Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Preparation and Storage
2.2. Laboratory Analysis
2.2.1. Enzyme Linked Immunosorbent Assay (ELISA)
2.2.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3. Spectra Processing and Data Analysis
2.3.1. Spectral Preprocessing and Visualization
2.3.2. Principal Component Analysis (PCA)
2.3.3. Partial Least Squares Discriminant Analysis (PLS-DA)
3. Results and Discussion
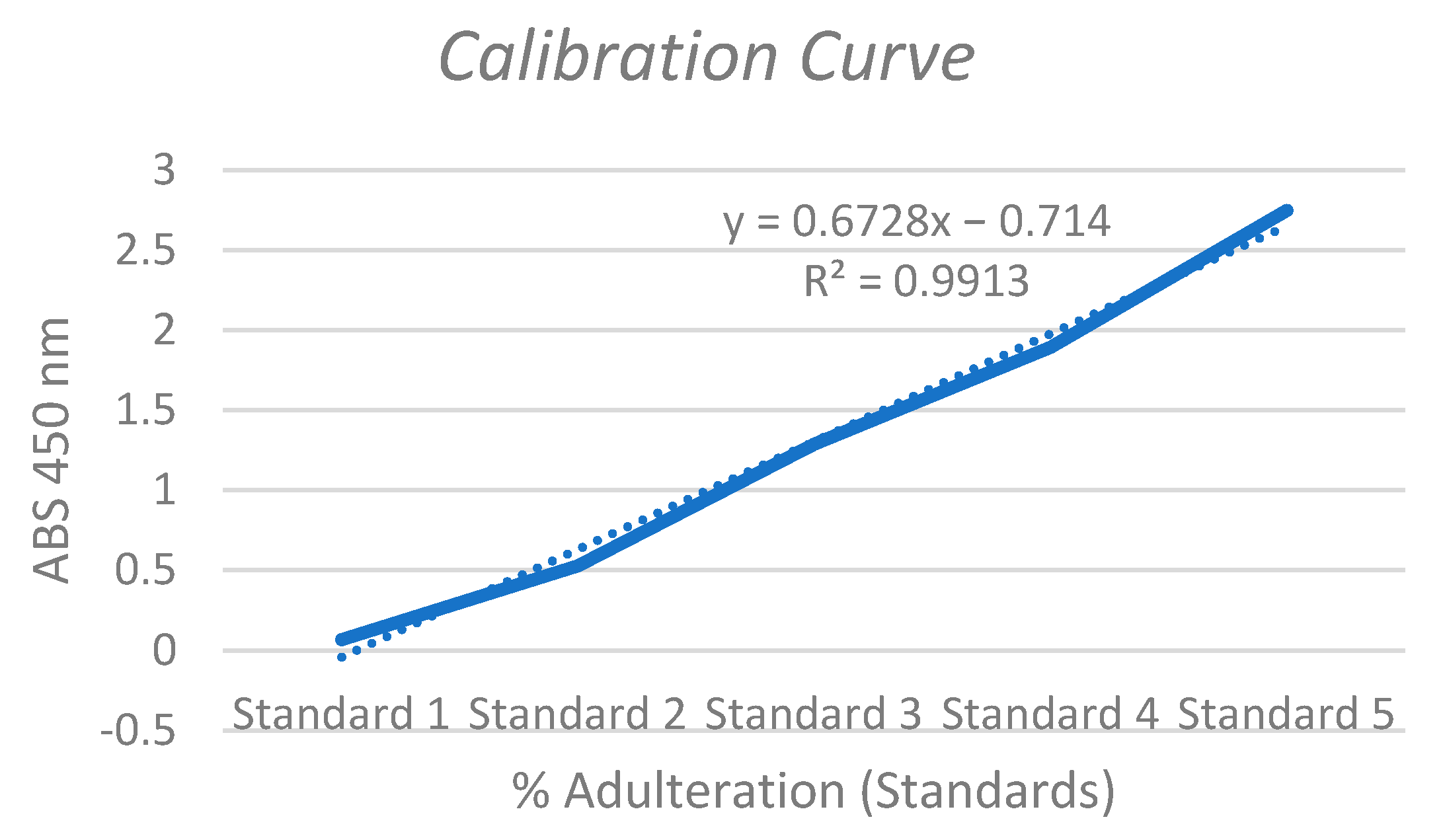
3.1. ELISA Analysis
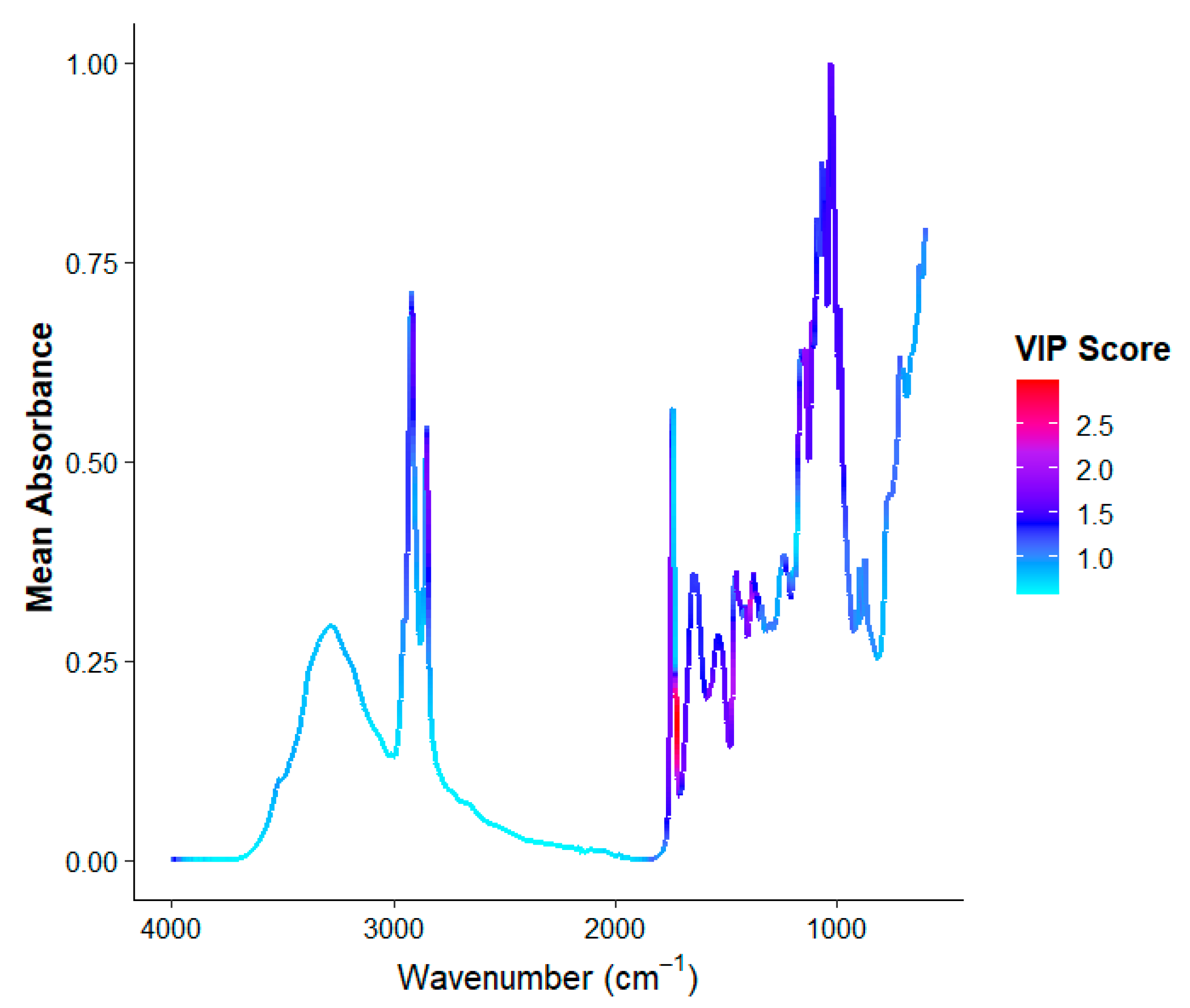
3.2. Analysis of FTIR Spectra
3.3. Principal Component Analysis
3.4. Partial Least Square Discriminant Analysis (PLS-DA)
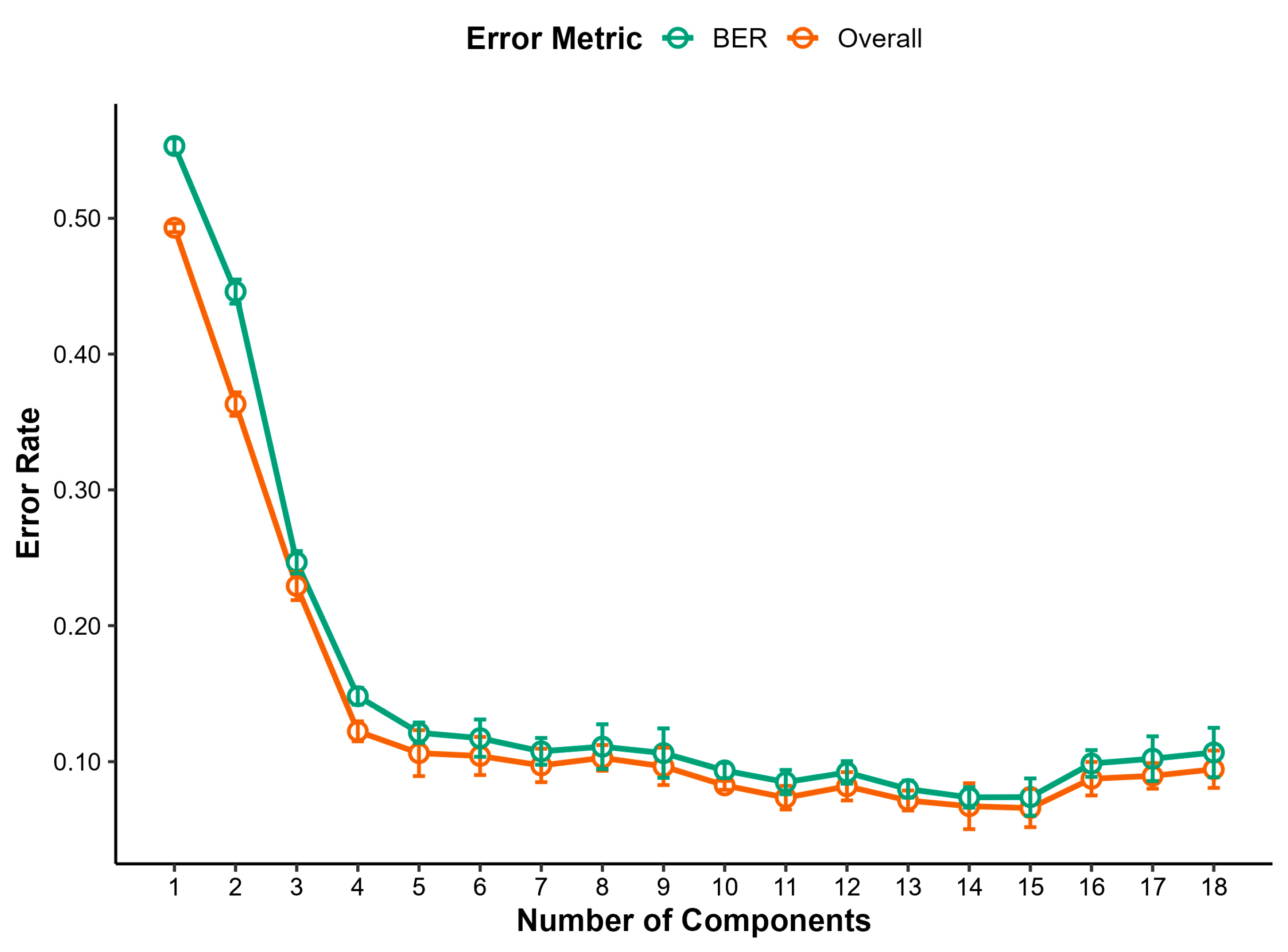
3.4.1. Raw Milk Samples
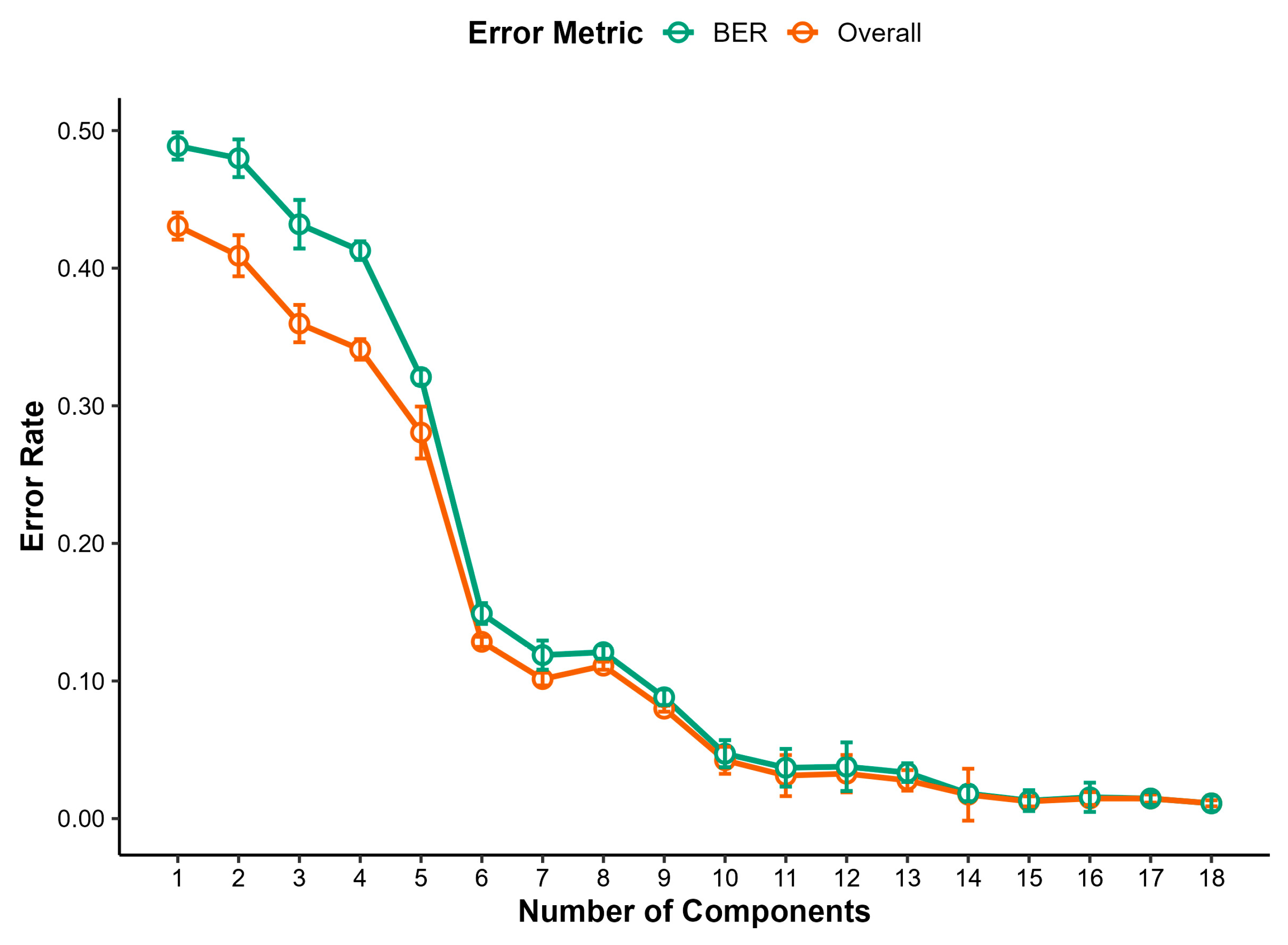
3.4.2. Freeze-Dried Milk Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | Linear dichroism |
| ATR-FTIR | Fourier Transform Infrared Attenuated Total Reflectance |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| PCA | Principal Component Analysis |
| BER | balanced error rate |
| LVs | latent variables |
| DTGS | Deuterated Triglycine Sulfate |
| VIP | Variable Importance in Projection |
| FTIR | Fourier transform infrared |
| ANN | Artificial Neural Network |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| SVM | Support Vector Machine |
| RF | Random Forest |
References
- Hodgkinson, A.J.; McDonald, N.A.; Kivits, L.J.; Hurford, D.R.; Fahey, S.; Prosser, C. Allergic responses induced by goat milk αS1-casein in a murine model of gastrointestinal atopy. J. Dairy Sci. 2012, 95, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yulyana, A.; Rohman, A.; Arifah, M.F.; Windarsih, A.; Nisa, K. Authentication analysis of goat milk from cow milk using Fourier TransformInfrared spectroscopy and chemometrics. Food Res. 2024, 8, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Agricultural Organization—Recording of Fresh Milk Production Data. Available online: https://www.elgo.gr/index.php?option=com_content&view=article&id=888&Itemid=1267&lang=el#%CF%83%CF%84%CE%B1%CF%84%CE%B9%CF%83%CF%84%CE%B9%CE%BA%CE%AC-%CE%BD%CF%89%CF%80%CE%BF%CF%8D-%CF%80%CF%81%CF%8C%CE%B2%CE%B5%CE%B9%CE%BF%CF%85-%CE%B3%CE%AF%CE%B4%CE%B9%CE%BD%CE%BF%CF%85-%CE%B3%CE%AC%CE%BB%CE%B1%CE%BA%CF%84%CE%BF%CF%82 (accessed on 25 April 2025).
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, A.S.; Aalizadeh, R.; Damalas, D.E.; Barla, I.V.; Baessmann, C.; Thomaidis, N.S. MALDI-TOF-MS integrated workflow for food authenticity investigations: An untargeted protein-based approach for rapid detection of PDO feta cheese adulteration. Food Chem. 2022, 370, 131057. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Kokkinofta, R.; Theocharis, C.R. Chemometric analysis combined with FTIR spectroscopy of milk and Halloumi cheese samples according to species’ origin. Food Sci. Nutr. 2020, 8, 3262–3273. [Google Scholar] [CrossRef]
- Calvano, C.D.; De Ceglie, C.; Monopoli, A.; Zambonin, C.G. Detection of sheep and goat milk adulterations by direct MALDI–TOF MS analysis of milk tryptic digests. J. Mass Spectrom. 2012, 47, 1141–1149. [Google Scholar] [CrossRef]
- Moyer, D.C.; DeVries, J.W.; Spink, J. The economics of a food fraud incident—Case studies and examples including Melamine in Wheat Gluten. Food Control 2017, 71, 358–364. [Google Scholar] [CrossRef]
- Yang, Y.; Huisman, W.; Hettinga, K.A.; Zhang, L.; Van Ruth, S.M. The Chinese milk supply chain: A fraud perspective. Food Control 2020, 113, 107211. [Google Scholar] [CrossRef]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Yadav, A.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid detection and quantification of sucrose adulteration in cow milk using Attenuated total reflectance-Fourier transform infrared spectroscopy coupled with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118628. [Google Scholar] [CrossRef]
- Cirak, O.; Icyer, N.C.; Durak, M.Z. Rapid detection of adulteration of milks from different species using Fourier Transform Infrared Spectroscopy (FTIR). J. Dairy Res. 2018, 85, 222–225. [Google Scholar] [CrossRef]
- Conceição, D.; Gonçalves, B.-H.; Da Hora, F.; Faleiro, A.; Santos, L.; Ferrão, S. Use of FTIR-ATR Spectroscopy Combined with Multivariate Analysis as a Screening Tool to Identify Adulterants in Raw Milk. J. Braz. Chem. Soc. 2019, 30, 780–785. [Google Scholar] [CrossRef]
- Julmohammad, N.; Suhaini, I.K.M.; Govindasamy, T.; Tan, E.; Soloi, S.; Julmohamad, N.; Akanda, M.J.H. Quantification of Adulterant Residues in UHT Milk Products using ATR-FTIR Spectroscopy Coupled with Multivariate Analysis. J. Adv. Res. Des. 2024, 115, 1–13. [Google Scholar] [CrossRef]
- Reid, L.M.; O’Donnell, C.P.; Downey, G. Recent technological advances for the determination of food authenticity. Trends Food Sci. Technol. 2006, 17, 344–353. [Google Scholar] [CrossRef]
- Salleh, N.A.; Selamat, J.; Meng, G.Y.; Abas, F.; Jambari, N.N.; Khatib, A. Fourier transform infrared spectroscopy and multivariate analysis of milk from different goat breeds. Int. J. Food Prop. 2019, 22, 1673–1683. [Google Scholar] [CrossRef]
- Kourkouli, A.; Thomaidis, N.; Dasenaki, M.; Markou, A. Novel and Sensitive Touchdown Polymerase Chain Reaction Assays for the Detection of Goat and Sheep Milk Adulteration with Cow Milk. Molecules 2024, 29, 1820. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.S.; Raptis, I.; Misiakos, K.; Livaniou, E.; Makarona, E.; Kakabakos, S. Rapid detection of mozzarella and feta cheese adulteration with cow milk through a silicon photonic immunosensor. Analyst 2021, 146, 529–537. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Pinto, P.A.; Anconi, A.C.S.A.; De Abreu, L.R.; Magalhães, E.J.; Nunes, C.A. Strategies to determine lactose in cow milk by mid infrared spectroscopy. J. Food Compos. Anal. 2021, 104, 104176. [Google Scholar] [CrossRef]
- Iñón, F.A.; Garrigues, S.; De La Guardia, M. Nutritional parameters of commercially available milk samples by FTIR and chemometric techniques. Anal. Chim. Acta 2004, 513, 401–412. [Google Scholar] [CrossRef]
- Murphy, E.G.; Fenelon, M.A.; Roos, Y.H.; Hogan, S.A. Decoupling Macronutrient Interactions during Heating of Model Infant Milk Formulas. J. Agric. Food Chem. 2014, 62, 10585–10593. [Google Scholar] [CrossRef]
- Kher, A.; Udabage, P.; McKinnon, I.; McNaughton, D.; Augustin, M.A. FTIR investigation of spray-dried milk protein concentrate powders. Vib. Spectrosc. 2007, 44, 375–381. [Google Scholar] [CrossRef]
- Gebhardt, R.; Takeda, N.; Kulozik, U.; Doster, W. Structure and Stabilizing Interactions of Casein Micelles Probed by High-Pressure Light Scattering and FTIR. J. Phys. Chem. B 2011, 115, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Alvarado, H.; Pérez-Cabal, M.A.; Tempelman, R.J.; Cecchinato, A.; Bittante, G.; de Los Campos, G.; Vazquez, A.I. Association between days open and milk spectral data in dairy cows. J. Dairy Sci. 2021, 104, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sala, P.; Hierro, M.T.G.; Martínez-Castro, I.; Santa-María, G. Triglyceride composition of ewe, cow, and goat milk fat. J. Am. Oil Chem. Soc. 1996, 73, 283–293. [Google Scholar] [CrossRef]
- Barron, L.J.R.; Hierro, M.T.G.; Santa-María, G. HPLC and GLC analysis of the triglyceride composition of bovine, ovine and caprine milk fat. J. Dairy Res. 1990, 57, 517–526. [Google Scholar] [CrossRef]
- Rachah, A.; Reksen, O.; Tafintseva, V.; Stehr, F.J.M.; Rukke, E.O.; Prestløkken, E.; Martin, A.; Kohler, A.; Afseth, N.K. Exploring Dry-Film FTIR Spectroscopy to Characterize Milk Composition and Subclinical Ketosis throughout a Cow’s Lactation. Foods 2021, 10, 2033. [Google Scholar] [CrossRef]
- Casarrubias-Torres, L.M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Mid-infrared spectroscopy and multivariate analysis for determination of tetracycline residues in cow’s milk. Acta Vet. Brno 2018, 87, 181–188. [Google Scholar] [CrossRef]
- Julmohammad, N.B.; Tan, E.; Yusop, M.R.; Samidin, S. Recent advances in detection techniques and chemometric methods for identifying adulterants in milk and dairy products. Food Chem. 2025, 483, 144202. [Google Scholar] [CrossRef]
- Sen, S.; Dundar, Z.; Uncu, O.; Ozen, B. Potential of Fourier-transform infrared spectroscopy in adulteration detection and quality assessment in buffalo and goat milks. Microchem. J. 2021, 166, 106207. [Google Scholar] [CrossRef]
- Silva, L.K.R.; Gonçalves, B.R.F.; Da Hora, F.F.; Santos, L.S.; Ferrão, S.P.B. Spectroscopic method (FTIR-ATR) and chemometric tools to detect cow’s milk addition to buffalo’s milk. Rev. Mex. Ing. Química 2019, 19, 11–20. [Google Scholar] [CrossRef]


| Confusion Matrix | Performance Metrics | |||||
|---|---|---|---|---|---|---|
| Cow | Goat | Sheep | Sensitivity | Specificity | Balanced Accuracy | |
| Predicted as Cow Milk | 12 | 0 | 0 | 1 | 0.96 | 0.98 |
| Predicted as Goat Milk | 0 | 8 | 0 | 1 | 1 | 1 |
| Predicted as Sheep Milk | 1 | 0 | 13 | 0.93 | 1 | 0.96 |
| Overall Accuracy: 0.97, 95% CI: (0.85, 1.00) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, L.; Koureas, M.; Pappas, C.; Manouras, A.; Kantas, D.; Malissiova, E. Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis. Sci 2025, 7, 87. https://doi.org/10.3390/sci7030087
Dimitriou L, Koureas M, Pappas C, Manouras A, Kantas D, Malissiova E. Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis. Sci. 2025; 7(3):87. https://doi.org/10.3390/sci7030087
Chicago/Turabian StyleDimitriou, Lamprini, Michalis Koureas, Christos Pappas, Athanasios Manouras, Dimitrios Kantas, and Eleni Malissiova. 2025. "Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis" Sci 7, no. 3: 87. https://doi.org/10.3390/sci7030087
APA StyleDimitriou, L., Koureas, M., Pappas, C., Manouras, A., Kantas, D., & Malissiova, E. (2025). Rapid Classification of Cow, Goat, and Sheep Milk Using ATR-FTIR and Multivariate Analysis. Sci, 7(3), 87. https://doi.org/10.3390/sci7030087







