Bioactive Properties of a Serine Protease Inhibitor Purified from Vicia ervilia Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Fungal Strains and Culture Conditions
2.3. Protein Quantification
2.4. Determination of Protease Inhibitory Activity
2.5. Purification of a Protease Inhibitor from Vicia ervilia L.
2.6. Electrophoretic Analysis
2.7. Biochemical Characterization of the Purified Protease Inhibitor
2.8. Antifungal Activity Assay
2.9. Antioxidant Activity Assay
2.10. DPPH Radical Scavenging Activity
2.11. Superoxide Anion-Scavenging Activity
2.12. Statistical Evaluation of the Results
3. Results
3.1. Purification and Characterization of a Protease Inhibitor from Bitter Vetch Seeds
3.1.1. Purification Procedure
3.1.2. Biochemical Characterization of the Purified vPI
3.2. Bioactive Properties of the Purified vPI
3.2.1. Antifungal Activity
3.2.2. Antioxidant Activity of the Protease Inhibitor Isolated from V. ervilia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MWCO | Molecular weight cut-off |
| PAGE | Polyacrylamide gel electrophoresis |
| FPLC | Fast protein liquid chromatography |
References
- Latef, A.A.H.A.; Ahmad, P. Legumes and breeding under abiotic stress: An overview. In Legumes Under Environmental Stress: Yield, Improvement and Adaptations; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 315. [Google Scholar]
- Martín-Pedrosa, M.; Varela, A.; Guillamon, E.; Cabellos, B.; Burbano, C.; Gomez-Fernandez, J.; de Mercado, E.; Gomez-Izquierdo, E.; Cuadrado, C.; Muzquiz, M. Biochemical characterization of legume seeds as ingredients in animal feed. Span. J. Agric. Res. 2016, 14, 0901. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; DuChaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional composition and bioactive content of legumes: Characterization of pulses frequently consumed in France and effect of the cooking method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef]
- Champ, M.M.J. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2002, 88, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Abu-Reidah, I.M.; Sharopov, F.; Karazhan, N.; Sharifi-Rad, J.; Akram, M.; Daniyal, M.; Khan, F.S.; Abbaass, W.; Zainab, R.; et al. Vicia plants—A comprehensive review on chemical composition and phytopharmacology. Phytother. Res. 2021, 35, 790–809. [Google Scholar] [CrossRef] [PubMed]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World; Oxford University Press: Oxford, UK, 2012; 264p, ISBN 9780199549061. [Google Scholar]
- Miller, N.F.; Enneking, D. Bitter Vetch (Vicia ervilia): Ancient Medicinal Crop and Farmers’ Favorite for Feeding Livestock. In New Lives for Ancient and Extinct Crops; University of Arizona Press: Tucson, AZ, USA, 2014; pp. 254–268. [Google Scholar]
- Okba, M.M.; Abdel Jaleel, G.A.; Yousif, M.F.; El Deeb, K.S.; Soliman, F.M. Vicia ervilia L. seeds newly explored biological activities. Cogent Biol. 2017, 3, 1299612. [Google Scholar] [CrossRef]
- Vioque, J.; Giron-Calle, J.; Torres-Salas, V.; Elamine, Y.; Alaiz, M. Characterizion of Vicia ervilia (bitter vetch) seed protein, free amino acids, and polyphenols. J. Food Biochem. 2020, 44, e13271. [Google Scholar] [CrossRef]
- López-Bellido, L. Neglected Crops: 1492 from a Different Perspective. In Plant Production and Protection; Hernándo Bermejo, J.E., León, J., Eds.; Series 26; FAO: Italy, Rome, 1994; pp. 273–288. [Google Scholar]
- Jaenicke, H.; Hoschle-Zeledon, I. Strategic Framework for Underutilized Plant Species Research and Development with Special Reference to Asia and the Pacific, and to Sub-Saharan Africa; International Centre for Underutilised Crops: Colombo, Sri Lanka; Global Facilitation Unit for Underutilized Species: Rome, Italy, 2006; p. 33. [Google Scholar]
- Russi, L.; Acuti, G.; Trabalza-Marinucci, M.; Porta, R.; Rubini, A.; Damiani, F.; Cristiani, S.; Dal Bosco, A.; Martuscelli, G.E.; Bellucci, M.; et al. Genetic characterisation and agronomic and nutritional value of bitter vetch (Vicia ervilia), an under-utilised species suitable for low-input farming systems. Crop Past. Sci. 2019, 70, 606–614. [Google Scholar] [CrossRef]
- Berger, J.D.; Robertson, L.D.; Cocks, P.S. Agricultural potential of Mediterranean grain and forage legumes: 2) Anti-nutritional factor concentrations in the genus Vicia. Genet. Res. Crop Evol. 2003, 50, 201–212. [Google Scholar] [CrossRef]
- Brown, W.E.; Takio, K.; Titani, K.; Ryan, C.A. Wound-Induced Trypsin Inhibitor in Alfalfa Leaves: Identity as a Member of the Bowman-Birk Inhibitor Family. Biochemistry 1985, 24, 2105–2108. [Google Scholar] [CrossRef]
- McManus, M.T.; Ryan, S.; Laing, W.A. The functions of proteinase inhibitors in seeds. Spec. Publ. Agron. Soc. N. Z. 2000, 12, 3–13. [Google Scholar]
- Chye, M.L.; Sin, S.F.; Xu, Z.F.; Yeung, E.C. Serine proteinase inhibitor proteins: Exogenous and endogenous functions. Vitr. Cell. Develop. Biol. Plant 2006, 42, 100–108. [Google Scholar] [CrossRef]
- Grosse-Holz, F.M.; van der Hoorn, R.A.L. Juggling jobs: Roles and mechanisms of multifunctional protease inhibitors in plants. New Phytol. 2016, 210, 794–807. [Google Scholar] [CrossRef]
- Kidrič, M.; Kos, J.; Sabotič, J. Proteases and their endogenous inhibitors in the plant response to abiotic stress. Bot. Serbica 2014, 38, 139–158. [Google Scholar]
- Vaseva, I.; Zehirov, G.; Kirova, E.; Simova-Stoilova, L. Transcript profiling of serine- and cysteine protease inhibitors in Triticum aestivum varieties with different drought tolerance. Cereal Res. Commun. 2016, 44, 79–88. [Google Scholar] [CrossRef]
- Guillamon, E.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; de Cortes Sánchez, M.; Muzquiz, M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chem. 2008, 107, 68–74. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Ivanova, K.; Simova-Stoilova, L. Bowman-Birk protease inhibitor gene expression in Phaseolus vulgaris L.—Organ specificity andinduction under abiotic stresses. Annu. Sofia Univ. “St. Kliment Ohridski” 2022, 4, 61–71. Available online: https://www.uni-sofia.bg/index.php/bul/universitet_t/fakulteti/biologicheski_fakultet2/oficialni_izdaniya/godishnik_na_sofijskiya_universitet_kniga_4_nauchni_sesii_na_biologicheskiya_fakultet/tom_107_2022_g_nauchna_konferenciya_klimentovi_dni_2021/irina_i_vaseva_et_al_bowman_birk_protease_inhibitor_gene_expression_in_phaseolus_vulgaris_organ_specificity_and_induction_under_abiotic_stresses (accessed on 1 September 2025).
- Ryan, C.A. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- Svendsen, I.B.; Hejgaard, J.; Chavan, J.K. Subtilisin inhibitor from seeds of broad bean (Vicia faba); purification, amino acid sequence and specificity of inhibition. Carlsberg Res. Commun. 1984, 49, 493–502. [Google Scholar] [CrossRef]
- Rodríguez-Sifuentes, L.; Marszalek, J.E.; Chuck-Hernández, C.; Serna-Saldívar, S.O. Legumes protease inhibitors as biopesticides and their defense mechanisms against biotic factors. Int. J. Mol. Sci. 2020, 21, 3322. [Google Scholar] [CrossRef]
- Cotabarren, J.; Lufrano, D.; Parisi, M.G.; Obregón, W.D. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci. 2020, 292, 110398. [Google Scholar] [CrossRef]
- Mosolov, V.V.; Valueva, T.A. Participation of proteolytic enzymes in the interaction of plants with phytopathogenic microorganisms. Biochemistry 2006, 71, 838–845. [Google Scholar] [CrossRef]
- Yike, I. Fungal proteases and their pathophysiological effects. Mycopathologia 2011, 171, 299–323. [Google Scholar] [CrossRef]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant-pathogen interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gongora, D.; Geddes-McAlister, J. From naturally-sourced protease inhibitors to new treatments for fungal infections. J. Fungi 2021, 7, 1016. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Lee, D.W.; Che, M.H. Alternaria-induced barrier dysfunction of nasal epithelial cells: Role of serine protease and reactive oxygen species. Int. Forum Allergy Rhinol. 2019, 9, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Abrashev, R.; Miteva-Staleva, J.; Gocheva, Y.; Stoyancheva, G.; Dishliyska, V.; Spasova, B.; Krumova, E.; Angelova, M. Cell response to oxidative stress in Antarctic filamentous fungi. Appl. Sci. 2025, 15, 5149. [Google Scholar] [CrossRef]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef]
- Missall, T.A.; Lodge, J.K.; McEwen, J.E. Mechanisms of resistance to oxidative and nitrosative stress: Implications for fungal survival in mammalian hosts. Eukaryot. Cell 2004, 3, 835–846. [Google Scholar] [CrossRef]
- Lingaiah, P.M.S.; Srinivas, L. Potent antioxidant activity of a protease Inhibitor hayanin from the seed coats of Horse gram (Macrotylomauniflorum (lam.) Verdc.). Int. J. Pharma. Res. Health Sci. 2016, 4, 1305–1310. [Google Scholar] [CrossRef]
- Simamora, A.; Santoso, A.W.; Timotius, K.H.; Rahayu, I. Antioxidant activity, enzyme Inhibition potentials, and phytochemical profiling of Premna serratifolia L. leaf extracts. Internat. J. Food Sci. 2020, 3436940. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Moein, M.; Sabahi, Z.; Moein, S.; Hafez Ghoran, S.; Naderian, M.; Zebarjad, Z. Antioxidant potentials, protease inhibitory, and cytotoxic activities of various Isolated extracts from Salvia aegyptiaca. Iran. Biomed. J. 2024, 29, 57–67. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Haas, A.; Cornely, O.A. Invasive aspergillosis. Drugs 2007, 67, 1567–1601. [Google Scholar] [CrossRef] [PubMed]
- van Diepeningen, A.D.; Brankovics, B.; Iltes, J.; van der Lee, T.A.J.; Waalwijk, C. Diagnosis of fusarium infections: Approaches to identification by the clinical mycology laboratory. Curr. Fungal Infect. Rep. 2015, 9, 135–143. [Google Scholar] [CrossRef]
- Martínez-Culebras, P.V.; Gandía, M.; Garrigues, S.; Marcos, J.V.; Manzanares, P. Antifungal peptides and proteins to control toxigenic fungi and mycotoxin biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Fatima, S. Characterization, biomedical and agricultural applications of protease inhibitors: A review. Int. J. Biol. Macromol. 2016, 91, 1120–1133. [Google Scholar] [CrossRef]
- Gitlin-Domagalska, A.; Maciejewska, A.; Dębowski, D. Bowman-Birk inhibitors: Insights into family of multifunctional proteins and peptides with potential therapeutical applications. Pharmaceuticals 2020, 13, 421. [Google Scholar] [CrossRef]
- Cid-Gallegos, M.S.; Corzo-Ríos, L.J.; Jiménez-Martínez, C.; Sánchez-Chino, X.M. Protease inhibitors from plants as therapeutic agents—A review. Plant Foods Hum. Nutr. 2022, 77, 20–29. [Google Scholar] [CrossRef]
- Gueven, N.; Dittmann, K.; Mayer, C.; Rodemann, H.P. The radioprotective potential of the Bowman–Birk protease inhibitor is independent of its secondary structure. Cancer Lett. 1998, 125, 77–82. [Google Scholar] [CrossRef]
- Dittmann, K.; Löffler, H.; Bamberg, M.; Rodemann, H.P. Bowman–Birk proteinase inhibitor (BBI) modulates radiosensitivity and radiation induced differentiation of human fibroblasts in culture. Radiother. Oncol. 1995, 34, 137–143. [Google Scholar] [CrossRef]
- Magee, P.J.; Owusu-Apenten, R.; McCann, M.J.; Gill, C.I.; Rowland, I.R. Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 2012, 64, 741–748. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kakade, M.L.; Rackis, J.J.; McGhee, J.E.; Puski, G. Determination of trypsin inhibitor activity of soy products: A collaborative analysis of an improved procedure. Cereal Chem. 1974, 51, 376–382. [Google Scholar]
- Prasad, E.R.; Dutta-Gupta, A.; Padmasree, K. Purification and characterization of a Bowman-Birk proteinase inhibitor from the seeds of black gram (Vigna mungo). Phytochemistry 2010, 71, 363–372. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analyt. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Deshimaru, M.; Hanamoto, R.; Kusano, C.; Yoshimi, S.; Terada, S. Purification and characterization of proteinase inhibitors from wild soja (Glycine soja) seeds. Biosci. Biotechnol. Biochem. 2002, 66, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Abrashev, R.; Miteva-Staleva, J.; Dishliyska, V.; Dolashki, A.; Spasova, B.; Dolashka, P.; Angelova, M.; Krumova, E. The Role of Oxidative Stress in the Antifungal Activity of Two Mollusk Fractions on Resistant Fungal Strains. Int. J. Mol. Sci. 2025, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analyt. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Asao, T.; Imai, F.; Tsuji, I.; Tashiro, M.; Iwami, K.; Ibuki, F. Purification and characterization of a Bowman-birk type proteinase inhibitor from faba beans (Vicia faba L.). Agricult. Biol. Chem. 1991, 55, 707–713. [Google Scholar] [CrossRef]
- Dantzger, M.; Vasconcelos, I.M.; Scorsato, V.; Aparicio, R.; Marangoni, S.; Macedo, M.L.R. Bowman–Birk proteinase inhibitor from Clitoria fairchildiana seeds: Isolation, biochemical properties and insecticidal potential. Phytochemistry 2015, 118, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.; Stoilova, T.; Velinov, V.; Vaseva, I.I.; Simova-Stoilova, L. Phenotypic Diversity and Abiotic Stress Tolerance Among Vicia ervilia (L.) Willd. Accessions. Plants 2025, 14, 1008. [Google Scholar] [CrossRef]
- Warsy, A.S.; Norton, G.; Stein, M. Protease inhibitors from broad bean isolation and purification. Phytochemistry 1974, 13, 2481–2486. [Google Scholar] [CrossRef]
- Wu, C.; Whitaker, J.R. Purification and partial characterization of four trypsin/chymotrypsin inhibitors from red kidney beans (Phaseolus vulgaris, var. Linden). J. Agricult. Food Chem. 1990, 38, 1523–1529. [Google Scholar] [CrossRef]
- Chan, Y.S.; Zhang, Y.; Ng, T.B. Brown kidney bean Bowman–Birk trypsin inhibitor is heat and pH stable and exhibits anti-proliferative activity. Appl. Biochem. Biotechnol. 2013, 169, 1306–1314. [Google Scholar] [CrossRef]
- Rawlings, N.D.; O’Brien, E.; Barrett, A. MEROPS: The protease database. J. Nucl. Acids Res. 2002, 30, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.; Poh, S.E.; Li, H. Secretory proteases of the human skin microbiome. Infect. Immun. 2022, 90, e00397-21. [Google Scholar] [CrossRef]
- Olivieri, F.; Godoy, A.V.; Escande, A.; Casalongué, C.A. Analysis of intercellular washing fluids of potato tubers and detection of increased proteolytic activity upon fungal infection. Physiol. Plant. 1998, 104, 232–238. [Google Scholar] [CrossRef]
- Budak, S.O.; Zhou, M.; Brouwer, C.; Wiebenga, A.; Benoit, I.; Di Falco, M.; Tsang, A.; de Vries, R.P. A genomic survey of proteases in Aspergilli. BMC Genom. 2014, 15, 523. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Satala, D.; Juszczak, M.; Bras, G.; Rapala-Kozik, M. Candida albicans aspartyl protease (Sap6) inhibits neutrophil function via a “Trojan horse” mechanism. Sci. Rep. 2025, 15, 6946. [Google Scholar] [CrossRef]
- Alves, M.H.; de Campos-Takaki, G.M.; Okada, K.; Pessoa, I.H.F.; Milanez, A.I. Detection of extracellular protease in Mucor species. Rev. Iberoamer. Micol. 2005, 22, 114. [Google Scholar] [CrossRef]
- Fernandez, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria pathogenesis in animals and plants. FEMS Microbiol. Rev. 2023, 47, fuad061. [Google Scholar] [CrossRef] [PubMed]
- Thery, T.; Lynch, K.M.; Arendt, E.K. Natural antifungal peptides/proteins as model for novel food preservatives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1327–1360. [Google Scholar] [CrossRef] [PubMed]
- Shwaiki, L.N.; Lynch, K.M.; Arendt, E.K. Future of antimicrobial peptides derived from plants in food application—A focus on synthetic peptides. Trends Food Sci. Technol. 2021, 112, 312–324. [Google Scholar] [CrossRef]
- Heskamp, M.L.; Barz, W. Characterization of proteases from Rhizopus species after growth on soybean protein. Z. Naturforsch. C 1997, 52, 595–604. [Google Scholar] [CrossRef]
- Lowe, R.G.; McCorkelle, O.; Bleackley, M.; Collins, C.; Faou, P.; Mathivanan, S.; Anderson, M. Extracellular peptidases of the cereal pathogen Fusarium graminearum. Front. Plant Sci. 2015, 6, 962. [Google Scholar] [CrossRef]
- Urbanek, H.; Yirdaw, G. Acid proteases produced by Fusarium species in cultures and in infected seedlings. Physiol. Plant Pathol. 1978, 13, 81–87. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Liu, Z.; Qian, Y.; Li, Y.; Yang, L.; Liu, S.; Liang, W.; Li, J. The secreted FolAsp aspartic protease facilitates the virulence of Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2023, 14, 1103418. [Google Scholar] [CrossRef]
- Shen, H.D.; Tam, M.F.; Tang, R.B.; Chou, H. Aspergillus and Penicillium allergens: Focus on proteases. Curr. Allergy Asthma Rep. 2007, 7, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef] [PubMed]
- Druey, K.M.; McCullough, M.; Krishnan, R. Aspergillus fumigatus protease alkaline protease 1 (Alp1): A new therapeutic target for fungal asthma. J. Fungi 2020, 6, 88. [Google Scholar] [CrossRef]
- Strader, M.B.; Saha, A.L.; Fernandes, C.; Sharma, K.; Hadiwinarta, C.; Calheiros, D.; Conde-de-Oliveira, G.; Gonçalves, T.; Slater, J.E. Distinct proteomes and allergen profiles appear across the life-cycle stages of Alternaria alternata. J. Allergy Clin. Immunol. 2024, 154, 424–434. [Google Scholar] [CrossRef]
- Jashni, M.K.; Dols, I.H.; Iida, Y.; Boeren, S.; Beenen, H.G.; Mehrabi, R.; Collemare, J.; de Wit, P.J. Synergistic action of a metalloprotease and a serine protease from Fusarium oxysporum f. sp. lycopersici cleaves chitin-binding tomato chitinases, reduces their antifungal activity, and enhances fungal virulence. Molec. Plant Microbe Interact. 2015, 28, 996–1008. [Google Scholar] [CrossRef]
- Benken, I.I.; Mosolov, V.V.; Fedurkina, N.V. Effects of an inhibitor of bean protease on plant pathogenic fungi. Mycol. Phytopathol. 1976, 10, 198–201. [Google Scholar]
- Valueva, T.A.; Kudryavtseva, N.N.; Sofyin, A.V.; Zaitchik, B.T.; Pobedinskaya, M.A.; Kokaeva, L.Y.; Elansky, S.N. Serine exoproteinases secreted by the pathogenic fungi of Alternaria genus. J. Plant Pathol. Microbiol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Silva, M.; Taveira, G.; Gebara, R.; Santos, L.; Cherene, M.; Souza, T.; Moreira, F.; Rodrigues, P.; Vieira-da-Motta, O.; Seabra, S.; et al. Understanding the mechanism of action of protease inhibitors in controlling the growth of the Candida genus: Potential candidates for development of new antifungal molecules. Arch. Microbiol. 2024, 206, 257. [Google Scholar] [CrossRef]
- Dabhade, A.R.; Mokashe, N.U.; Patil, U.K. Purification, characterization, and antimicrobial activity of nontoxic trypsin inhibitor from Albizia amara Boiv. Process Biochem. 2016, 51, 659–674. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, S.-C.; Kim, J.-Y.; Lee, S.Y.; Lim, H.-T.; Cheong, H.; Hahm, K.-S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 81–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hu, X.P.; Bai, Y.; Zhao, Q.Y.; Yu, S.Q.; Tian, Y.X.; Bian, Y.Y.; Li, J.; Li, S.H.; Li, T.P. Preparation and antioxidative stability of the potato protease inhibitors (PPIs) from potato starch waste-water. LWT 2020, 134, 109963. [Google Scholar] [CrossRef]
- Tamura, K.; Manabe, T.; Imanishi, K.; Nonaka, A.; Asano, N.; Yamaki, K.; Tobe, T. Effect of synthetic protease inhibitors on superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical production by human polymorphonuclear leukocytes. Hepato-Gastroenterol. 1992, 39, 59–61. [Google Scholar] [PubMed]
- Khan, S.; Arshad, S.; Arif, A.; Tanveer, R.; Amin, Z.S.; Abbas, S.; Maqsood, A.; Raza, M.; Munir, A.; Latif, A.; et al. Trypsin inhibitor isolated from Glycine max (soya bean) extraction, purification, and characterization. Dose-Response 2022, 20, 4. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Gupta, S. Analysis plant protease inhibitors and their antiviral activities—Potent therapeutics for SARS CoV-2. J. Infec. Dis. Treat. 2020, 7, 5205. [Google Scholar]
- Liu, Y.; Bian, Y.; Bai, Y.; Yu, S.; Tian, Y.; Li, J.; Li, S.; Li, T. Potato protease inhibitors, a functional food material with antioxidant and anticancer potential. Food Sci. Human. Wellness 2023, 12, 1762–1771. [Google Scholar] [CrossRef]
- Zhu, M.J.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. Isolation and characterization of a Kunitz-type trypsin inhibitor with antiproliferative activity from Gymnocladus chinensis (Yunnan bean) seeds. Protein J. 2011, 30, 240–246. [Google Scholar] [CrossRef]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- García-Gasca, T.; García-Cruz, M.; Hernandez-Rivera, E.; López-Matínez, J.; Castaneda-Cuevas, A.L.; Yllescas-Gasca, L.; Rodríguez-Méndez, A.J.; Mendiola-Olaya, E.; Castro-Guillén, J.L.; Blanco-Labra, A. Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and semipure lectin fractions on cancer cells. Nutr. Cancer 2012, 64, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, M.; Jiang, L. Advancements in serine protease inhibitors: From mechanistic Insights to clinical applications. Catalysts 2024, 14, 787. [Google Scholar] [CrossRef]

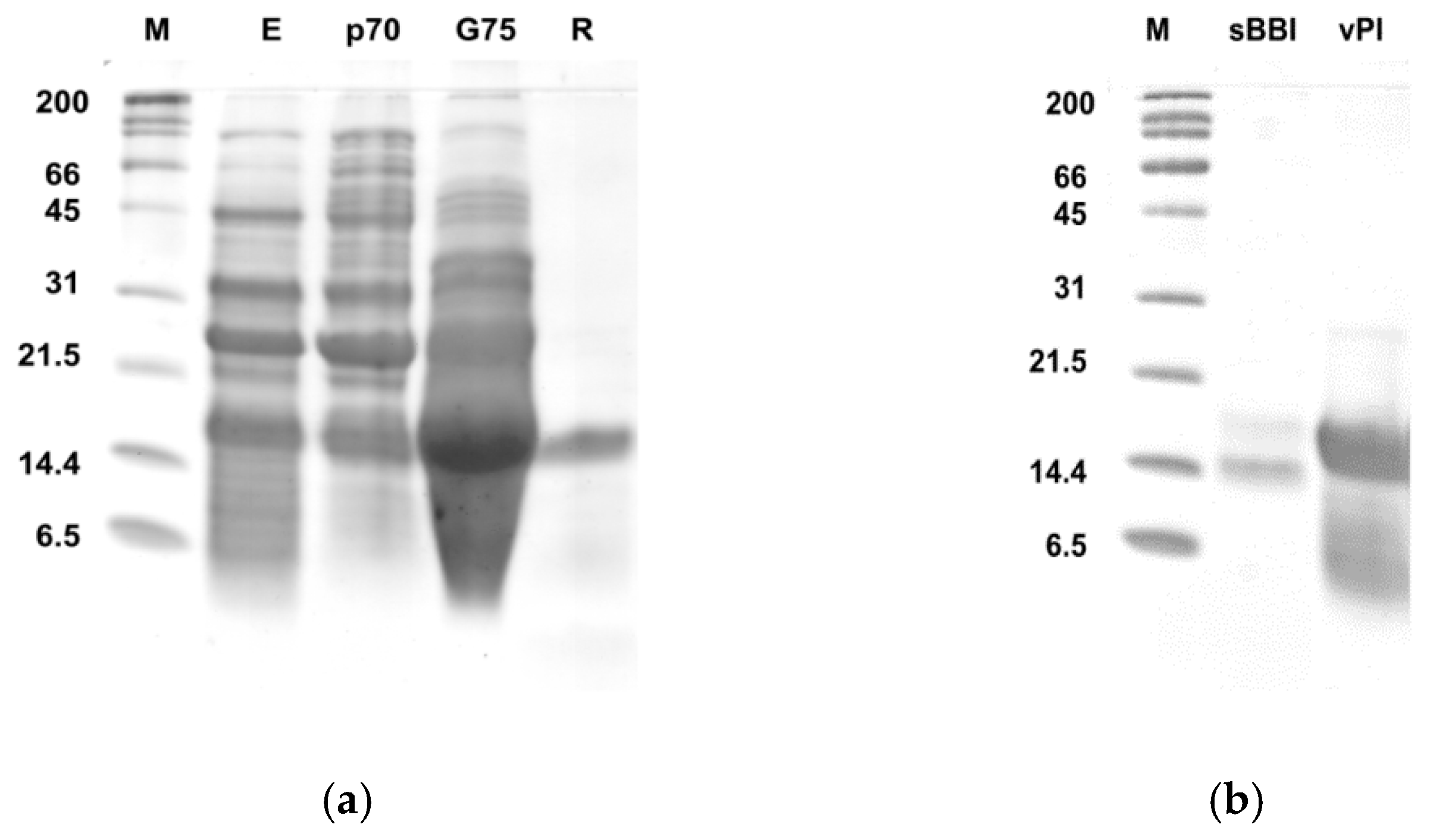
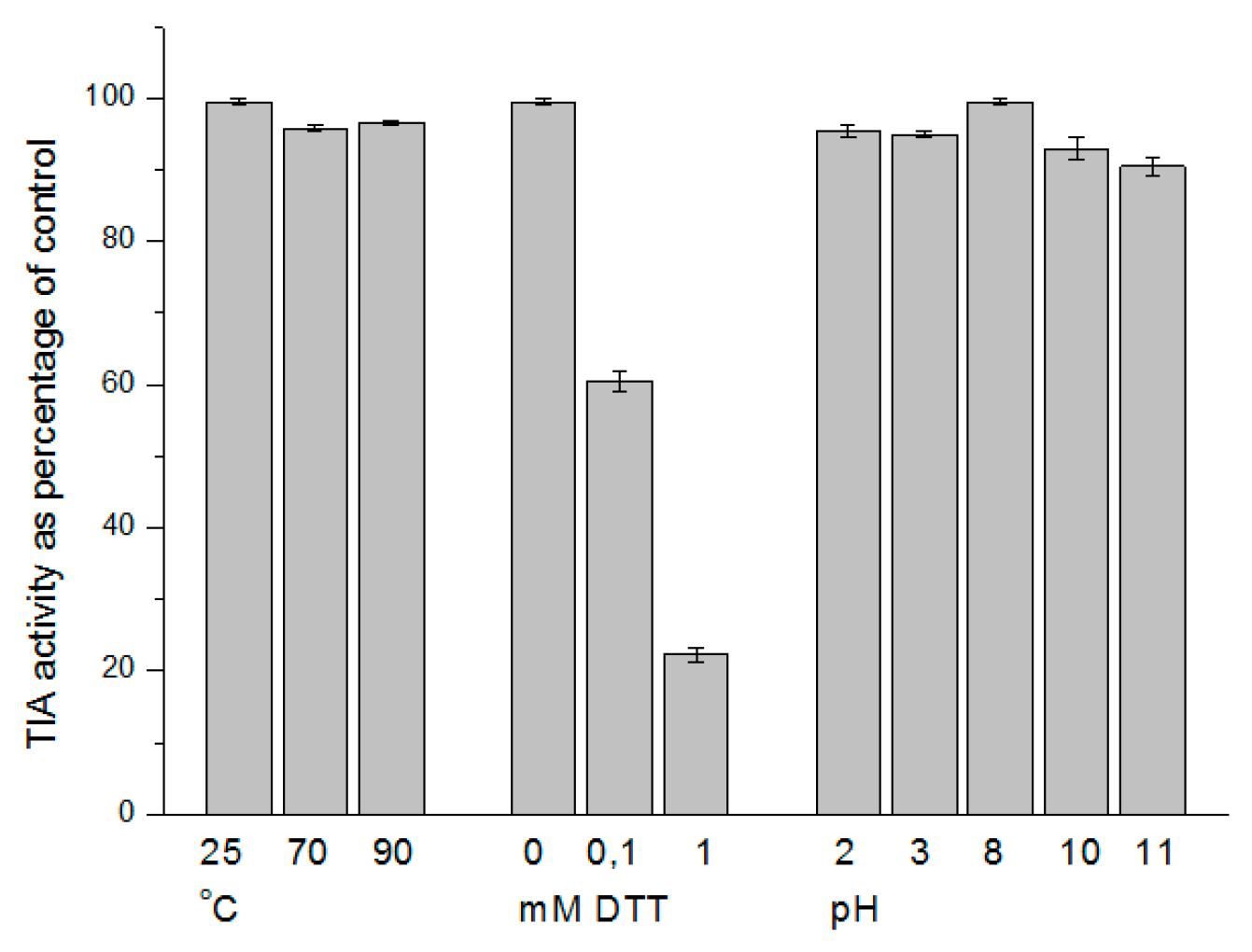
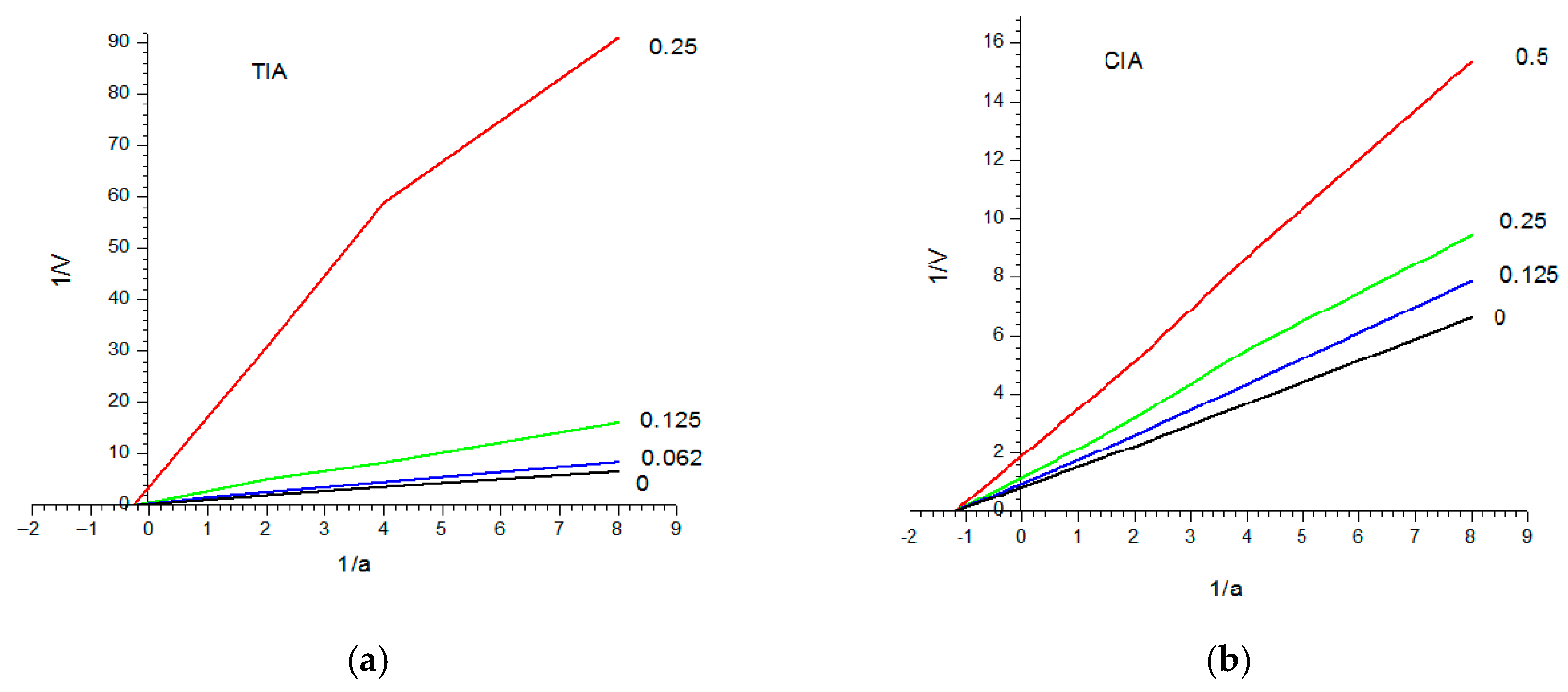
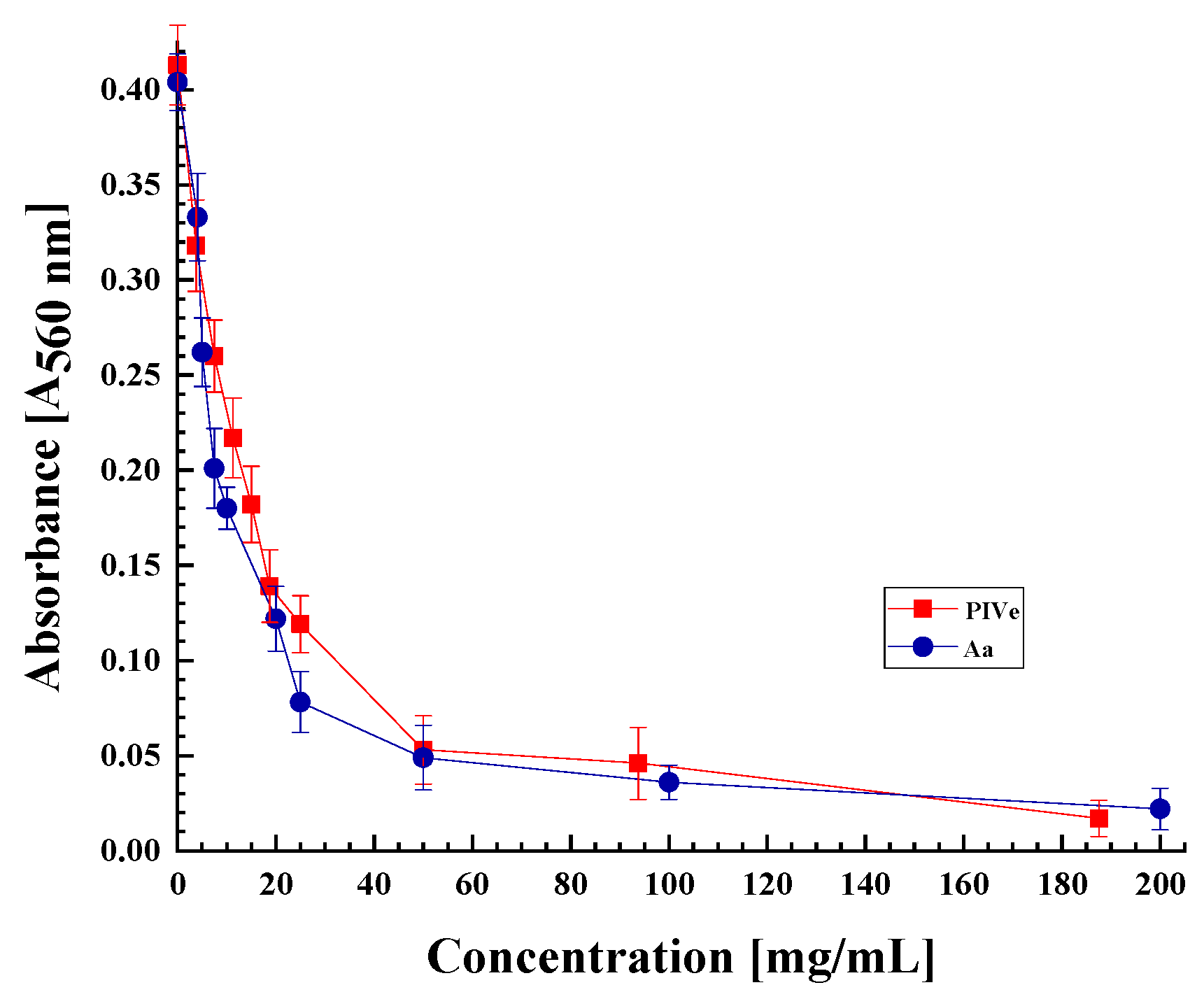
| Purification Step | Total Protein (mg) | Total TIA U·104 | Specific TIA U·mg−1 Pr·102 | Purif. Fold (Spec. TIA) | Yield % Total TIA |
|---|---|---|---|---|---|
| Extract | 850 | 2.64 | 31.06 | 1 | 100 |
| 30–70% (NH4)2SO4 | 680 | 2.55 | 37.5 | 1.187 | 96.59 |
| Sephadex G75 | 100 | 2.5 | 250 | 8.04 | 94.7 |
| Rechromatography | 9.6 | 0.33 | 343.75 | 11.07 | nd * |
| % Inhibition | ||||
|---|---|---|---|---|
| Strain | Protease inhibitor of V. ervilia | Nystatin | ||
| 24 h | 48 h | 24 h | 48 h | |
| A. alternata | 88.97 | 0 | 67.02 | 0 |
| A. solani | 96.12 | 87.72 | 90.88 | 86.32 |
| A. fumigatus | 94.09 | 0 | 76.35 | 75.21 |
| A. niger | 96.12 | 0 | 0 | 0 |
| C. albicans | 90.12 | 41.63 | 96.98 | 0 |
| F. solani | 95.52 | 87.13 | 98.47 | 45.34 |
| M. michei | 0 | 0 | 0 | 0 |
| P. griseofulvum | 93.36 | 40.52 | 81.8 | 0 |
| R. oryzae | 97.68 | 0 | 72.62 | 71.12 |
| Strain | MIC [mg/mL] | |
|---|---|---|
| 24 h | 48 h | |
| A. alternata | 0.175 | 0.35 |
| A. solani | 0.175 | 0.35 |
| A. fumigatus | 0.175 | 0.35 |
| A. niger | 0.35 | 0.35 |
| C. albicans | 0.7 | - |
| F. solani | 0.35 | 0.35 |
| M. michei | - | - |
| P. griseofulvum | 0.175 | 0.35 |
| R. oryzae | 0.35 | 0.35 |
| Variants | Concentration (mg/mL)/% Inhibition | IC50 Value (mg/mL) | ||
|---|---|---|---|---|
| DPPH | ABTS | DPPH | ABTS | |
| vPI | 1.25/19.37 | 0.375/33.02 | >4 | >2.5 |
| Ascorbic acid | 0.1/85.30 | 0.1/74.20 | 0.005 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrashev, R.; Krumova, E.; Angelova, M.; Miteva-Staleva, J.; Dishliyska, V.; Ralchev, N.; Stoyanova, Z.; Rodeva, R.; Simova-Stoilova, L. Bioactive Properties of a Serine Protease Inhibitor Purified from Vicia ervilia Seeds. Sci 2025, 7, 129. https://doi.org/10.3390/sci7030129
Abrashev R, Krumova E, Angelova M, Miteva-Staleva J, Dishliyska V, Ralchev N, Stoyanova Z, Rodeva R, Simova-Stoilova L. Bioactive Properties of a Serine Protease Inhibitor Purified from Vicia ervilia Seeds. Sci. 2025; 7(3):129. https://doi.org/10.3390/sci7030129
Chicago/Turabian StyleAbrashev, Radoslav, Ekaterina Krumova, Maria Angelova, Jeny Miteva-Staleva, Vladislava Dishliyska, Nikola Ralchev, Zornitsa Stoyanova, Rossitza Rodeva, and Lyudmila Simova-Stoilova. 2025. "Bioactive Properties of a Serine Protease Inhibitor Purified from Vicia ervilia Seeds" Sci 7, no. 3: 129. https://doi.org/10.3390/sci7030129
APA StyleAbrashev, R., Krumova, E., Angelova, M., Miteva-Staleva, J., Dishliyska, V., Ralchev, N., Stoyanova, Z., Rodeva, R., & Simova-Stoilova, L. (2025). Bioactive Properties of a Serine Protease Inhibitor Purified from Vicia ervilia Seeds. Sci, 7(3), 129. https://doi.org/10.3390/sci7030129








