleandra, M.; Nagoda, E.; Badea, I.; Chifiriuc, M. Chemical and biological studies of Achillea setacea herba essential oil—First report on some antimicrobial and antipathogenic features. Antibiotics 2023, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Janda-Milczarek, K.; Styburski, D.; Lukomska, A. Goutweed (Aegopodium podagraria L.)—Botanical characteristics and prohealthy properties. Postęp. Hyg. Med. Dośw. 2020, 74, 28–35. [Google Scholar] [CrossRef]
- Pawłowska, K.; Hałasa, R.; Dudek (Jamróz), M.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and anti-inflammatory activity of bistort (Bistorta officinalis) aqueous extract and its major components. Justification of the usage of the medicinal plant material as a traditional topical agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Bragina, O.; Kuhtinskaja, M.; Reile, I.; Laanet, P.-R.; Kulp, M.; Vaher, M. Extraction and fractionation of bioactives from Dipsacus fullonum L. leaves and evaluation of their anti-Borrelia activity. Pharmaceuticals 2022, 15, 87. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Aggarwal, G.; Kaur, G.; Bhardwaj, G.; Mutreja, V.; Sohal, H.S.; Nayik, G.A.; Bhardwaj, A.; Sharma, A. Traditional uses, phytochemical composition, pharmacological properties, and the biodiscovery potential of the genus Cirsium. Chemistry 2022, 4, 1161–1192. [Google Scholar] [CrossRef]
- Botalova, A.; Bombela, T.; Zubov, P.; Segal, M.; Korkotian, E. The flavonoid acetylpectolinarin counteracts the effects of low ethanol on spontaneous network activity in hippocampal cultures. J. Ethnopharmacol. 2019, 229, 22–28. [Google Scholar] [CrossRef]
- Radulović, N.S.; Mladenović, M.Z.; Vukićević, D.R.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Boylan, F. Pulicaria dysenterica (L.) Bernh.—Rightfully earned name? Identification and biological activity of new 3-methoxycuminyl esters from P. dysenterica essential oil. Plants 2022, 11, 3340. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and identification of compounds from bioactive extracts of Taraxacum officinale Weber ex F. H. Wigg. (Dandelion) as a potential source of antibacterial agents. Evid. Based Complement. Alternat. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef]
- Kong, Y.D.; Qi, Y.; Cui, N.; Zhang, Z.H.; Wei, N.; Wang, C.F.; Zeng, Y.N.; Sun, Y.P.; Kuang, H.X.; Wang, Q.H. The traditional herb Polygonum hydropiper from China: A comprehensive review on phytochemistry, pharmacological activities and applications. Pharm. Biol. 2023, 61, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Najafian, Y.; Hamedi, S.S.; Farshchi, M.K.; Feyzabadi, Z. Plantago major in traditional Persian medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Pol, M.; Schmidtke, K.; Lewandowska, S. Plantago lanceolata—An overview of its agronomically and healing valuable features. Open Agric. 2021, 6, 479–488. [Google Scholar] [CrossRef]
- Budzianowska, A.; Derda, M.; Budzianowski, J.; Szopa, A.; Kikowska, M. Comparative study of Plantago media extracts in the treatment of Acanthamoeba sp. Trophozoites. Appl. Sci. 2023, 13, 7075. [Google Scholar] [CrossRef]
- Kenari, H.M.; Kordafshari, G.; Moghimi, M.; Eghbalian, F.; TaherKhani, D. Review of pharmacological properties and chemical constituents of Pastinaca sativa. J. Pharmacopunct. 2021, 24, 14–23. [Google Scholar] [CrossRef]
- Shabih, S.; Hajdari, A.; Mustafa, B.; Quave, C.L. Chapter 3—Medicinal Plants in the Balkans with Antimicrobial Properties. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 103–138. ISBN 9780323909990. [Google Scholar]
- Denisow, B. Pollen Production of Selected Ruderal Plant Species in the Lublin Area; University of Life Sciences in Lublin Press: Lublin, Poland, 2011; Volume 351, p. 86. [Google Scholar]
- Nichols, R.N.; Goulson, D.; Holland, J.M. The best wildflowers for wild bees. J. Insect Conserv. 2019, 23, 819–830. [Google Scholar] [CrossRef]
- Denisow, B.; Wrzesień, M. The anthropogenic refuge areas for bee flora in agricultural landscape. Acta Agrobot. 2007, 60, 147–157. [Google Scholar] [CrossRef][Green Version]
- Boix-Fayos, C.; Martínez-Mena, M.; Arnau-Rosalén, E.; Calvo-Cases, A.; Castillo, V.; Albaladejo, J. Measuring soil erosion by field plots: Understanding the sources of variation. Earth Sci. Rev. 2006, 78, 267–285. [Google Scholar] [CrossRef]
- Jim, C.Y. Sustainable urban greening strategies for compact cities in developing and developed economies. Urb. Ecosyst. 2013, 16, 741–761. [Google Scholar] [CrossRef]
- Kleyer, M. Enhancing landscape planning: Vegetation-mediated ecosystem services predicted by plant traits. Landsc. Urb. Plan. 2021, 215, 104220. [Google Scholar] [CrossRef]
- Palliwoda, J.; Banzhaf, E.; Priess, J.A. How do the green components of urban green infrastructure influence the use of ecosystem services? Examples from Leipzig, Germany. Landsc. Ecol. 2020, 35, 1127–1142. [Google Scholar] [CrossRef]
- Jansen, J.; Hobohm, C. Urban Habitats: Cities and Their Potential for Nature Protection. In Perspectives for Biodiversity and Ecosystems; World Bank Group: Washington, DC, USA, 2021; pp. 425–447. [Google Scholar]
- Mogîldea, E.D.; Mitoi, M.E.; Biță-Nicolae, C.; Murariu, D. Urban flora riches: Unraveling metabolic variation long altitudinal gradients in two spontaneous plant species. Plants 2024, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Sukopp, H. Human-caused impact on preserved vegetation. Landsc. Urb. Plan. 2004, 68, 347–355. [Google Scholar] [CrossRef]
- Kowarik, I.; Fischer, L.K.; Kendal, D. Biodiversity conservation and sustainable urban development. Sustainability 2020, 12, 4964. [Google Scholar] [CrossRef]

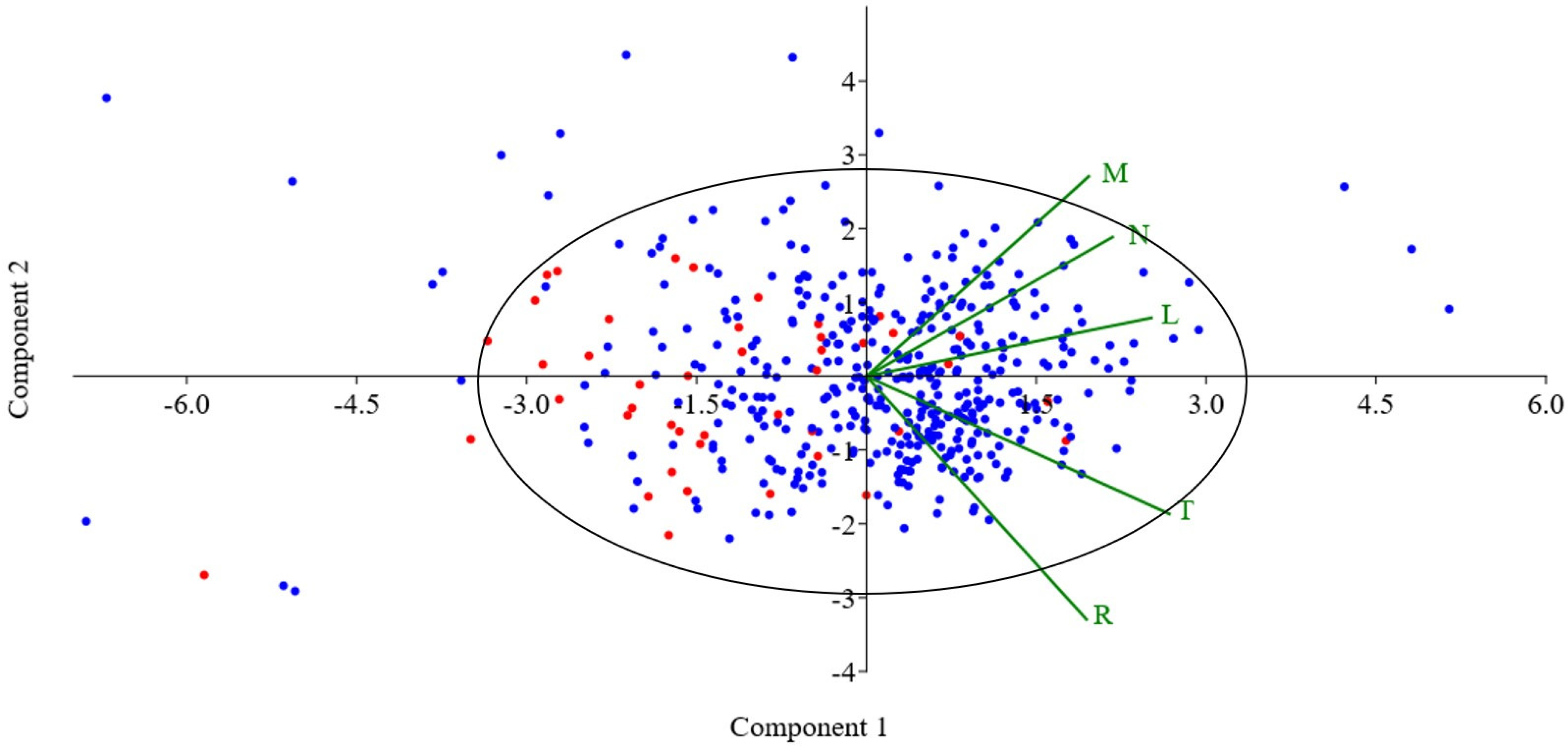
| Environment Index | Principal Component 1 | Principal Component 2 |
|---|---|---|
| M | 0.38619 | 0.53236 |
| N | 0.42751 | 0.37052 |
| R | 0.38224 | −0.64835 |
| L | 0.49499 | 0.15559 |
| T | 0.52627 | −0.36709 |
| Species | Use in Phytoremediation | Reference |
|---|---|---|
| Achillea millefolium | high Cd and Zn accumulation | Antoniadis et al., 2021 [32] |
| Agrostis stolonifera subsp. stolonifera | Hyperaccumulator Pb | Reeves, R. and Baker, A., 2000 [33] |
| Arrhenatherum elatius | high Cd and Zn accumulation; hyperaccumulation Pb | Antoniadis et al., 2021; Reeves, R. and Baker, A., 2000 [32,33] |
| Artemisia vulgaris | high Cd and Zn accumulation | Antoniadis et al., 2021 [32] |
| Bromopsis inermis | high Cd and Zn accumulation | Antoniadis et al., 2021 [32] |
| Cannabis sativa | Removal of environmental contamination by phytoremediation Cd, Cr, Ni, Pb, Fe | Kumar, Sanjeev et al., 2017 [34] |
| Chaerophyllum hirsutum | high Hg levels in roots | Bini et al., 2018 [35] |
| Eupatorium cannabinum | accumulated a high amount of As in its roots | González et al., 2019 [36] |
| Galium mollugo | high Cd and Zn accumulation | Antoniadis et al., 2021 [32] |
| Lolium perenne | Ni, Co, and Fe phytoextraction | Kafle et al., 2022 [37] |
| Rumex acetosa | Hyperaccumulation of Zn, Pb | Reeves, R. and Baker, A., 2000 [33] |
| Silene vulgaris | high Cd and Zn accumulation | Antoniadis et al., 2021 [32] |
| Solanum nigrum | Strong ability to accumulate Cd | Yu et al., 2015 [38] |
| Stellaria holostea | high Cd and Zn accumulation | Antoniadis et al., 2021 [33] |
| Taraxacum sect. Taraxacum | suitable for phytoremediation of Cd | Kano et al., 2021 [39] |
| Species | Use for Green Roof | Reference |
|---|---|---|
| Achillea millefolium | yes | D’Arco et al., 2022; Seyedabadi et al., 2021 [40,41] |
| Ajuga reptans | cool, dry climate | Erwin and Hensley, 2019 [42] |
| Antennaria dioica | score 5 of 5 * | Hawke R., 2015 [43] |
| Bellis perennis | yes | Seyedabadi et al., 2021 [41] |
| Calamagrostis epigejos | yes | Jacobs et al., 2023 [44] |
| Cichorium intybus | yes | Walters et al., 2018 [45] |
| Equisetum arvense | yes | Jacobs et al., 2023 [44] |
| Galium odoratum | yes | Seyedabadi et al., 2021 [41] |
| Galium verum | score 4 of 5 * | Hawke R., 2015 [43] |
| Geranium robertianum | yes | Jacobs et al., 2023 [44] |
| Leucanthemum vulgare | score 3 of 5 * | Hawke R., 2015 [43] |
| Lotus corniculatus | yes | Piana and Carlisle, 2015 [46] |
| Medicago lupulina | yes | Jacobs et al., 2023 [44] |
| Sonchus asper | yes | Jacobs et al., 2023 [44] |
| Sonchus oleraceus | yes | Jacobs et al., 2023 [44] |
| Teucrium chamaedrys | yes | Jacobs et al., 2023 [44] |
| Trifolium arvense | yes | Jacobs et al., 2023 [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogîldea, D.; Biță-Nicolae, C. Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability. Urban Sci. 2024, 8, 159. https://doi.org/10.3390/urbansci8040159
Mogîldea D, Biță-Nicolae C. Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability. Urban Science. 2024; 8(4):159. https://doi.org/10.3390/urbansci8040159
Chicago/Turabian StyleMogîldea, Daniela, and Claudia Biță-Nicolae. 2024. "Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability" Urban Science 8, no. 4: 159. https://doi.org/10.3390/urbansci8040159
APA StyleMogîldea, D., & Biță-Nicolae, C. (2024). Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability. Urban Science, 8(4), 159. https://doi.org/10.3390/urbansci8040159






