Leptospirosis and Extensive Urbanization in West Africa: A Neglected and Underestimated Threat?
Abstract
:1. Leptospirosis: A Widespread but Poorly Documented Disease
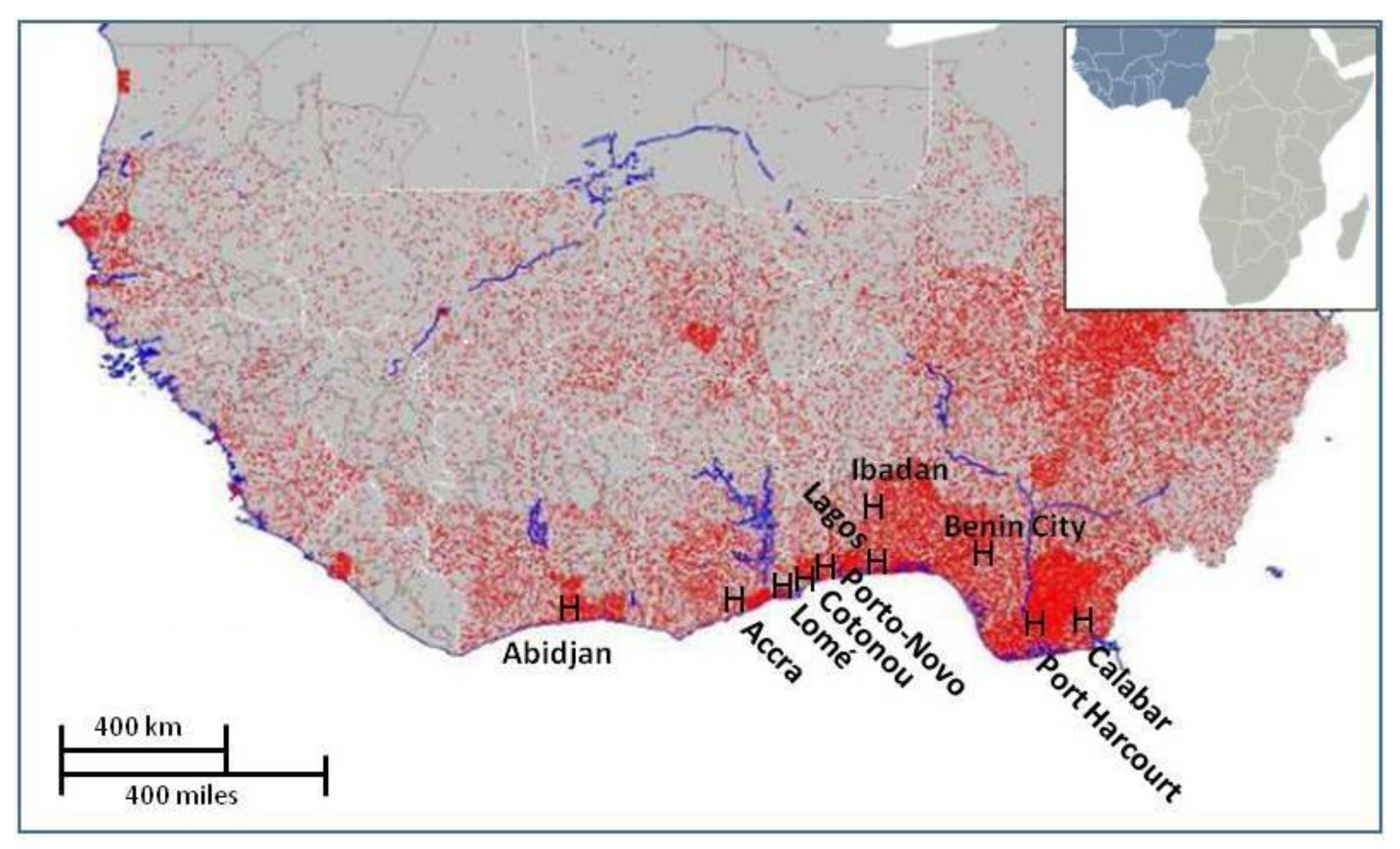
2. Highly Favorable Conditions for Leptospirosis Propagation in the West African Coastal Conurbation
3. What We Know about Leptospira in the WACC
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [PubMed]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e3898. [Google Scholar] [CrossRef] [PubMed]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.A.; Zinsstag, J.; Hattendorf, J. Environmental and behavioural determinants of leptospirosis transmission: A systematic review. PLoS Trop. Negl. Dis. 2015, 9, e3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudarmono, P.; Aman, A.T.; Arif, M.; Syarif, A.K.; Kosasih, H.; Karyana, M.; Chotpitayasunondh, T.; Vandepitte, W.P.; Boonyasiri, A.; Lapphra, K.; et al. Causes and outcomes of sepsis in Southeast Asia: A multinational multicentre cross-sectional study. Lancet Glob. Health 2017, 5, e157–e167. [Google Scholar]
- Durski, K.N.; Jancloes, M.; Chowdhary, T.; Bertherat, E. A global multi-disciplinary, multi-sectorial initiative to combat leptospirosis: Global Leptospirosis Environmental Action Network (GLEAN). Int. J. Environ. Res. Public Health 2014, 11, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013, 141, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Dobigny, G.; Garba, M.; Tatard, C.; Loiseau, A.; Galan, M.; Kadaouré, I.; Rossi, J.; Picardeau, M.; Bertherat, E. Urban market gardening and rodent-borne pathogenic Leptospira in arid zones: A case study in Niamey, Niger. PLoS Trop. Negl. Dis. 2015, 9, e4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosson, J.F.; Picardeau, M.; Mielcarek, M.; Tatard, C.; Chaval, Y.; Suputtamongkol, Y.; Buchy, P.; Jittapalapong, S.; Herbreteau, V.; Morand, S. Epidemiology of Leptospira transmitted by rodents in Southeast Asia. PLoS Trop. Negl. Dis. 2014, 8, e2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Zanzi, C.; Mason, M.R.; Encina, C.; Astroza, A.; Romero, A. Leptospira contamination in household and environmental water in rural communities in Southern Chile. Int. J. Environ. Res. Public Health 2014, 11, 6666–6680. [Google Scholar] [CrossRef] [PubMed]
- Della Rossa, P.; Tantrakarnapa, K.; Sutdan, D.; Kasetsinsombat, K.; Cosson, J.F.; Supputamongkol, Y.; Chaisiri, K.; Tran, A.; Supputamongkol, S.; Binot, A.; et al. Environmental factors and public health policy associated with human and rodent infection by leptospirosis: A land cover-based study in Nan province, Thailand. Epidemiol. Infect. 2015, 144, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Reis, M.G.; Ribeiro Dourado, C.M.; Johnson, W.D.; Riley, L.W. The Salvador Leptospirosis Study Group. Urban epidemic of severe leptospirosis in Brazil. Lancet 1999, 354, 820–825. [Google Scholar] [CrossRef]
- Reis, R.B.; Ribeiro, G.S.; Felzemburgh, R.D.M.; Santana, F.S.; Mohr, S.; Melendez, A.X.T.O.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Trop. Negl. Dis. 2008, 2, e228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, J.E.B.; Knobel, D.L.; Allan, K.J.; de Bronsvoort, A.B.M.; Handel, I.; Agwanda, I.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; et al. Urban leptospirosis in Africa: A cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. Am. J. Trop. Med. Hyg. 2013, 89, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, W. A plague of rats. Science 2016, 352, 912–915. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.G.; Visser, B.J.; Nagel, I.M.; Goris, M.G.A.; Hartskeerl, R.A.; Grobush, M.P. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 2014, 28, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.J.; Biggs, H.M.; Halliday, J.E.B.; Kazwala, R.R.; Maro, V.P.; Cleaveland, S.; Crump, J.A. Epidemiology of leptospirosis in Africa: A systematic review of a neglected zoonosis and a paradigm for “One Health” in Africa. PLoS Trop. Negl. Dis. 2015, 9, e3899. [Google Scholar] [CrossRef] [PubMed]
- Doudou, M.H.; Mahamadou, A.; Ouba, I.; Lazoumar, R.; Boubacar, B.; Arzika, I.; Zamanka, H.; Ibrahim, M.L.; Labbo, R.; Maiguizo, S.; et al. A refined estimate of the malaria burden in Niger. Malar. J. 2012, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mattar, S.; Tique, V.; Miranda, J.; Montes, E.; Garzon, D. Undifferentiated tropical febrile illness in Cordoba, Colombia: Not everything is dengue. J. Infect. Public Health 2017, 10, 507–512. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Investigation of Yellow Fever Epidemics in Africa; Field Guide; WHO/HSE/EPR/2008.5; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Bertherat, E.; Renaut, A.; Nabias, R.; Dubreuil, G.; Georges-Courbot, M.C. Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon. Am. J. Trop. Med. Hyg. 1999, 60, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.M.; Docha de Sa, D.A.; Turra, T.Z.; Ferreira Borges, N.M.; Nascimento, U.M.; Damasceno, E.A. Hantavirus pulmonary syndrome in Brasilia periphery: A diagnostic challenge. J. Infect. Dev. Ctries 2009, 3, 639–643. [Google Scholar] [PubMed]
- Bertherat, E.; Mueller, M.J.; Shako, J.C.; Picardeau, M. Discovery of a leptospirosis cluster amidst a pneumonic plague outbreak in a miners’camp in the Democratic Republic of Congo. Int. J. Environ. Res. Public Health 2014, 11, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Awosanya, E.J.; Nguku, P.; Oyemakinde, A.; Omobowale, O. Factors associated with probable cluster of leptospirosis among kennel workers in Abuja, Nigeria. Pan Afr. J. Med. 2013, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- United Nation, Department of Economic and Social Affairs, Population Division. World Urbanization Prospect; UN/ST/ESA/SER.A/352; United Nation, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2014. [Google Scholar]
- United Nation, Habitat. L’état des Villes Africaines: Réinventer la Transition Urbaine; ONU Habitat: Nairobi, Kenya, 2014. [Google Scholar]
- Neumann, B.; Vafeidis, A.T.; Zimmerman, J.; Nicholls, R.J. Future coastal population growth and sea-level and coastal flooding: A global assessment. PLoS ONE 2015, 10, e0118571. [Google Scholar] [CrossRef] [PubMed]
- AETS Consulting. Analyse de la Vulnérabilité au Changement Climatique de Cotonou et de Trois Villes Secondaires du Bénin; Programme d’Adaptation des Villes au Changement Climatique; French Agency for Development: Cotonou, Benin, 2016. [Google Scholar]
- Inondations dans le Grand Cotonou: Facteurs Humains, Vulnérabilité des Populations et Stratégies de Lutte et de Gestion. Programme de Protection de la Communauté Urbaine de Cotonou Face aux Changements Climatiques. PCUG3C Final Report; Abomey-Calavi, Benin, 2012. Available online: https://idl-bnc-idrc.dspacedirect.org/handle/10625/50607 (accessed on 22 March 2018).
- Khairani-Bejo, S.; Bahaman, A.R.; Zamri-Saad, M.; Mutalib, A.R. The survival of Leptospira interrogans serovar Hardjo in the Malaysian environment. J. Anim. Vet. Adv. 2004, 3, 123–129. [Google Scholar]
- Rossati, A. Global warming and its health impact. Int. J. Occup. Environ. Med. 2017, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Denis, E.; Moriconi-Ebrard, F.; Harre-Roger, D.; Thiam, O.; Séjourné, M.; Chatel, C. Dynamiques de L’urbanisation, 1952–2020: Approches Géostatistique, Afrique de L’ouest. Report of the French Agency for Development; Paris, France, 2008. Available online: https://hal.archives-ouvertes.fr/hal-00357271 (accessed on 22 March 2018).
- Ezeh, A.O.; Adesiyun, A.A.; Addo, P.B.; Ellis, W.A.; Makinde, A.A.; Bello, C.S. Serological and cultural examination for human leptospirosis in Plateau State, Nigeria. Cent. Afr. J. Med. 1991, 37, 11–15. [Google Scholar] [PubMed]
- Onyemelukwe, N.F. A serological survey for leptospirosis in the Enugu area of eastern Nigeria among people at occupational risk. J. Trop. Med. Hyg. 1993, 96, 301–304. [Google Scholar] [PubMed]
- Agunloye, C.A.; Alabi, F.O.; Odemuyiwa, S.O.; Olaleye, O.D. Leptospirosis in Nigerians: A seroepidemiological survey. Indian Vet. J. 2001, 78, 371–375. [Google Scholar]
- Isa, S.E.; Onyedibe, K.I.; Okolo, M.O.; Abiba, A.E.; Mafuka, J.S.; Simji, G.S.; Nathan, S.Y.; Udoh, U.A.; Awang, S.K.; Egah, D.Z.; et al. A 21-year old student with fever and profound jaundice. PLoS Negl. Trop. Dis. 2014, 8, e2534. [Google Scholar] [CrossRef] [PubMed]
- Hogerzeil, H.V.; Terpstra, W.J.; De Geus, A.; Korver, H. Leptospirosis in rural Ghana. Trop. Geogr. Med. 1986, 38, 162–166. [Google Scholar] [PubMed]
- Nimo Paintsil, S.C.; Fichet-Calvet, E.; Mohareb, E.; Morales, M.; Bonney, J.H.; Obiri-Danso, K.; Ampofo, W.K.; Schoepp, R.J.; Kronmann, K.C. Rodent species and their correlation with human seropositivity for zoonotic infections in Ghana. Am. J. Trop. Med. Hyg. 2013, 89, 422. [Google Scholar]
- Rothstein, N. Leptospirosis in Ghana: A preliminary serological survey. Ghana Med. J. 1964, 3, 90–92. [Google Scholar]
- Kinebuchi, H.; Afoakwa, S.N. Leptospirosis in Ghana. Ghana Med. J. 1973, 12, 190–193. [Google Scholar] [PubMed]
- Kronman, K.C.; Pimentel, G.; Puplampu, N.; Odoom, S.; Tagoe, J.; Nyarko, E.; Agbenohevi, P.; Raczniak, G.; Dejli, J.; Wilson, M; et al. Laboratory confirmed diagnoses of acute febrile illness in Ghana. Am. J. Trop. Med. Hyg. 2009, 221–222. [Google Scholar]
- Tagoe, J.A.; Puplampu, N.; Odoom, S.C.; Adbul-Rahman, B.; Habashy, E.E.; Pimentel, B.; Kronmann, K.; Koram, K.; Wilson, M.; Abdel, M.; et al. Serosurvey of leptospirosis among patients with acute febrile illness in Accra. Am. J. Trop. Med. Hyg. 2010, 83, 306. [Google Scholar]
- Roqueplo, C.; Marié, J.L.; André-Fontaine, G.; Kodjo, A.; Davoust, B. Serological survey of canine leptospirosis in three countries of tropical Africa: Sudan, Gabon and Ivory Coast. Comp. Immunol. Microbiol. Infect. Dis. 2015, 38, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Houéménou, G.; Ahmed, A.; Libois, R.; Hartskeerl, R.A. Leptospira spp. prevalence in small mammal populations in Cotonou, Benin. ISRN Epdemiol. 2013, 2013, 502638. [Google Scholar]
- Houngbo, P.T.; N’Gouizé, J. Premiers Résultats de Dépistage Sérologique de la Leptospirose à Cotonou et ses Environs; DIT Report; TBH/ABM: Cotonou, Benin; CPU/UNB: Fredericton, NB, Canada; Saint John, NB, Canada, 1995. [Google Scholar]
- Bello, C.I. Prévalence et Facteurs Associés de la Séropositivité à la lEptospirose dans Certains Quartiers de Cotonou (Bénin). Ph.D. Thesis, Université d’Abomey-Calavi, Cotonou, Benin, 2011. [Google Scholar]
- Houéménou, G. Les Petits Mammifères de la ville de Cotonou (Bénin) Peuvent-ils Constituer un Risque Pour la Santé Humaine? Etude de Quelques Pathogènes. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2013. [Google Scholar]
- Faine, S. Leptospiroses: Guide Pour la Lutte Contre les Leptospiroses; WHO: Geneva, Switzerland, 1987. [Google Scholar]
- Dossou-Yovo, P.O. Contribution à l’étude de la leptospirose chez l’enfant au CNHU de Cotonou: Aspects épidémiologiques, Diagnostiques et Thérapeutiques. Master’s Thesis, Faculty of Health Science, Abomey-Calavi University, Cotonou, Benin, 1999. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobigny, G.; Gauthier, P.; Houéménou, G.; Choplin, A.; Dossou, H.-J.; Badou, S.; Etougbétché, J.; Bourhy, P.; Koffi, S.; Durski, K.N.; et al. Leptospirosis and Extensive Urbanization in West Africa: A Neglected and Underestimated Threat? Urban Sci. 2018, 2, 29. https://doi.org/10.3390/urbansci2020029
Dobigny G, Gauthier P, Houéménou G, Choplin A, Dossou H-J, Badou S, Etougbétché J, Bourhy P, Koffi S, Durski KN, et al. Leptospirosis and Extensive Urbanization in West Africa: A Neglected and Underestimated Threat? Urban Science. 2018; 2(2):29. https://doi.org/10.3390/urbansci2020029
Chicago/Turabian StyleDobigny, Gauthier, Philippe Gauthier, Gualbert Houéménou, Armelle Choplin, Henri-Joël Dossou, Sylvestre Badou, Jonas Etougbétché, Pascale Bourhy, Stéphane Koffi, Kara N. Durski, and et al. 2018. "Leptospirosis and Extensive Urbanization in West Africa: A Neglected and Underestimated Threat?" Urban Science 2, no. 2: 29. https://doi.org/10.3390/urbansci2020029
APA StyleDobigny, G., Gauthier, P., Houéménou, G., Choplin, A., Dossou, H.-J., Badou, S., Etougbétché, J., Bourhy, P., Koffi, S., Durski, K. N., Bertherat, E., & Picardeau, M. (2018). Leptospirosis and Extensive Urbanization in West Africa: A Neglected and Underestimated Threat? Urban Science, 2(2), 29. https://doi.org/10.3390/urbansci2020029




