Characterization of All Allotropes of Phosphorus
Abstract
1. Introduction
2. Structures of Phosphorus Allotropes
2.1. White Phosphorus (WP)
2.2. Red Phosphorus (RP)
2.3. Black Phosphorus (BP)
2.4. Blue Phosphorus (BuP)
3. Raman Spectra of Phosphorus Allotropes
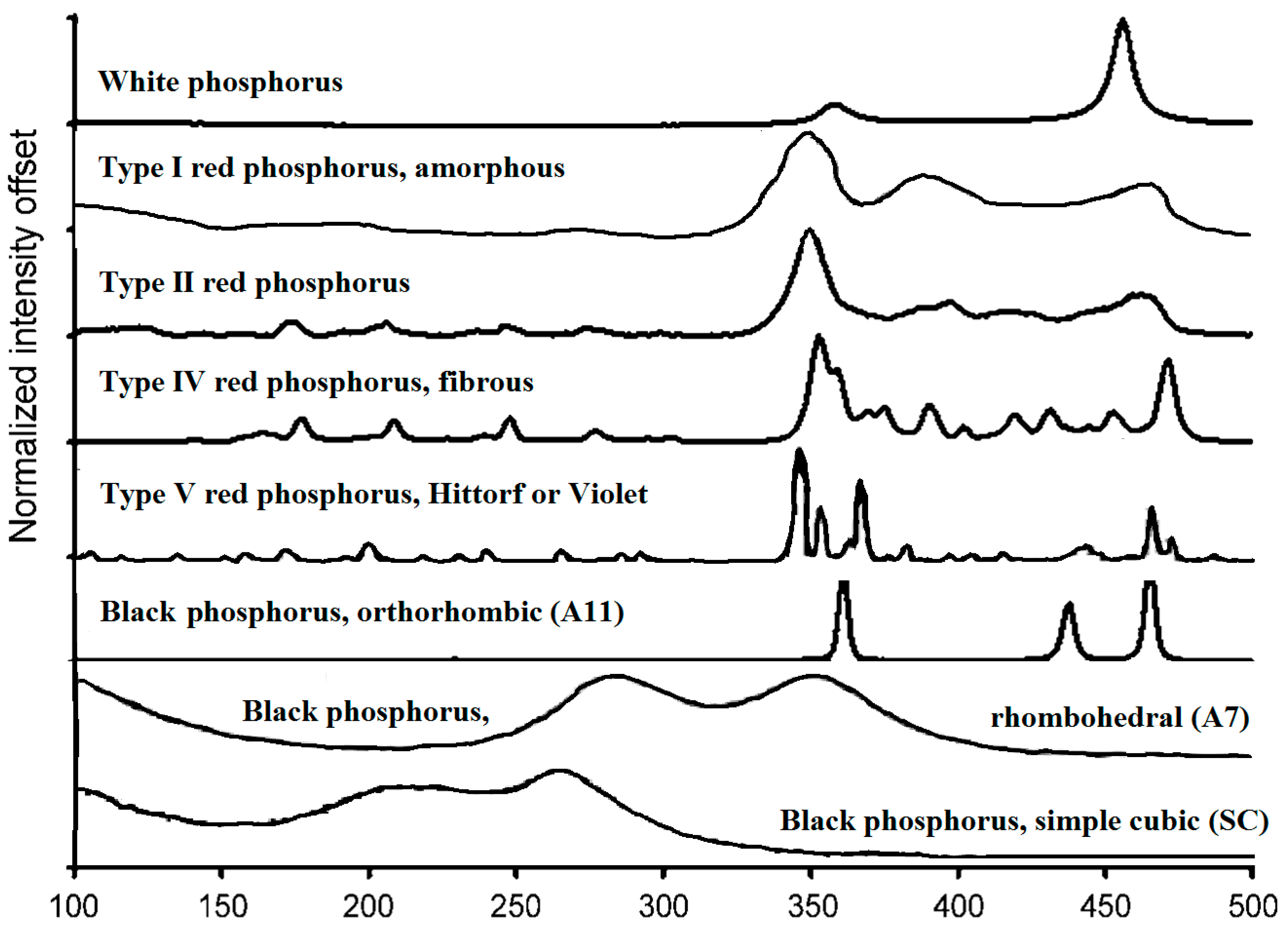
| Phosphorus Allotropes | Peak Locations and Assignments, cm−1 |
|---|---|
| White | F2 (457 [13], 461 [23]), E (357, 365, same sequence above), A1 (600, above, not shown in Figure 2). |
| a-RP (Type I) [35] | 350, 387, 464 (The assignment of the peaks remains to be determined). |
| Type II red | 350, 398, 417, 462 (Not assigned in the publication) [37] |
| Type III red | Not reported. It can only be prepared in a very narrow temperature range, and mixed with either type II or type IV in the sample. |
| Type IV red | Not specifically discussed in literature, but similar to those assigned for type V below. |
| Type V red [36] | Breathing waves along the tube (430–480); Longitudinal (420) and transverse breathing modes (360–400) of P8 and P9; Bond angle distortions (290–175); Bending waves or rotational deformation waves along the tubes (<160). |
| Orthorhombic black [19] | , longitudinal displacement along the trigonal b-axis (362 [19], 365 [38,39], 362 [40]), B2g, transverse motions (439, 442, 436, 439), , deformation of the zigzag chain along the a-axis (first paper) (466, 470, 471, 467) , displacement between zigzag chains parallel to the c-axis (228, 223, 230) B1g, displacement between zigzag chains parallel to the a-axis (192, 197, 193) |
| Rhombohedral black [19] | Eg, pressure dependent. (308 at 5 GPa, 290 at 7 GPa, 280 at 10 GPa), decrease at −5.5 cm−1/GPa. A1g pressure dependent. (388 at 5 GPa, 370 at 7 GPa, 340 at 10 GPa), decrease at −10 cm−1/GPa. |
| Simple cubic black | 230, 275. No explanation on the origin of the bands. [19] |
4. Powder X-Ray Diffraction
5. Single Crystal XRD
6. Transmission Electron Microscopy (TEM)/Selected Area Electron Diffraction (SAED)
7. High-Resolution Transient Electron Microscopy (HRTEM)
8. A Summary of the Characterization Features for Phosphorus Allotropes and a Guideline for Choosing and Interpreting Characterization Techniques
9. Common Pitfalls and Ambiguities
10. Conclusions
Funding
Conflicts of Interest
References
- Ruck, M.; Hoppe, D.; Wahl, B.; Simon, P.; Wang, Y.; Seifert, G. Fibrous Red Phosphorus. Angew. Chem. Int. Ed. 2005, 44, 7616–7619. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black Phosphorus Field-Effect Transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Corbridge, D.E.C.; Lowe, E.J. Structure of White Phosphorus: Single Crystal X-Ray Examination. Nature 1952, 170, 629. [Google Scholar] [CrossRef]
- Simon, A.; Borrmann, H.; Horakh, J. On the Polymorphism of White Phosphorus. Chem. Ber. 1997, 130, 1235–1240. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhao, S.; Han, C.; Wang, Z.; Zhong, S.; Sun, S.; Guo, R.; Zhou, X.; Gu, C.D.; Yuan, K.D.; et al. Epitaxial Growth of Single Layer Blue Phosphorus: A New Phase of Two-Dimensional Phosphorus. Nano Lett. 2016, 16, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Nachtrieb, N.H.; Handler, G.S. Self-Diffusion in α White Phosphorus. J. Chem. Phys. 1955, 23, 1187–1193. [Google Scholar] [CrossRef]
- Simon, A.; Borrmann, H.; Craubner, H. Crystal Structure of Ordered White Phosphorus(β-P). Phosphorous Sulfur Relat. Elem. 1987, 30, 507–510. [Google Scholar] [CrossRef]
- Okudera, H.; Dinnebier, R.E.; Simon, A. The Crystal Structure of γ -P 4, a Low Temperature Modification of White Phosphorus. Z. Krist.-Cryst. Mater. 2005, 220, 259–264. [Google Scholar] [CrossRef]
- Kohn, M. The Discovery of Red Phosphorus (1847) by Anton von Schrötter (1802–1875). J. Chem. Educ. 1944, 21, 522. [Google Scholar] [CrossRef]
- Roth, W.; DeWitt, T.; Smith, A.J. Polymorphism of Red Phosphorus. J. Am. Chem. Soc. 1947, 69, 2881–2885. [Google Scholar] [CrossRef]
- Hittorf, W. Zur Kenntniss des Phosphors. Ann. Phys. Chem. 1865, 202, 193–228. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, H.; Liu, Z.; Ju, H.; Lu, Z.; Zhang, H.; Chi, L.; Cui, S. Towards Unveiling the Exact Molecular Structure of Amorphous Red Phosphorus by Single-Molecule Studies. Angew. Chem. 2019, 131, 1673–1677. [Google Scholar] [CrossRef]
- Winchester, R.A.L.; Whitby, M.; Shaffer, M.S.P. Synthesis of Pure Phosphorus Nanostructures. Angew. Chem. 2009, 121, 3670–3675. [Google Scholar] [CrossRef]
- Thurn, H.; Krebs, H. Über Struktur Und Eigenschaften Der Halbmetalle. XXII. Die Kristallstruktur Des Hittorfschen Phosphors. Acta Crystallogr. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 125–135. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus, 6th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Maruyama, Y.; Suzuki, S.; Kobayashi, K.; Tanuma, S. Synthesis and Some Properties of Black Phosphorus Single Crystals. Physica B+C 1981, 105, 99–102. [Google Scholar] [CrossRef]
- Hultgren, R.; Gingrich, N.S.; Warren, B.E. The Atomic Distribution in Red and Black Phosphorus and the Crystal Structure of Black Phosphorus. J. Chem. Phys. 1935, 3, 351–355. [Google Scholar] [CrossRef]
- Cartz, L.; Srinivasa, S.R.; Riedner, R.J.; Jorgensen, J.D.; Worlton, T.G. Effect of Pressure on Bonding in Black Phosphorus. J. Chem. Phys. 1979, 71, 1718–1721. [Google Scholar] [CrossRef]
- Akahama, Y.; Kobayashi, M.; Kawamura, H. Raman Study of Black Phosphorus up to 13 GPa. Solid State Commun. 1997, 104, 311–315. [Google Scholar] [CrossRef]
- Kikegawa, T.; Iwasaki, H. An X-Ray Diffraction Study of Lattice Compression and Phase Transition of Crystalline Phosphorus. Acta Crystallogr. B Struct. Sci. 1983, 39, 158–164. [Google Scholar] [CrossRef]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@Black Phosphorus: An Easy Access to Black Phosphorus. Inorg. Chem. 2007, 46, 4028–4035. [Google Scholar] [CrossRef]
- Köpf, M.; Eckstein, N.; Pfister, D.; Grotz, C.; Krüger, I.; Greiwe, M.; Hansen, T.; Kohlmann, H.; Nilges, T. Access and in Situ Growth of Phosphorene-Precursor Black Phosphorus. J. Cryst. Growth 2014, 405, 6–10. [Google Scholar] [CrossRef]
- Rissi, E.N.; Soignard, E.; McKiernan, K.A.; Benmore, C.J.; Yarger, J.L. Pressure-Induced Crystallization of Amorphous Red Phosphorus. Solid State Commun. 2012, 152, 390–394. [Google Scholar] [CrossRef]
- Chang, K.J.; Cohen, M.L. Structural Stability of Phases of Black Phosphorus. Phys. Rev. B 1986, 33, 6177–6186. [Google Scholar] [CrossRef]
- Iwasaki, H.; Kikegawa, T.; Fujimura, T.; Endo, S.; Akahama, Y.; Akai, T.; Shimomura, O.; Yamaoka, S.; Yagi, T.; Akimoto, S.; et al. Synchrotron Radiation Diffraction Study of Phase Transitions in Phosphorus at High Pressures and Temperatures. Physica B+C 1986, 139–140, 301–304. [Google Scholar] [CrossRef]
- Akai, T.; Endo, S.; Akahama, Y.; Koto, K.; Marljyama, Y. The Crystal Structure and Oriented Transformation of Black Phosphorus under High Pressure. High Press. Res. 1989, 1, 115–130. [Google Scholar] [CrossRef]
- Akahama, Y.; Endo, S.; Narita, S. Electrical Properties of Single-Crystal Black Phosphorus under Pressure. Physica B+C 1986, 139–140, 397–400. [Google Scholar] [CrossRef]
- Bachhuber, F.; Von Appen, J.; Dronskowski, R.; Schmidt, P.; Nilges, T.; Pfitzner, A.; Weihrich, R. Van Der Waals Interactions in Selected Allotropes of Phosphorus. Z. Krist.-Cryst. Mater. 2015, 230, 107–115. [Google Scholar] [CrossRef]
- Zhu, Z.; Tománek, D. Semiconducting Layered Blue Phosphorus: A Computational Study. Phys. Rev. Lett. 2014, 112, 176802. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.L.; Chen, W.; Li, Z. Structure of Blue Phosphorus Grown on Au(111) Surface Revisited. J. Phys. Chem. C 2020, 124, 2024–2029. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhao, S.; Sun, S.; Ding, H.; Hu, J.; Li, Y.; Xu, Q.; Yu, X.; Telychko, M.; Su, J.; et al. Synthesis of Monolayer Blue Phosphorus Enabled by Silicon Intercalation. ACS Nano 2020, 14, 3687–3695. [Google Scholar] [CrossRef]
- Durig, J.R.; Casper, J.M. On the Vibrational Spectra and Structure of Red phosphorus. J. Mol. Struct. 1970, 5, 351–358. [Google Scholar] [CrossRef]
- Shanabrook, B.V.; Lannin, J.S. Structural and Vibrational Properties of Amorphous Phosphorus. Phys. Rev. B 1981, 24, 4771–4780. [Google Scholar] [CrossRef]
- Lannin, J.S.; Shanabrook, B.V. Raman Scattering and Infrared Absorption in Bulk Amorphous Red Phosphorous. Solid State Commun. 1978, 28, 497–500. [Google Scholar] [CrossRef]
- Olego, D.J.; Baumann, J.A.; Kuck, M.A.; Schachter, R.; Michel, C.G.; Raccah, P.M. The Microscopic Structure of Bulk Amorphous Red Phosphorus: A Raman Scattering Investigation. Solid State Commun. 1984, 52, 311–314. [Google Scholar] [CrossRef]
- Fasol, G.; Cardona, M.; Hönle, W.; von Schnering, H.G. Lattice Dynamics of Hittorf’s Phosphorus and Identification of Structural Groups and Defects in Amorphous Red Phosphorus. Solid State Commun. 1984, 52, 307–310. [Google Scholar] [CrossRef]
- Olego, D.J.; Baumann, J.A.; Schachter, R. The Microscope Structures of Amorphous Phosphorus. Solid State Commun. 1985, 53, 905–908. [Google Scholar] [CrossRef]
- Sugai, S.; Shirotani, I. Raman and Infrared Reflection Spectroscopy in Black Phosphorus. Solid State Commun. 1985, 53, 753–755. [Google Scholar] [CrossRef]
- Sugai, S.; Ueda, T.; Murase, K. Pressure Dependence of the Lattice Vibration in the Orthorhombic and Rhombohedral Structures of Black Phosphorus. J. Phys. Soc. Jpn. 1981, 50, 3356–3361. [Google Scholar] [CrossRef]
- Vanderborgh, C.A.; Schiferl, D. Raman Studies of Black Phosphorus from 0.25 to 7.7 GPa at 15 K. Phys. Rev. B 1989, 40, 9595–9599. [Google Scholar] [CrossRef]
- Lannin, J.S.; Shanabrook, B.V.; Gompf, F. Intermediate Range Order in Amorphous Phosphorous. J. Non-Cryst. Solids 1982, 49, 209–219. [Google Scholar] [CrossRef]
- Ruan, B.; Wang, J.; Shi, D.; Xu, Y.; Chou, S.; Liu, H.; Wang, J. A Phosphorus/N-Doped Carbon Nanofiber Composite as an Anode Material for Sodium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19011–19017. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, Z.; Wang, W.; Lee, S.-F.; Chan, D.K.L.; Li, Y.; Gu, T.; Yu, J.C. Crystalline Phosphorus Fibers: Controllable Synthesis and Visible-Light-Driven Photocatalytic Activity. Nanoscale 2014, 6, 14163–14167. [Google Scholar] [CrossRef]
- Eckstein, N.; Hohmann, A.; Weihrich, R.; Nilges, T.; Schmidt, P. Synthesis and Phase Relations of Single-Phase Fibrous Phosphorus. Z. Anorg. Allg. Chem. 2013, 639, 2741–2743. [Google Scholar] [CrossRef]
- Nilges, T.; Kersting, M.; Pfeifer, T. A Fast Low-Pressure Transport Route to Large Black Phosphorus Single Crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]
- Clark, S.M.; Zaug, J.M. Compressibility of Cubic White, Orthorhombic Black, Rhombohedral Black, and Simple Cubic Black Phosphorus. Phys. Rev. B 2010, 82, 134111. [Google Scholar] [CrossRef]
- Natta, G.; Passerini, L. The Crystal Structure of White Phosphorus. Nature 1930, 125, 707–708. [Google Scholar] [CrossRef]
- Jamieson, J.C. Crystal Structures Adopted by Black Phosphorus at High Pressures. Science 1963, 139, 1291–1292. [Google Scholar] [CrossRef]
- Smith, J.B.; Hagaman, D.; DiGuiseppi, D.; Schweitzer-Stenner, R.; Ji, H. Ultra-Long Crystalline Red Phosphorus Nanowires from Amorphous Red Phosphorus Thin Films. Angew. Chem. Int. Ed. 2016, 55, 11829–11833. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, H.; Niu, X.; Wang, W.; Guan, L.; Sha, J.; Wang, Y. Growth Mechanism and Enhanced Yield of Black Phosphorus Microribbons. Cryst. Growth Des. 2016, 16, 1096–1103. [Google Scholar] [CrossRef]
- Sun, L.-Q.; Li, M.-J.; Sun, K.; Yu, S.-H.; Wang, R.-S.; Xie, H.-M. Electrochemical Activity of Black Phosphorus as an Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 14772–14779. [Google Scholar] [CrossRef]
- Zhang, C.D.; Lian, J.C.; Yi, W.; Jiang, Y.H.; Liu, L.W.; Hu, H.; Xiao, W.D.; Du, S.X.; Sun, L.L.; Gao, H.J. Surface Structures of Black Phosphorus Investigated with Scanning Tunneling Microscopy. J. Phys. Chem. C 2009, 113, 18823–18826. [Google Scholar] [CrossRef]
- Goodman, N.B.; Ley, L.; Bullett, D.W. Valence-Band Structures of Phosphorus Allotropes. Phys. Rev. B 1983, 27, 7440–7450. [Google Scholar] [CrossRef]


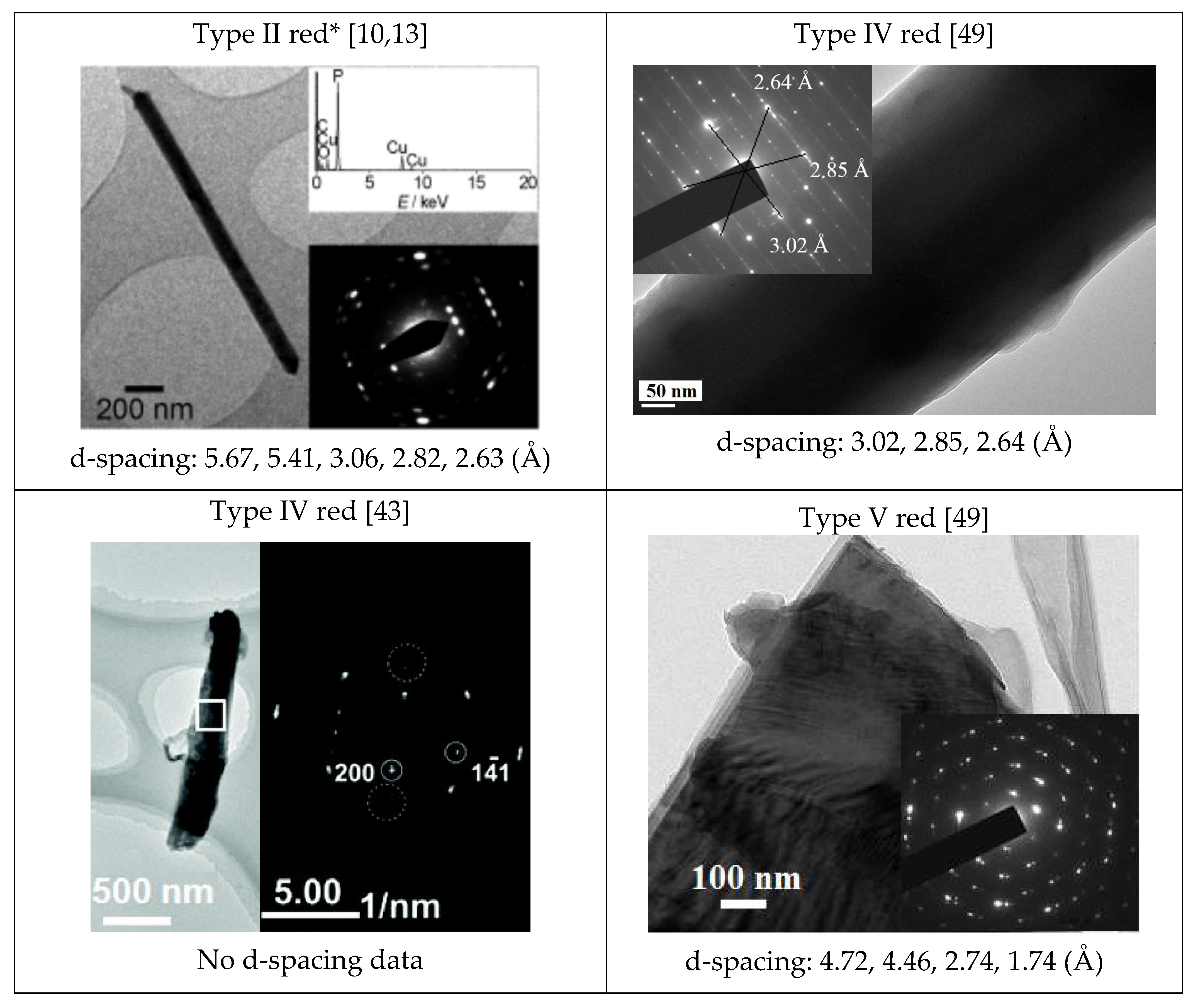
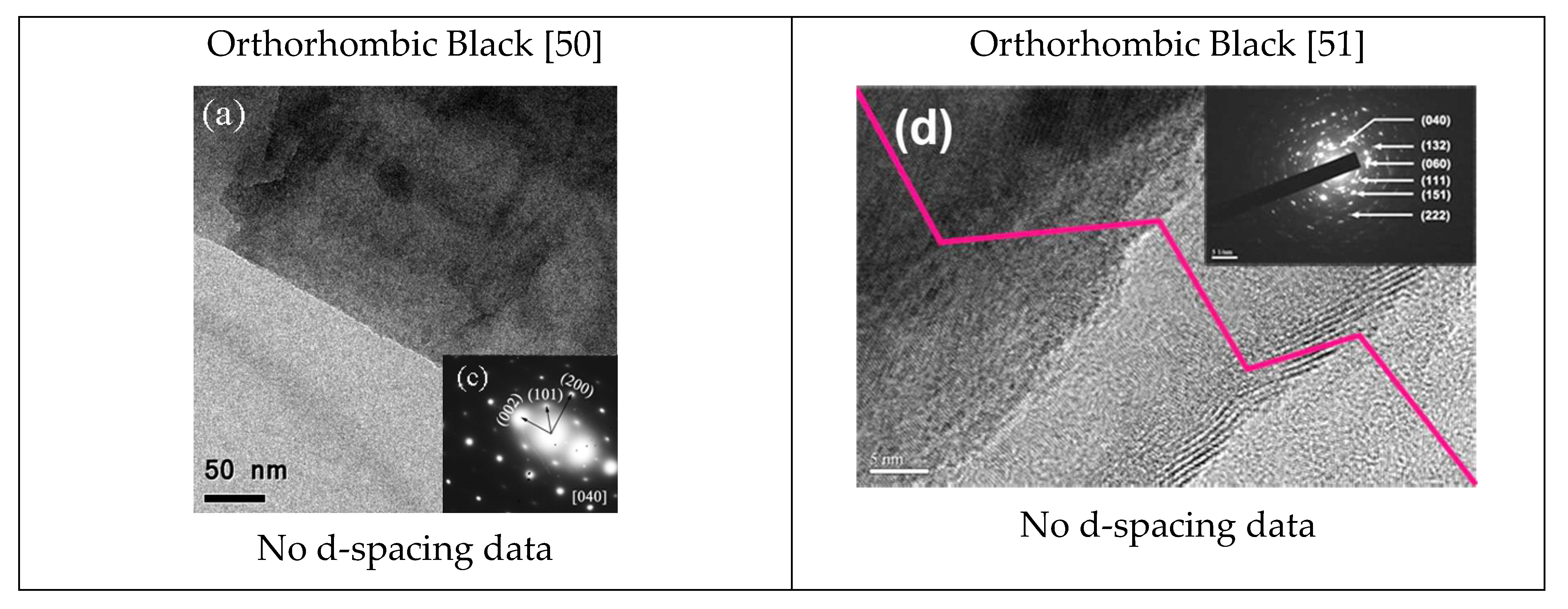

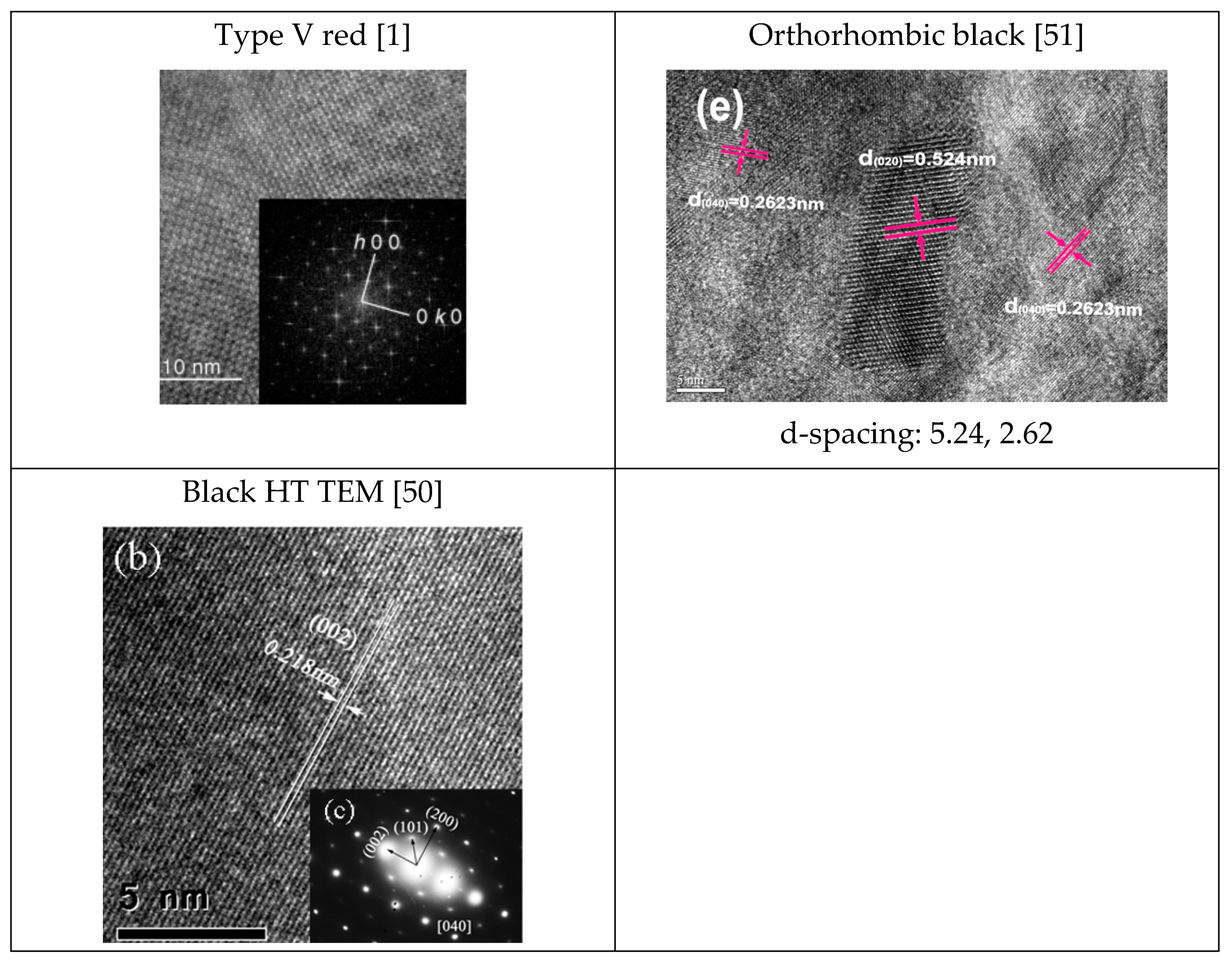
| Phosphorus Allotropes | Notable Peak Locations, 2θ (Degree) |
|---|---|
| a-RP (Type I) [42] | 15, 34, 56 * |
| Type II red * [10,13] | 15.8, 16.5, ~27, 31.6, 34 |
| Type IV red [44] | 15, 16, 17, 28, 31, 34, 57 |
| Type V red [45] | 15, 29, 52 |
| Orthorhombic black [45] | 35, 51, 56 |
| White Phosphorus | a = 7.17 Å for α-WP, a ≈ 7.43 Å, c ≈ 18.9 Å for β-WP, and γ-WP Unknown [47]. |
| Type II red | No single crystal data reported. |
| Type IV red | Triclinic. Cell constants: a = 1219.8 pm, b = 1298.6 pm, c = 707.5 pm; α = 116.99°, β = 106.31°, γ = 97.91°. P-P bond lengths are between 219 pm and 231 pm; the mean length is 221.9 pm [1]. |
| Type V red | Monoclinic. Cell constants: a = 9.21 Å, b = 9.15 Å, c = 22.60 Å, β = 106.1°, Z = 84. The average P-P bond length is 2.219 Å [14]. |
| Orthorhombic black | a = 3.31 Å, b = 4.38 Å, c = 10.50 Å. P-P bond length 2.18 Å [9], α = β = γ = 90°. |
| Rhombohedral black | a = 3.377 Å, c = 8.806 Å, P-P bond length 2.13 Å, α = 57.25° [48] |
| Simple cubic black | a = 2.377 Å, α = 60° [48]. |
| Allotrope | Raman Peaks (cm−1) | Powder XRD Peaks (2θ, °) | Single Crystal | TEM d-Spacings (Å) |
|---|---|---|---|---|
| Red P—Type I | 350, 387, 464 | 15, 34, 56 | No data. | Not reported |
| Red P—Type II | 350, 398, 417, 462 | 15.8, 16.5, ~27.20 *, 28.4 **, 31.6, 34.1 | Not reported. | 18.3 (HR), 5.67, 5.41, 3.05, 2.82, 2.63 |
| Red P—Type III | Not reported | 15.5, 19.8, 27.3, 28.7, 30.8, 31.0, 34.1 | Not reported. | Not reported |
| Red P—Type IV | Similar to Type V | 15, 16, 17, 28, 31, 34, 57 | Triclinic. Cell constants: a = 1219.8 pm, b = 1298.6 pm, c = 707.5 pm; α = 116.99°, β = 106.31°, γ = 97.91°. | 3.02, 2.85, 2.64, 2.78, 5.81 |
| Red P—Type V | <160, 290–175, 420, 360–400, 430–480. | 15, 29, 52 | Monoclinic. Cell constants: a = 9.21 Å, b = 9.15 Å, c = 22.60 Å, β = 106.1°, Z = 84. | 4.72, 4.46, 2.74, 1.74 |
| Black P—Ortho | 192–197, 228–230, 362–365, 439–442, 466–471 | 35, 51, 56 | a = 3.31 Å, b = 4.38 Å, c = 10.50 Å. β = 99°. | 5.24, 2.62, 2.18 |
| Sample Type | Recommended Technique | What to Look For | Notes |
|---|---|---|---|
| Bulk crystalline sample | Powder XRD or Single-Crystal XRD | Phase identification via 2θ peaks or cell parameters | Use XRD for types II, IV, V, and Black P (orthorhombic) |
| Thin film | Raman + GIXRD | Raman fingerprints, weak XRD peaks | Useful for a-RP; Raman shows broad features |
| Nanowires or nanoribbons | TEM/HRTEM + Raman | Lattice spacings, morphology, vibrational modes | HRTEM can show lattice periodicity for Types II, IV, V |
| Amorphous powders | Raman + PXRD | Broad PXRD humps, Raman cluster features (P8/P9) | Look for similarity to types II/V in Raman |
| Exfoliated monolayers | Raman + STEM | Raman peak positions (strain sensitive), sheet morphology | Use polarized Raman if available |
| Pitfall/Ambiguity | Cause | Tips to Resolve/Distinguish |
|---|---|---|
| Confusing a-RP with type II or V | Broad Raman peaks can overlap; amorphous character masks structure | Compare Raman to known P8/P9 modes (430–480 cm−1); supplement with XRD for crystalline content |
| Overlapping Raman bands among red P polymorphs | Similar backbone structures (e.g., P9 cages in type IV and V) | Use both Raman and XRD together; look for characteristic peak broadness in type I, and sharper peaks in V |
| Assigning mixed-phase samples as pure | Samples (especially red P) may contain coexisting allotropes | TEM or XRD may reveal multiple lattice spacings or 2θ peaks; always report the possibility of mixed-phase presence |
| Low crystallinity leads to weak/absent XRD peaks | Amorphous or poorly ordered samples (e.g., a-RP) | Use Raman as primary tool; avoid over-interpreting noisy XRD patterns |
| Contamination from substrate (e.g., BuP on Au) | Elemental intercalation distorts crystal structure | Verify purity via EDX or intercalation control (e.g., use silicon buffer layers) |
| Assuming blue phosphorus has the same features as black | Structural changes dramatically alter Raman/XRD signatures | Cross-check against theoretical predictions; expect distinct Raman and regular hexagonal features |
| Misinterpretation of TEM d-spacing due to orientation effects | TEM lattice spacing depends on imaging axis | Confirm zone axis orientation or use FFT patterns; pair with HRTEM |
| Technique | Main Strength | Why It Is Omitted Here | When It May Become Essential |
|---|---|---|---|
| FTIR/ATR-IR | Sensitive to P–H, P–O, and surface-oxidized species | Overlaps heavily with Raman for bulk modes; most allotropes are IR-silent in the mid-IR | Oxidation studies of BP devices; probing functionalized phosphorene |
| X-ray Photoelectron Spectroscopy (XPS) | Quantifies oxidation states and surface chemistry | Surface-sensitive only; does not resolve long-range crystal order | Tracking ambient degradation of thin BP; validating P-elemental doping |
| Scanning Tunnelling Microscopy (STM)/AFM | Real-space atomic imaging; topography | Requires ultra-clean, atomically flat surfaces rarely available for red-P polymorphs | Band-edge mapping of exfoliated monolayer BP or BuP |
| Neutron or Electron Diffraction (ED) | High sensitivity to light atoms; dynamic in-situ studies | Limited beam-time access; phosphorus is a strong neutron absorber; ED often duplicates TEM SAED | Phase transitions under rapid heating or electrochemical cycling |
| EELS/EXAFS/XANES | Bond-specific, oxidation-state-sensitive | Requires synchrotron/electron microscope add-ons and complex modelling | Tracking local coordination in a-RP and black-to-blue reconstructions |
| Magnetic, Optical Pump–Probe, THz, etc. | Electronic/phonon dynamics | Beyond structural scope of this review | Device-level performance optimization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walters, J.T.; Cao, M.; Lam, Y.; Schwenk, G.R.; Ji, H.-F. Characterization of All Allotropes of Phosphorus. Sci 2025, 7, 128. https://doi.org/10.3390/sci7030128
Walters JT, Cao M, Lam Y, Schwenk GR, Ji H-F. Characterization of All Allotropes of Phosphorus. Sci. 2025; 7(3):128. https://doi.org/10.3390/sci7030128
Chicago/Turabian StyleWalters, John T., Meijuan Cao, Yuki Lam, Gregory R. Schwenk, and Hai-Feng Ji. 2025. "Characterization of All Allotropes of Phosphorus" Sci 7, no. 3: 128. https://doi.org/10.3390/sci7030128
APA StyleWalters, J. T., Cao, M., Lam, Y., Schwenk, G. R., & Ji, H.-F. (2025). Characterization of All Allotropes of Phosphorus. Sci, 7(3), 128. https://doi.org/10.3390/sci7030128







