Abstract
In this study, the physiological response of a sensitive lichen species (Evernia prunastri) exposed for three months in a complex urban area (Milan, Italy) was evaluated in order to verify if the air pollution abatement measures adopted over the years resulted in a suitable air quality for the survival of this sensitive species. Parameters investigated rely on the photosynthetic activity of the photobiont (Fv/Fm, PIabs, and OJIP curves), damage to mycobiont (membrane damage and antiradical activity), and the production of secondary metabolites involved in the protective functions of the organisms. Results showed that although air quality in Milan still suffers from heavy pollution from PM and NOx, the overall situation is not as severe as to induce the death of this sensitive biomonitor, at least in the short term. Nevertheless, the vital status of the samples exposed in the study area showed a significant impairment compared to that of samples exposed in a control area, indicating that the current air quality in Milan still prevents the optimal survival of E. prunastri.
1. Introduction
Air pollution in urban areas is of great concern worldwide, being well documented to cause a wide array of diseases and premature deaths [1]. The main emission sources in urban environments are vehicular traffic and heating systems, which emit a mixture of contaminants, among which potentially toxic elements (PTEs), which are a consistent part of PM10 and are known to cause serious health injuries [2,3,4]. To abate air pollution and improve air quality, many policies have been addressed both at local and national levels, such as the ban of leaded gasoline, the promotion of ecofriendly vehicles, and the creation of pedestrian areas [5]. Monitoring of air quality may, thus, serve both as a routine control and for the assessment of the success of any environmental policy. However, chemical-physical measurement of air contaminants provided by monitoring devices is not sufficient for evaluating the overall effect of pollutants on the ecosystem. Biological monitoring (biomonitoring) complements this approach by providing biological information about the effect and the interaction with contaminants [6].
Lichens are the best bioindicators of air pollution owing to their well-known sensitivity to phytotoxic gases such as SO2 and NOx [7] and their capacity to bioaccumulate PTEs reflecting atmospheric deposition [8]. Their use is so accepted that they are employed also for environmental justice assessment [9] and in environmental forensics [10]. The effects of air pollution are quickly indicated by changes in physiological parameters such as alteration of cell membrane permeability [11,12] impairment of the photosynthetic apparatus of the photobiont [13], damage to DNA [14], and peroxidation of membrane lipids [15], as well as changes in the level of some specific molecule, e.g., antioxidants [16]. Since these alterations appear long before a visible damage to the entire organisms is apparent, they are comprehensively referred to as “early warning signals” and have been widely used to assess the improvement or worsening of air quality in short-term studies [7,11,12,17,18]. On the other hand, the bioaccumulation of trace elements far above their nutritional requirements allows lichens to be extensively used in determining the patterns of metal deposition in urban and industrial areas [9,19].
This study aims to evaluate the biological effects of air pollution on some physiological parameter of lichens in a complex and large urban area that promoted over many years many pollution abatement measurements and air quality improvement strategies, in order to verify whether current air quality conditions are compatible with the survival of sensitive bioindicators.
2. Materials and Methods
2.1. Study Area
The study was carried in the municipal area of Milan (N Italy), one of the most important cities in Italy. Milan (1.4 million inhabitants) is located in the Po valley, a huge plain characterized by massive urbanization and a continental-like climate, with the stagnation of air masses and frequent thermal inversion making this area among the most polluted in Europe [20]. Over the last years, the administrators of Milan addressed several policies to improve air quality, such as the institution of pedestrian areas (the so-called “Ecopass area“ in 2008, that then turned in ”Area C” in 2012, which is a traffic-limited area in the city center, and the “Area B” in 2019 that coincides with the municipality borders and is prohibited for obsolete vehicles), the limitation of obsolete vehicles circulation [21,22], and the promotion of ecofriendly transports, among others [23].
2.2. Lichen Material
The lichen Evernia prunastri (L.) Ach. was chosen for this study owing to its sensitivity to air pollution and its great capacity to bioaccumulate PTEs, which made this species widely used in biomonitoring surveys [19,24,25]. To obtain a homogeneous pool of samples, the transplant technique was selected. The lichen material was harvested from a remote area of central Italy (43°07′27″ N, 11°18′40″ E), far from any local source of air pollution, selecting apparently healthy thalli of similar size (i.e., similar age [26]) from tree branches at ca. 1.5 m above the ground. Before the exposure, samples were rid of extraneous material with plastic tweezers and arranged in lichen bags made of plastic net loosely wrapped. The material was kept inside a climatic chamber at temperature = 16 °C, relative humidity = 65%, illumination = 40 µmol m−2 s−1 photosynthetic photon flux density with photoperiod of 12 h until transplantation, that occurred within 1 week. The lichen samples were exposed during the winter period (1 December 2018–28 February 2019) to ensure the highest metabolic activity of the organisms.
2.3. Sampling Design
Samples were transplanted in the study area according to a stratified sampling design based on the distance from the city center. Three concentric belts were taken into account (“Central”, “Semi-periphery”, and “Periphery”), and a total of 50 sampling sites were investigated (Figure 1). A control site was selected in a remote hilly location 50 km N of Milan (45°51′46″ N, 9°25′54″ E). In each site, 3 samples were transplanted at 2 m above the ground. After the exposure, the material was retrieved, transported back to the laboratory, air-dried, and stored at −20 °C until the analysis [27]. The results of a previous study in the same area [28] showed a severe bioaccumulation of Cr, Cu, Fe, Pb, and Sb and a clear decreasing trend from the central area (more polluted) to the peripheral area (less polluted).
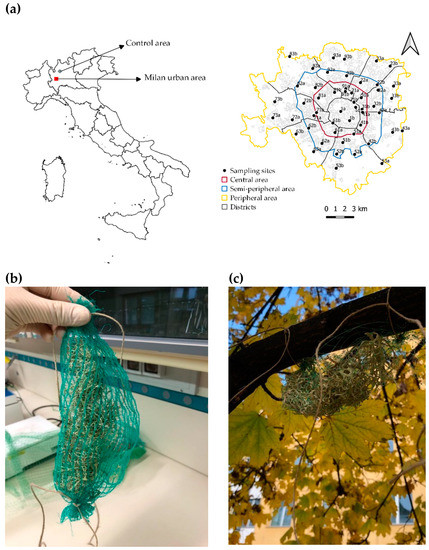
Figure 1.
(a) Study area with indication of sampling sites. Milan municipality is represented with the three concentric belts designed for the stratified sampling design. The red square on the map of Italy (left side) indicates the location of Milan municipality; (b) A lichen-bag before the exposure; (c) a lichen bag transplanted in the study area.
2.4. Physiological Parameters
Photosynthetic efficiency, cell membrane integrity, and total antioxidant activity were selected as response physiological parameters, being among the most commonly used parameters in biomonitoring studies, since they are quickly affected by environmental changes, providing an accurate overview of the biological effects of air pollution.
2.4.1. Photosynthetic Efficiency
Environmental stress can severely impair photosynthetic activity [29] with consequences on the vitality of the organism. A rapid test to assess the functionality of the photosynthetic apparatus consists of evaluating the chlorophyll a fluorescence emission, through the measurements of 2 parameters: Fv/FM and performance index (PIabs). Samples were previously humidified and kept overnight in a climatic chamber to fully recover the vital status [30]. Samples were dark-adapted for 10 min using a clip and then lightened with a saturating red light for 1 s (650 nm, 3000 µmol photons m−2s−1) with a light-emitting diode (LED). Measurements of chlorophyll a fluorescence were carried out with a plant efficiency analyzer fluorimeter (Hand PEA, Hansatech, Norfolk, UK). At T0, all the reaction centers of PSII are opened, and the fluorescence is minimum (F0), along with time the centers close until the fluorescence is maximum (Fm), when all the centers are closed. The ratio Fv/Fm (where Fv = Fm − F0) is an indicator of the maximum quantum yield of PS II. The PIabs summarizes the contribution of all parameters involved in the functionality of the electron flow through PSII; thus, it is used as a global indicator of the photosynthetic performance [29]. The transients of chlorophyll fluorescence (OJIP curves) were plotted on a logarithmic scale as an integrative analysis of the status of the photosynthetic apparatus [31,32].
2.4.2. Cell Membrane Integrity
The alteration of membrane permeability to electrolytes is a reliable indicator of mycobiont stress [12] and can be easily assessed with the electrical conductivity test. About 50 mg of each sample was washed for 3 s with deionized water to remove superficial dust and then soaked in 50 mL of deionized water for 1 h, and the conductivity of the water was recorded through a conductivity meter (Crison Basic 30). Samples were then stored at −20 °C overnight to induce the maximum damage to membranes and then newly soaked in 50 mL of deionized water for 1 h recording the electrical conductivity. The percentage of membrane damage (MD%) was expressed as the ratio between the first soaking (1 h) and the second (1 h after freezing).
2.4.3. Antioxidant Activity
The DPPH assay is a simple and functional spectrophotometric method to evaluate the response of the total antioxidant activity after the exposure to stressful environmental conditions. Samples of ca. 50 mg were homogenized in 1 mL of a solution of ethanol/water (80:20; v/v), and 100 µL of the homogenate was added to 1 mL of a 100 µM DPPH solution prepared by dissolving 3.9 mg of this compound in 100 mL of methanol/water (80:20; v/v) solution. The reaction occurs in 1 h, and then the samples were read at 517 nm, and the results were expressed as % antiradical activity (ARA%) according to the formula:
where controlabsorbance = the absorbance of the reagents only.
2.5. Assessment of Secondary Metabolites Production
A total of 500 mg of sample was treated with 5 mL of dichloromethane and left under stirring for 24 h. The solid fraction was then filtered off, and the solvent evaporated under the nitrogen stream. The residue was analyzed through GC-MS analysis. In total, 0.5 mg of the residue was dissolved in 1 mL of CH2Cl2 (HPLC purity) and analyzed using an ISQ™ QD single quadrupole GC-MS (Thermo Fisher) and an Agilent technology VF-5ms (30 m × 0.25 mm i.d. × 0.25 µm) GC column.
Parameters used in the GC oven were as follows: 50 °C held for 2 min, 50–120 °C at 10 °C/min, 120 °C held for 5 min, 120–300 °C at 10 °C/min, and 300 °C held for 10 min.
Carrier gas helium (purity ≥ 99.999%) with a flow rate of 1.2 mL/min, injection temperature 280 °C, injection volume of 1 µL, and a split flow of 6.0 mL/min.
MS apparatus transfer line and ion source temperatures were set at 270 °C with a delay time of 5 min. The m/z range was set between 45 and 1000.
A blank analysis was performed with CH2Cl2 alone.
2.6. Statistical Analysis
Data normality was checked with the Shapiro–Wilk test (p < 0.05). The significance of differences between control and exposed samples according to concentric belts was checked with one-way analysis of variance (ANOVA) using the Tukey test for post hoc comparisons (p < 0.05). Physiological data were also compared with the accumulation of PTEs in the same samples measured by [28], using the Pearson correlation coefficient.
To produce distribution maps of the physiological parameters investigated, the deterministic IDW (inverse distance weight) interpolation algorithm and the QGIS 3.8 software were used.
3. Results
Figure 2 shows the distribution pattern of the physiological parameters investigated. Disentangling the ecophysiological response according to concentric belts (Figure 3), statistically significant (p < 0.05) differences emerged only with control samples for all the investigated parameters, while values measured within the three belts were overall similar. On average, the photosynthetic performance (Fv/FM) was 10% lower in exposed samples compared to in control samples, and the antioxidant activity (ARA%) and cell membrane damage (MD%) were 11% and 38% higher in exposed than in control samples, respectively.
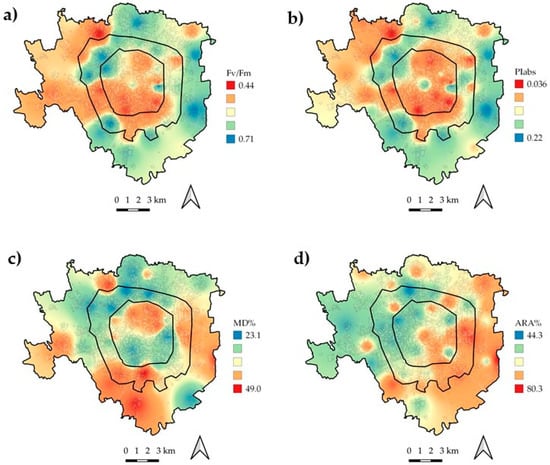
Figure 2.
Distribution maps of the physiological parameters over the study area. (a) Fv/FM, (b) PIABS, (c) MD%, and (d) ARA%.
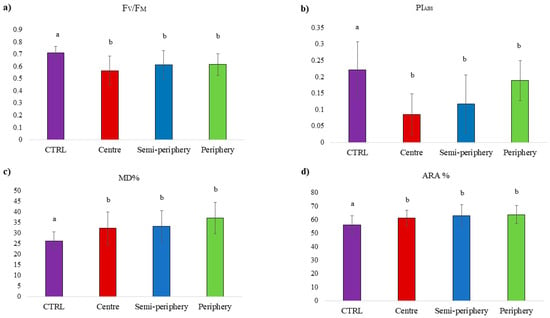
Figure 3.
Ecophysiological parameters (mean ± standard error) in control samples (CTRL) and in samples exposed in the three concentric belts. Different letters indicate statistically significant (p < 0.05) differences. (a) Fv/FM, (b) PIABS, (c) MD%, and (d) ARA%.
Compared with control samples, the OJIP transient curves of exposed samples (Figure 4) showed an evident flattening, while controls showed the classical shape of unstressed plants [29].
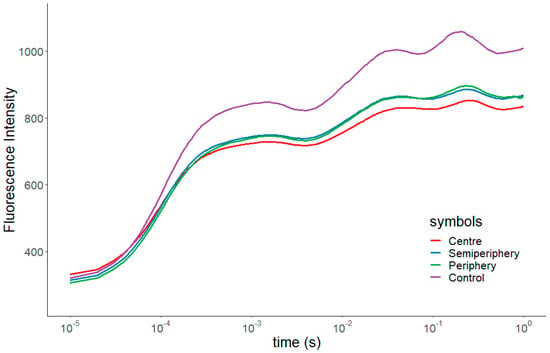
Figure 4.
OJIP transients in control samples and in samples exposed in the three concentric belts.
GC-MS chromatograms of control (Figure 5a) and exposed (Figure 5b) samples showed a similar chromatographic response, indicating a similar chemical composition. The metabolites detected are commonly found in lichens [33,34]. The main difference between control and exposed samples was the higher quantity of ethyl chlorohematommate in exposed samples, as highlighted by the peak at 17.16 min (Figure 5b).
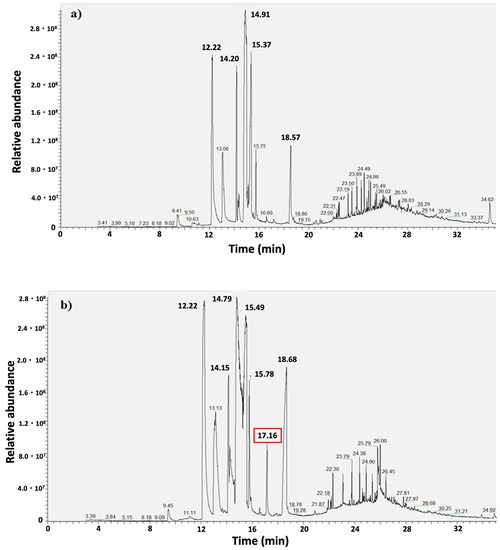
Figure 5.
Chromatograms of control (a) and exposed samples (b). Exposed samples differ from the controls for the peak at 17.16 min, identifying chlorohematommate.
In order to test the correlation between the physiological parameters and the bioaccumulation of trace elements evaluated in the same samples by [28], the mean element concentration in exposed and control samples is reported (Table 1), as well as the map of the contamination index (Figure 6) and the values of the contamination index sorted for concentric belts (Figure 7). The correlation matrix between physiological parameters and the accumulation of trace elements is shown in Table 2. Only one statistically significant (p < 0.05) weak positive correlation emerged between Cu and cell membrane damage (r = 0.28).

Table 1.
Mean (±standard deviation) values (µg/g dw) of trace elements accumulated in control and exposed samples (from [28]).
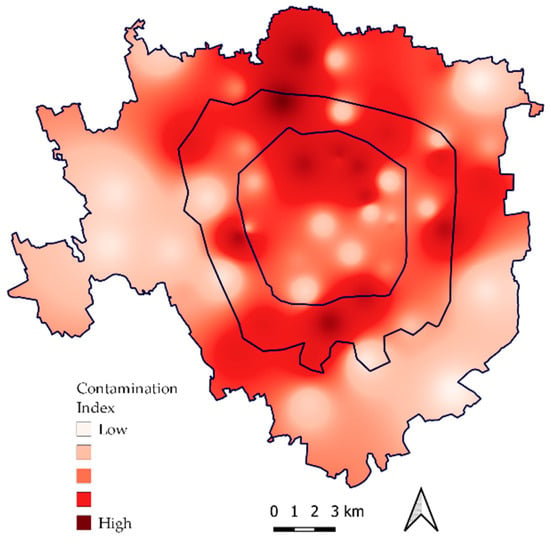
Figure 6.
Map of the contamination index obtained from the bioaccumulation of selected trace elements, modified after [28].
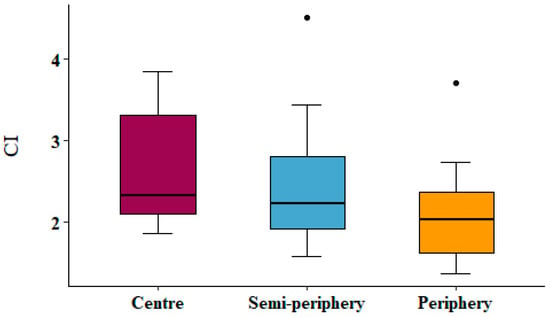
Figure 7.
Boxplots showing the value of the contamination index sorted according to concentric belts.

Table 2.
Pearson correlation matrix between bioaccumulated trace elements and the physiological parameters investigated. * = statistically significant (p < 0.05) correlation.
4. Discussion
In this study, the biological effects of air pollution on the physiology of the lichen E. prunastri were evaluated after 3 months of exposure in Milan. The Municipality promoted over the years many pollution abatement measurements and air quality improvement strategies; thus, through this work we verified whether current air quality conditions are now compatible with the survival of sensitive bioindicators and indirectly, if the abatement policies were effective at improving the environmental situation.
Contardo et al. [28] found in the same lichen samples a marked accumulation of Sb, Cu, Cr, and Fe, which were clearly originated from nonexhaust emissions of vehicles, mainly brake abrasion, as confirmed also by magnetic measurements [35]. Nevertheless, as reported in many other similar studies [36,37], the bioaccumulation of these elements was not at all correlated with the physiological status of the lichen samples, except for a very weak positive correlation between Cu and cell membrane damage, explaining less than 8% of the variability in the latter parameter. The overall limited toxic effect of trace elements on lichen physiology is explained taking into account that in such urban environments, metals are mainly deposited as particles (PM) over the thallus surface [38] and at least accumulated extracellularly [39], while intracellular uptake is very limited [24]. The role of Cu on lichen physiology is well known, being an important component of many enzymes, and its uptake may also be active, but when in excess, uptake is only passive [40]. Copper toxicity may cause strong plasmolysis, resulting in a loss of K ions proportionally to the Cu supply, as found for several lichen species [37,41], thus contributing to increasing the value of MD%.
The results of the present study showed a decrease in the photosynthetic efficiency and an increase in the antioxidant activity and cell membrane damage in samples exposed in Milan compared with control samples, irrespective of the concentric belt. The effect on photosynthesis and antioxidant activity was ca. 10%, while a four-fold stronger effect of ca. 40% was found for cell membrane damage. Increased cell membrane permeability is diagnostic of acute exposure to nitrogen compounds [12], and membrane lipid peroxidation has been observed as a consequence of exposure to NOx [42].
After the drop of SO2 in the past decades [43], NOx is the main phytotoxic gaseous pollutant in urban areas. Mean annual concentration of SO2 in Milan dropped from ca. 200 µg m−3 in 1980 to <10 µg m−3 from 2000 onward [44]. It is noteworthy that 10 µg m−3 is the guideline value indicated by the WHO [45] for the protection of sensitive vegetation from the effects of SO2, and this value has been established based on sensitive lichen species. As a consequence, it is logical to discard SO2 as the main responsible for the toxic effects observed on lichen physiology. In the last 20 years, the concentration of NOx in Milan decreased from mean annual values of 100–120 µg m−3 in early 2000 to ca 30–70 µg m−3 in recent years [44]. These values are within the limit of 82 µg m−3 of NOx reported in [46] as a threshold compatible with the colonization by E. prunastri in London. However, the mean annual guideline value for NOx indicated by the WHO [45] for all receptors, including lichens, is 30 µg m−3. In addition, it was reported [42] that the maximum hourly concentration of NOx compatible with the colonization of E. prunastri is 120 µg m−3, a value which has to be compared with the 75 µg m−3 reported as guideline for the vegetation by WHO [45]. Hourly mean NOx values measured in Milan during our exposure period ranged from 165 to 240 µg m−3. It is thus reasonable to suggest that acute NOx pollution is the main responsible for the physiological damage that occurred to our transplants. NOx derives from the combustion process, and in urban areas, exhaust from vehicles and heating of buildings are the most diffuse sources [20].
For the study area, the NOx data are available from five municipal monitoring stations (Figure 8, where MA = Viale Marche (45°29′44″ N, 9°11′39″ E), CS = Città Studi (45°28′42″ N, 9°13′48″ E), VE = Via Verziere (45°27′47″ N, 9°11′43″ E), LI = Viale Liguria (45°26′38″ N, 9°10′12″ E), and SE= Via Senato (45°28′16″ N, 9°11′4″ E)), located in residential areas of the city, at 2–4 mt above ground, according to the Italian guidelines [47]. To compare the mean NOx values measured during the exposure period with the physiological parameters, the values of the latter were extrapolated from the interpolated maps (Table 3). Statistically significant (p < 0.05) correlations did not emerge between the atmospheric concentration of this gaseous pollutant and the physiological parameters (Figure 9).
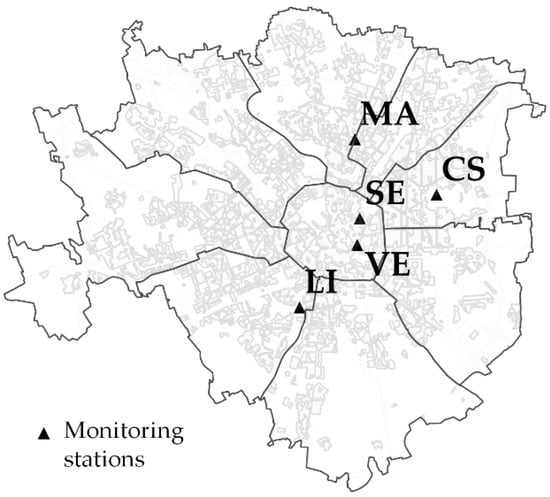
Figure 8.
Location of the monitoring stations that measured the NOx during the exposure period.

Table 3.
Hourly mean concentrations (µg m−3) of NOx are reported for each monitoring station. Physiological parameters values in a 100 mt area surrounding the station are also reported.
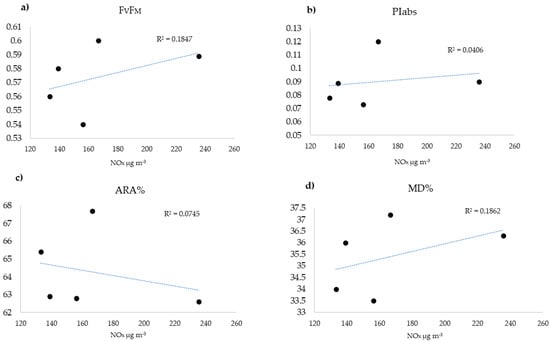
Figure 9.
Linear relation between NOx and physiological parameters measured in the surrounding of the monitoring stations. No significant relations emerged. (a) Fv/FM, (b) PIABS, (c) MD%, and (d) ARA%.
The oxidation induced by NOx can explain the increased antioxidant activity of exposed samples compared to controls. In this regard, GC-MS analyses pointed out a higher presence of ethyl chlorohematommate compared with control samples. This molecule originates from the degradation of chloroatranorin, which is a depside commonly detected in E. prunastri, which has a protective role against biotic and abiotic stress [48]. In particular, as indicated by several studies [49], an increased production of this molecule can also be promoted by the exposure to air pollution, besides other forms of environmental stress.
Although in some cases, under slightly NOx-enriched conditions, an enhanced photosynthetic activity has been reported owing to a higher N availability [50,51]. Samples exposed in Milan showed a decreased photosynthetic efficiency, but it should be considered that NOx concentrations in Milan are so high that the benefits of a higher nutrient supply are probably counterbalanced and overwhelmed by the phytotoxic effects. Nevertheless, the fact that samples were still alive at the end of the exposure period indicates that air quality conditions in Milan, although hostile for lichen growth, are not so severe to induce a rapid death of these organisms and hence to definitely hamper the success of these sensitive bioindicators.
Nevertheless, recent studies have shown that NH3 originated from vehicles equipped with catalytic converters is an important gas in urban environments [52]. It is known that the physiology of E. prunastri is impaired by NH3 concentration higher than 8 µg m−3 [53]. The value of 8 µg m−3 of NH3 is also the guideline value for vegetation indicated by the WHO [45]. During the exposure period, NH3 concentrations recorded at the only monitoring station measuring this gas averaged 5.3 µg m−3. Based on these features, we argue that NH3 contributed only very minimally to the physiological damage of E. prunastri and that pollutants hypothesized to be the primary explanatory variables, based on studies elsewhere and measured atmospheric concentrations in Milan, are NOx and perhaps derived pollutants such as nitric acid [54] and nitrates [55].
It is noteworthy that the exposure period was characterized by particularly high temperatures (6.3 °C vs. an average of 3.8 °C for the same period in the last 30 years) and particularly low precipitations (45 vs. 177 mm for the same period in the last 30 years), with the total absence of heavy rains that could have reduced the concentrations of NOx and PM in the atmosphere (the legal limit of 50 µg/m3 for PM10 was exceeded for more than 50% of the exposure days, with an average value of 57.3 µg/m3).
5. Conclusions
Cities are addressing efforts to improve air quality by implementing environmental policies and regulations. This is crucially important in large conurbations where millions of inhabitants live. Air quality in Milan suffers from heavy pollution by PM and NOx, but the overall situation is not as severe as to induce the death of sensitive biomonitors, at least in the short term. Nevertheless, the current concentrations of NOx and related pollutants probably prevent the normal vital status of the lichen Evernia prunastri, contributing to impair at least 10% of important physiological parameters such as photosynthetic efficiency and up to 40% of cell membrane integrity. The most important contaminant is likely NOx, which plays several roles in determining colonization by lichen species.
Author Contributions
Conceptualization, S.L. and T.C.; methodology, S.L. and T.C.; software, T.C.; formal analysis, S.L.; investigation, T.C., M.A.O., S.G. and A.V.; data curation, T.C.; writing—original draft preparation, T.C. and S.L.; writing—review and editing, S.L., T.C., M.A.O., S.G. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oberdörster, G.; Utell, M.J. Ultrafine Particles in the Urban Air: To the Respiratory Tract–and Beyond? Environ. Health Perspect. 2002, 110, A440–A441. [Google Scholar] [CrossRef]
- Medina, S.; Plasencia, A.; Ballester, F.; Mücke, H.G.; Schwartz, J. Apheis: Public Health Impact of PM10 in 19 European Cities. J. Epidemiol. Community Health 2004, 58, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Sternbeck, J.; Sjödin, Å.; Andréasson, K. Metal Emissions from Road Traffic and the Influence of Resuspension—Results from Two Tunnel Studies. Atmos. Environ. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Fenger, J. Air Pollution in the Last 50 Years–From Local to Global. Atmos. Environ. 2009, 43, 13–22. [Google Scholar] [CrossRef]
- Purvis, O.; Pawlik-Skowrońska, B. Chapter 12. Lichens and Metals. British Mycological Society Symposia Series. In Stress in Yeasts and Filamentous Fungi; Academic Press: Cambridge, MA, USA, 2008; Volume 27, pp. 175–200. [Google Scholar]
- Hawksworth, D.L.; Rose, F. Qualitative Scale for Estimating Sulphur Dioxide Air Pollution in England and Wales Using Epiphytic Lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, J.; Bach, L.; Asmund, G. Modelling Atmospheric Bulk Deposition of Pb, Zn and Cd near a Former Pb–Zn Mine in West Greenland Using Transplanted Flavocetraria nivalis Lichens. Chemosphere 2013, 90, 2549–2556. [Google Scholar] [CrossRef]
- Occelli, F.; Bavdek, R.; Deram, A.; Hellequin, A.-P.; Cuny, M.-A.; Zwarterook, I.; Cuny, D. Using Lichen Biomonitoring to Assess Environmental Justice at a Neighbourhood Level in an Industrial Area of Northern France. Ecol. Indic. 2016, 60, 781–788. [Google Scholar] [CrossRef]
- Purvis, O.W.; Williamson, B.J.; Spiro, B.; Udachin, V.; Mikhailova, I.N.; Dolgopolova, A. Lichen Monitoring as a Potential Tool in Environmental Forensics: Case Study of the Cu Smelter and Former Mining Town of Karabash, Russia. Geol. Soc. Lond. Spec. Publ. 2013, 384, 133–146. [Google Scholar] [CrossRef]
- Garty, J.; Cohen, Y.; Kloog, N.; Karnieli, A. Effects of Air Pollution on Cell Membrane Integrity, Spectral Reflectance and Metal and Sulfur Concentrations in Lichens. Environ. Toxicol. Chem. 1997, 16, 1396–1402. [Google Scholar] [CrossRef]
- Gaio-Oliveira, G.; Dahlman, L.; Palmqvist, K.; Martins-Loução, M.A.; Máguas, C. Nitrogen Uptake in Relation to Excess Supply and Its Effects on the Lichens Evernia prunastri (L.) Ach and Xanthoria parietina (L.) Th. Fr. Planta 2005, 220, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Skye, E. Air Pollution Affects Pattern of Photosynthesis in Parmelia sulcata, a Corticolous Lichen. Science 1965, 148, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Cansaran-Duman, D.; Atakol, O.; Aras, S. Assessment of Air Pollution Genotoxicity by RAPD in Evernia prunastri L. Ach. from around Iron-Steel Factory in Karabük, Turkey. J. Environ. Sci. 2011, 23, 1171–1178. [Google Scholar] [CrossRef]
- Majumder, S.; Mishra, D.; Ram, S.S.; Jana, N.K.; Santra, S.; Sudarshan, M.; Chakraborty, A. Physiological and Chemical Response of the Lichen, Flavoparmelia caperata (L.) Hale, to the Urban Environment of Kolkata, India. Environ. Sci. Pollut. Res. 2013, 20, 3077–3085. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B. Antioxidant and Antimicrobial Properties of Some Lichens and Their Constituents. J. Med. Food 2011, 14, 1624–1630. [Google Scholar] [CrossRef]
- Nimis, P.L.; Castello, M.; Perotti, M. Lichens as Biomonitors of Sulphur Dioxide Pollution in La Spezia (Northern Italy). Lichenologist 1990, 22, 333–344. [Google Scholar] [CrossRef]
- Carreras, H.A.; Pignata, M.L. Biomonitoring of Heavy Metals and Air Quality in Cordoba City, Argentina, Using Transplanted Lichens. Environ. Pollut. 2002, 117, 77–87. [Google Scholar] [CrossRef]
- Loppi, S.; Ravera, S.; Paoli, L. Coping with Uncertainty in the Assessment of Atmospheric Pollution with Lichen Transplants. Environ. Forensics 2019, 20, 228–233. [Google Scholar] [CrossRef]
- European Environment Agency. Air Quality in Europe: 2015 Report; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Invernizzi, G.; Ruprecht, A.; Mazza, R.; De Marco, C.; Močnik, G.; Sioutas, C.; Westerdahl, D. Measurement of Black Carbon Concentration as an Indicator of Air Quality Benefits of Traffic Restriction Policies within the Ecopass Zone in Milan, Italy. Atmos. Environ. 2011, 45, 3522–3527. [Google Scholar] [CrossRef]
- Percoco, M. Is Road Pricing Effective in Abating Pollution? Evidence from Milan. Transp. Res. Part Transp. Environ. 2013, 25, 112–118. [Google Scholar] [CrossRef]
- Luè, A.; Colorni, A.; Nocerino, R.; Paruscio, V. Green Move: An Innovative Electric Vehicle-Sharing System. Procedia-Soc. Behav. Sci. 2012, 48, 2978–2987. [Google Scholar] [CrossRef]
- Vannini, A.; Paoli, L.; Nicolardi, V.; Di Lella, L.A.; Loppi, S. Seasonal Variations in Intracellular Trace Element Content and Physiological Parameters in the Lichen Evernia prunastri Transplanted to an Urban Environment. Acta Bot. Croat. 2017, 76, 171–176. [Google Scholar] [CrossRef][Green Version]
- El Rhzaoui, G.; Divakar, P.K.; Crespo, A.; Tahiri, H. Biomonitoring of Air Pollutants by Using Lichens (Evernia prunastri) in Areas between Kenitra and Mohammedia Cities in Morocco. Mediterr. Bot. 2015, 36, 21. [Google Scholar] [CrossRef]
- McCune, B.; Derr, C.C.; Muir, P.S.; Shirazi, A.; Sillett, S.C.; Daly, W.J. Lichen Pendants for Transplant and Growth Experiments. Lichenologist 1996, 28, 161. [Google Scholar] [CrossRef]
- Paoli, L.; Munzi, S.; Pisani, T.; Guttová, A.; Loppi, S. Freezing of Air-Dried Samples of the Lichen Evernia Prunastri (L.) Ach. Ensures That Thalli Remain Healthy for Later Physiological Measurements. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2013, 147, 141–144. [Google Scholar] [CrossRef]
- Contardo, T.; Vannini, A.; Sharma, K.; Giordani, P.; Loppi, S. Disentangling Sources of Trace Element Air Pollution in Complex Urban Areas by Lichen Biomonitoring. A Case Study in Milan (Italy). Chemosphere 2020, 256, 127155. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Paoli, L.; Munzi, S.; Guttová, A.; Senko, D.; Sardella, G.; Loppi, S. Lichens as Suitable Indicators of the Biological Effects of Atmospheric Pollutants around a Municipal Solid Waste Incinerator (S Italy). Ecol. Indic. 2015, 52, 362–370. [Google Scholar] [CrossRef]
- Malaspina, P.; Giordani, P.; Pastorino, G.; Modenesi, P.; Mariotti, M.G. Interaction of Sea Salt and Atmospheric Pollution Alters the OJIP Fluorescence Transient in the Lichen Pseudevernia furfuracea (L.) Zopf. Ecol. Indic. 2015, 50, 251–257. [Google Scholar] [CrossRef]
- Malaspina, P.; Modenesi, P.; Giordani, P. Physiological Response of Two Varieties of the Lichen Pseudevernia furfuracea to Atmospheric Pollution. Ecol. Indic. 2018, 86, 27–34. [Google Scholar] [CrossRef]
- Marante, F.T.; Castellano, A.G.; Rosas, F.E.; Aguiar, J.Q.; Barrera, J.B. Identification and Quantitation of Allelochemicals from the Lichen Lethariella canariensis: Phytotoxicity and Antioxidative Activity. J. Chem. Ecol. 2003, 29, 2049–2071. [Google Scholar] [CrossRef] [PubMed]
- Staples, R.; LaDuca, R.L.; Roze, L.V.; Laivenieks, M.; Linz, J.E.; Beaudry, R.; Fryday, A.; Schilmiller, A.L.; Koptina, A.V.; Smith, B. Structure and Chemical Analysis of Major Specialized Metabolites Produced by the Lichen Evernia prunastri. Chem. Biodivers. 2020, 17, e1900465. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Contardo, T.; Vannini, A.; Sorbo, S.; Basile, A.; Loppi, S. Magnetic Emissions from Brake Wear Are the Major Source of Airborne Particulate Matter Bioaccumulated by Lichens Exposed in Milan (Italy). Appl. Sci. 2020, 10, 2073. [Google Scholar] [CrossRef]
- Garty, J.; Galun, M.; Kessel, M. Localization of Heavy Metals and Other Elements Accumulated in the Lichen Thallus. New Phytol. 1979, 82, 159–168. [Google Scholar] [CrossRef]
- Branquinho, C.; Brown, D.H.; Catarino, F. The Cellular Location of Cu in Lichens and Its Effects on Membrane Integrity and Chlorophyll Fluorescence. Environ. Exp. Bot. 1997, 38, 165–179. [Google Scholar] [CrossRef]
- Vannini, A.; Paoli, L.; Russo, A.; Loppi, S. Contribution of Submicronic (PM1) and Coarse (PM > 1) Particulate Matter Deposition to the Heavy Metal Load of Lichens Transplanted along a Busy Road. Chemosphere 2019, 231, 121–125. [Google Scholar] [CrossRef]
- Rola, K. Insight into the Pattern of Heavy-Metal Accumulation in Lichen Thalli. J. Trace Elem. Med. Biol. 2020, 61, 126512. [Google Scholar] [CrossRef]
- Hauck, M.; Willenbruch, K.; Leuschner, C. Lichen Substances Prevent Lichens from Nutrient Deficiency. J. Chem. Ecol. 2009, 35, 71–73. [Google Scholar] [CrossRef]
- Puckett, K.J. The Effect of Heavy Metals on Some Aspects of Lichen Physiology. Can. J. Bot. 1976, 54, 2695–2703. [Google Scholar] [CrossRef]
- Godinho, R.M.; Freitas, M.C.; Wolterbeek, H.T. Assessment of Lichen Vitality during a Transplantation Experiment to a Polluted Site. J. Atmos. Chem. 2004, 49, 355–361. [Google Scholar] [CrossRef]
- Koolen, C.D.; Rothenberg, G. Air Pollution in Europe. Chemsuschem 2019, 12, 164–172. [Google Scholar] [CrossRef] [PubMed]
- La Gaccia, L.; Colombi, C.; Algieri, A.; Chiesa, M.; Cigolini, G.; Corbella, L.; Gianelle, V. Rapporto Sulla Qualità dell’Aria della Città Mteropolitana di Milano. 2019. Available online: https://www.arpalombardia.it/qariafiles/RelazioniAnnuali/RQA_MI_2019.pdf (accessed on 11 August 2021).
- World Health Organization. Guidelines for Air Quality; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Davies, L.; Bates, J.W.; Bell, J.N.B.; James, P.W.; Purvis, O.W. Diversity and Sensitivity of Epiphytes to Oxides of Nitrogen in London. Environ. Pollut. 2007, 146, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Linee guida per la predisposizione delle reti di monitoraggio della qualità dell’aria in Italia. Available online: https://www.isprambiente.gov.it/files/aria/lineeguidaretimonitoraggio.pdf (accessed on 11 August 2021).
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia prunastri and Pseudoevernia furfuraceae Lichens and Their Major Metabolites as Antioxidant, Antimicrobial and Anticancer Agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Valencia-Islas, N.; Zambrano, A.; Rojas, J.L. Ozone Reactivity and Free Radical Scavenging Behavior of Phenolic Secondary Metabolites in Lichens Exposed to Chronic Oxidant Air Pollution from Mexico City. J. Chem. Ecol. 2007, 33, 1619–1634. [Google Scholar] [CrossRef]
- Von Arb, C.; Mueller, C.; Ammann, K.; Brunold, C. Lichen Physiology and Air Pollution: II. Statistical Analysis of the Correlation between SO2, NO2, NO and O3, and Chlorophyll Content, Net Photosynthesis, Sulphate Uptake and Protein Synthesis of Parmelia Sulcata Taylor. New Phytol. 1990, 115, 431–437. [Google Scholar] [CrossRef]
- Sujetoviene, G.; Sliumpaite, I. Response of Evernia prunastri Transplanted to an Urban Area in Central Lithuania. Atmos. Pollut. Res. 2013, 4, 222–228. [Google Scholar] [CrossRef]
- Fenn, M.E.; Bytnerowicz, A.; Schilling, S.L.; Vallano, D.M.; Zavaleta, E.S.; Weiss, S.B.; Morozumi, C.; Geiser, L.H.; Hanks, K. On-Road Emissions of Ammonia: An Underappreciated Source of Atmospheric Nitrogen Deposition. Sci. Total Environ. 2018, 625, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Paoli, L.; Benesperi, R.; Proietti Pannunzi, D.; Corsini, A.; Loppi, S. Biological Effects of Ammonia Released from a Composting Plant Assessed with Lichens. Environ. Sci. Pollut. Res. 2014, 21, 5861–5872. [Google Scholar] [CrossRef]
- Riddell, J.; Nash, T.H.; Padgett, P. The Effect of HNO3 Gas on the Lichen Ramalina menziesii. Flora-Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 47–54. [Google Scholar] [CrossRef]
- Jovan, S.; Riddell, J.; Padgett, P.E.; Nash, T.H. Eutrophic Lichens Respond to Multiple Forms of N: Implications for Critical Levels and Critical Loads Research. Ecol. Appl. 2012, 22, 1910–1922. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).