Sex-Specific Metabolic, Immunologic, and Behavioral Effects of Perfluorooctane Sulfonic Acid (PFOS) in BTBR-mtB6 Mice
Abstract
1. Introduction
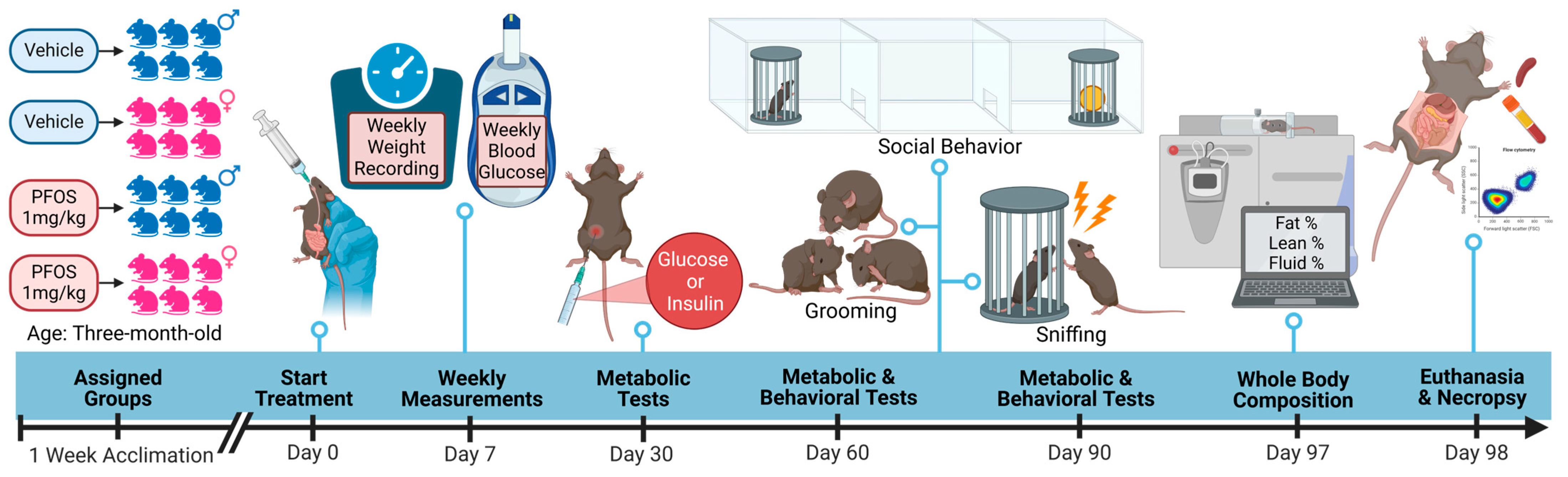
2. Materials and Methods
2.1. Animal Husbandry and Housing
2.2. Animal Exposure
2.3. Measurement of Body Weight and Blood Glucose Levels
2.4. Measurement of Free Body Fluid, Fat and Lean Tissue
2.5. Glucose and Insulin Tolerance Tests Following Intraperitoneal Injection
2.6. Behavioral Tests and Analysis
2.7. Necropsy and Flow Cytometric Analysis of Splenic Leukocytes
2.8. Statistical Analysis
3. Results
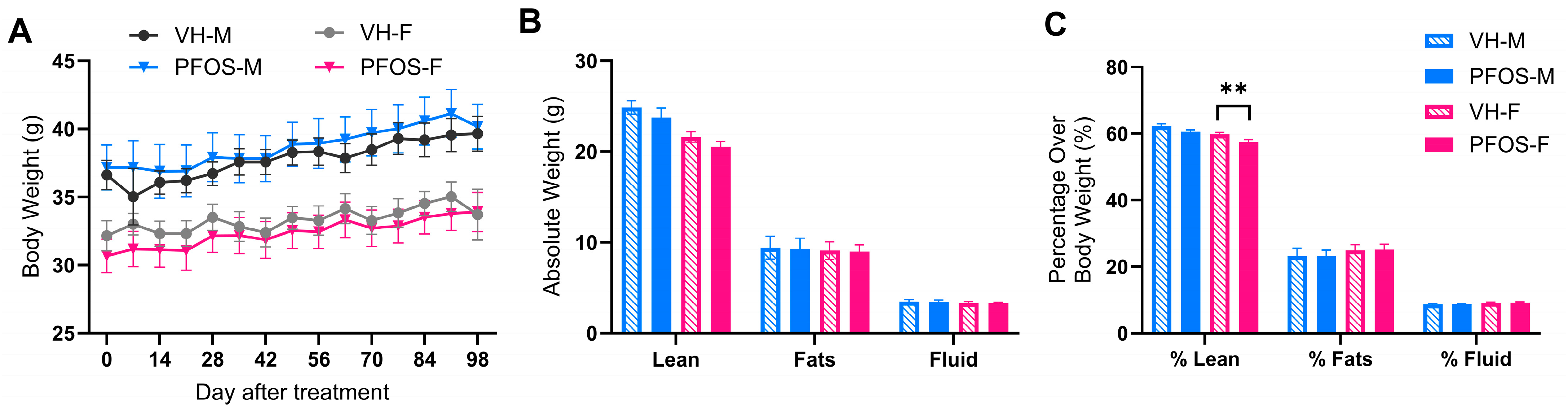
3.1. Effects on Body Weights, Organ Weights, and Body Compositions
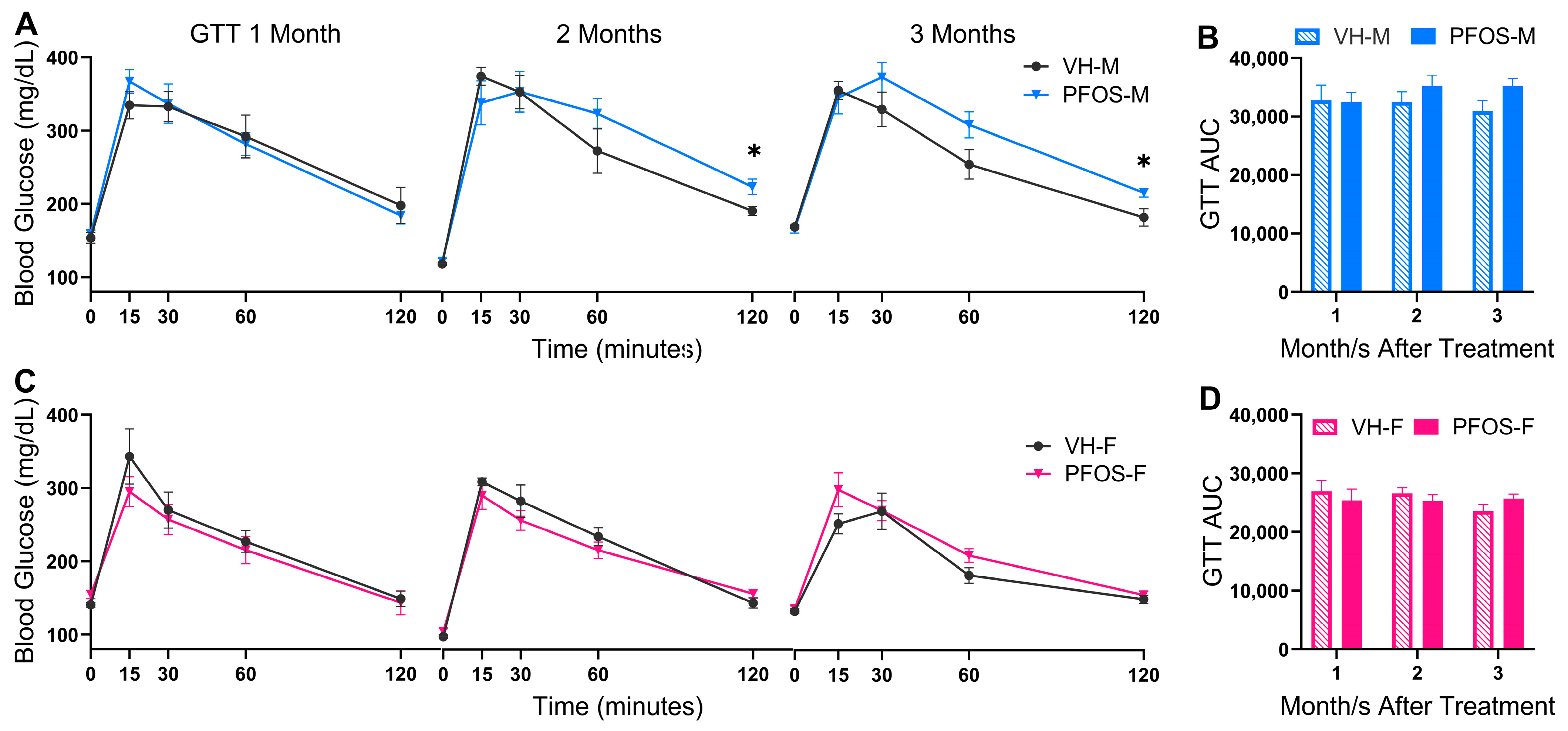
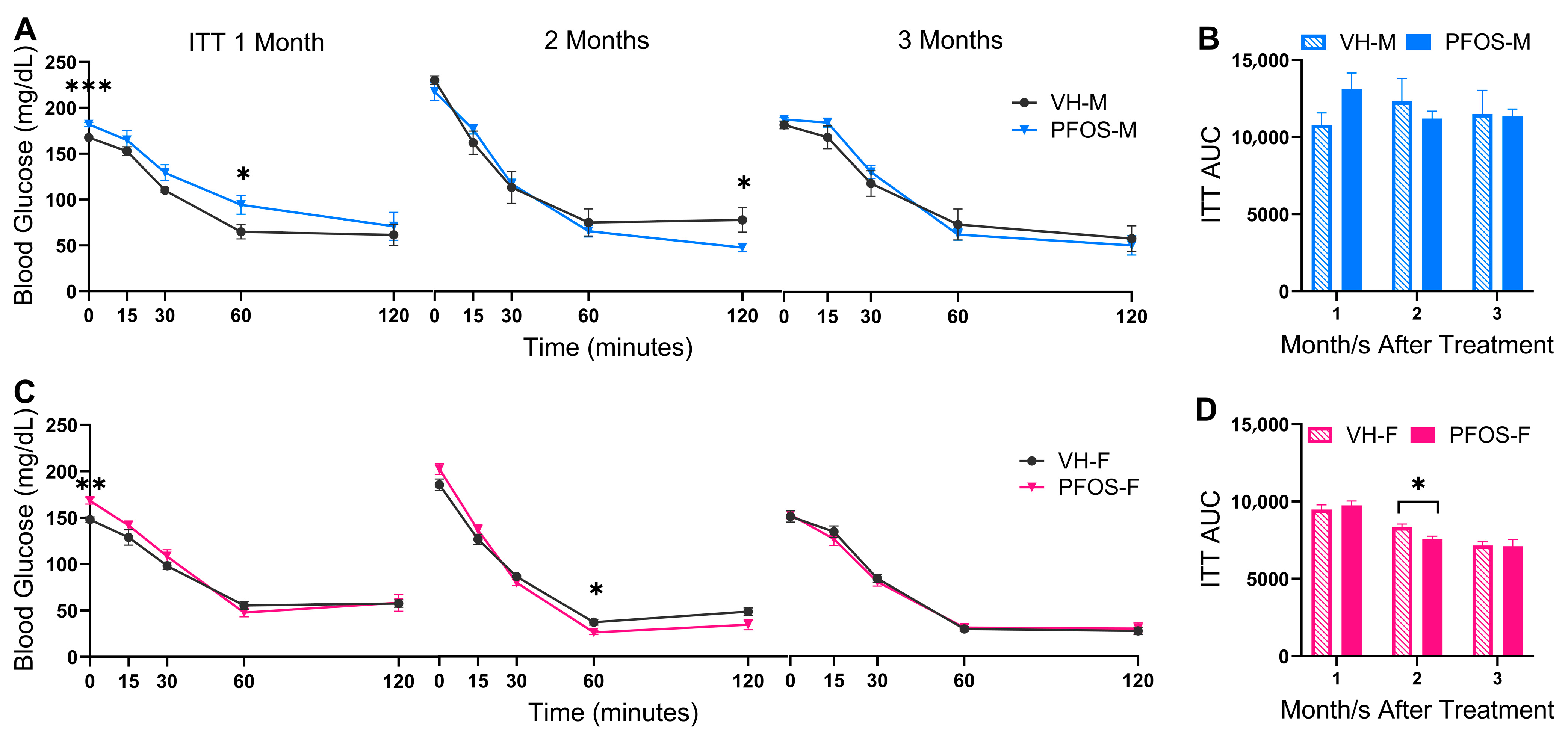
3.2. Effects on Blood Glucose Homeostasis
3.3. Effects on Behaviors
3.4. Effects on Immunity
4. Discussion and Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- NIH. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). 2024. Available online: https://www.niehs.nih.gov/health/topics/agents/pfc (accessed on 31 May 2025).
- EPA. Key EPA Actions to Address PFAS. Available online: https://www.epa.gov/pfas/key-epa-actions-address-pfas (accessed on 2 July 2024).
- Fuentes, S.; Vicens, P.; Colomina, M.T.; Domingo, J.L. Behavioral effects in adult mice exposed to perfluorooctane sulfonate (PFOS). Toxicology 2007, 242, 123–129. [Google Scholar] [CrossRef]
- Dong, G.-H.; Liu, M.-M.; Wang, D.; Zheng, L.; Liang, Z.-F.; Jin, Y.-H. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch. Toxicol. 2011, 85, 1235–1244. [Google Scholar] [CrossRef]
- Wan, H.T.; Cheung, L.Y.; Chan, T.F.; Li, M.; Lai, K.P.; Wong, C.K.C. Characterization of PFOS toxicity on in-vivo and ex-vivo mouse pancreatic islets. Environ. Pollut. 2021, 289, 117857. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, G.-H.; Jin, Y.-H.; He, Q.-C. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch. Toxicol. 2009, 83, 679–689. [Google Scholar] [CrossRef]
- Giulivi, C.; Zhang, Y.-F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial Dysfunction in Autism. JAMA 2010, 304, 2389. [Google Scholar] [CrossRef]
- Yui, K.; Sato, A.; Imataka, G. Mitochondrial Dysfunction and Its Relationship with mTOR Signaling and Oxidative Damage in Autism Spectrum Disorders. Mini-Rev. Med. Chem. 2015, 15, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Pallejà, A.; Torrell, H.; Muntané, G.; Cortés, M.J.; Martínez-Leal, R.; Abasolo, N.; Alonso, Y.; Vilella, E.; Martorell, L. Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Hum. Mol. Genet. 2018, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Foguth, R.M.; Flynn, R.W.; De Perre, C.; Iacchetta, M.; Lee, L.S.; Sepúlveda, M.S.; Cannon, J.R. Developmental exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) selectively decreases brain dopamine levels in Northern leopard frogs. Toxicol. Appl. Pharmacol. 2019, 377, 114623. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.; Fredriksson, A.; Eriksson, P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neuro Toxicol. 2008, 29, 160–169. [Google Scholar] [CrossRef]
- Yu, N.; Wei, S.; Li, M.; Yang, J.; Li, K.; Jin, L.; Xie, Y.; Giesy, J.P.; Zhang, X.; Yu, H. Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Sci. Rep. 2016, 6, 23963. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, J.; Yan, S.; Li, Y.; Wang, Y.; Liu, X.; Xiao, C. Exposure to Perfluorooctanoic Acid Induces Cognitive Deficits via Altering Gut Microbiota Composition, Impairing Intestinal Barrier Integrity, and Causing Inflammation in Gut and Brain. J. Agric. Food Chem. 2020, 68, 13916–13928. [Google Scholar] [CrossRef]
- Patel, R.; Bradner, J.; Stout, K.; Caudle, W. Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo. Med. Sci. 2016, 4, 13. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Ji, G.; Yan, L.; Hu, F.; Gu, A. Neurotoxicity of Perfluorooctane Sulfonate to Hippocampal Cells in Adult Mice. PLoS ONE 2013, 8, e54176. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Liu, W.; Wang, F.; Hao, S. Possible mechanism of perfluorooctane sulfonate and perfluorooctanoate on the release of calcium ion from calcium stores in primary cultures of rat hippocampal neurons. Toxicol. Vitr. 2011, 25, 1294–1301. [Google Scholar] [CrossRef]
- Jiang, D.Q.Y.; Guo, T.L. Interaction between Per- and Polyfluorinated Substances (PFAS) and Acetaminophen in Disease Exacerbation—Focusing on Autism and the Gut–Liver–Brain Axis. Toxics 2024, 12, 39. [Google Scholar] [CrossRef]
- Oh, J.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Roa, D.L.; Schmidt, R.J.; Hertz-Picciotto, I.; Shin, H.-M. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ. Int. 2021, 147, 106328. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Oh, J.; Bennett, D.H.; Calafat, A.M.; Schmidt, R.J.; Shin, H.-M. Prenatal exposure to per- and polyfluoroalkyl substances and child behavioral problems. Environ. Res. 2024, 251, 118511. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-M.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: A case-control study. Environ. Res. 2020, 186, 109514. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Shin, H.-M.; Kannan, K.; Busgang, S.A.; Schmidt, R.J.; Schweitzer, J.B.; Hertz-Picciotto, I.; Bennett, D.H. Childhood exposure to per- and polyfluoroalkyl substances and neurodevelopment in the CHARGE case-control study. Environ. Res. 2022, 215, 114322. [Google Scholar] [CrossRef]
- Mascari, M.; Cohen, N.; Yao, M.; Huang, J.; Lane, J.; Reeves, K.W.; Balasubramanian, R.; Liu, Z.; Laouali, N.; Daniel, L.M.; et al. Associations of cord blood concentrations of perfluoroalkyl substances with autistic traits in Singaporean children: The growing up in Singapore towards healthy outcomes study. Chemosphere 2025, 371, 144040. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Jin, N.; He, W.-T.; Zhang, Y.-T.; Huang, J.-W.; Liang, L.-X.; Zhou, J.-X.; Zhang, Z.-Q.; Wang, X.; Gui, Z.-H.; et al. Associations of childhood per- and polyfluoroalkyl substances with autistic traits and symptom severities among children with and without autism spectrum disorder. Hyg. Environ. Health Adv. 2025, 15, 100131. [Google Scholar] [CrossRef]
- Yao, Y.; Uddin, M.N.; Manley, K.; Lawrence, D.A. Improvements of autism-like behaviors but limited effects on immune cell metabolism after mitochondrial replacement in BTBR T Itpr3/J mice. J. Neuroimmunol. 2022, 368, 577893. [Google Scholar] [CrossRef]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant Immune Responses in a Mouse with Behavioral Disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, D.; Kluetzman, K.; Mendoza, A.; Bolivar, V.J.; Reilly, A.; Jolly, J.K.; Lawrence, D.A. The maternal autoimmune environment affects the social behavior of offspring. J. Neuroimmunol. 2013, 258, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; O’Neill, S.M.; Narayan, S.; Tiwari, A.; Arnold, E.; Samaroo, H.D.; Du, F.; Ring, R.H.; Campbell, B.; Pletcher, M.; et al. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol. Autism 2011, 2, 7. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Jahrami, H.A.; Alwehaidah, M.S.; Alshammari, Y.; Husni, M. Mitochondrial DNA copy number in autism spectrum disorder and attention deficit hyperactivity disorder: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1196035. [Google Scholar] [CrossRef]
- EPA. Final Toxicity Assessment for Perfluorooctane Sulfonic Acid (PFOS); U.S. Environmental Protection Agency: Washington, DC, USA, 2024.
- Peden-Adams, M.M.; Keller, J.M.; Eudaly, J.G.; Berger, J.; Gilkeson, G.S.; Keil, D.E. Suppression of Humoral Immunity in Mice following Exposure to Perfluorooctane Sulfonate. Toxicol. Sci. 2008, 104, 144–154. [Google Scholar] [CrossRef]
- Xing, J.; Wang, G.; Zhao, J.; Wang, E.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ. Pollut. 2016, 210, 388–396. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Mandel, J.H.; Zobel, L.R. Serum Perfluorooctane Sulfonate and Hepatic and Lipid Clinical Chemistry Tests in Fluorochemical Production Employees. J. Occup. Environ. Med. 1999, 41, 799–806. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Mandel, J.H.; Zobel, L.R. Epidemiologic Assessment of Worker Serum Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA) Concentrations and Medical Surveillance Examinations. J. Occup. Environ. Med. 2003, 45, 260–270. [Google Scholar] [CrossRef]
- East, A.; Egeghy, P.P.; Hubal, E.A.C.; Slover, R.; Vallero, D.A. Computational estimates of daily aggregate exposure to PFOA/PFOS from 2011 to 2017 using a basic intake model. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 56–68. [Google Scholar] [CrossRef]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, H.; Zhou, Z.; Pan, Y.; Liu, G.; Wang, T.; Wang, Y.; Xiao, M.; Chen, S.; Liang, Y. Perfluorooctanesulfonate Induces Hepatomegaly and Lipoatrophy in Mice through Phosphoenolpyruvate Carboxykinase-Mediated Glyceroneogenesis Inhibition. Environ. Sci. Technol. Lett. 2020, 7, 185–190. [Google Scholar] [CrossRef]
- Wan, H.T.; Zhao, Y.G.; Wei, X.; Hui, K.Y.; Giesy, J.P.; Wong, C.K.C. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim. Biophys. Acta BBA-Gen. Subj. 2012, 1820, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Curran, I.; Hierlihy, S.L.; Liston, V.; Pantazopoulos, P.; Nunnikhoven, A.; Tittlemier, S.; Barker, M.; Trick, K.; Bondy, G. Altered Fatty Acid Homeostasis and Related Toxicologic Sequelae in Rats Exposed to Dietary Potassium Perfluorooctanesulfonate (PFOS). J. Toxicol. Environ. Health Part A 2008, 71, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Litvin, M.; Clark, A.L.; Fisher, S.J. Defective Counterregulation and Hypoglycemia Unawareness in Diabetes. Endocrinol. Metab. Clin. N. Am. 2013, 42, 15–38. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, K.S.; Jacobs, D.R.; Lee, D.H. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes. Rev. 2017, 18, 129–139. [Google Scholar] [CrossRef]
- Butruille, L.; Jubin, P.; Martin, E.; Aigrot, M.S.; Lhomme, M.; Fini, J.B.; Demeneix, B.; Stankoff, B.; Lubetzki, C.; Zalc, B.; et al. Deleterious functional consequences of perfluoroalkyl substances accumulation into the myelin sheath. Environ. Int. 2023, 180, 108211. [Google Scholar] [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2021, 11, 612320. [Google Scholar] [CrossRef]
- Pryweller, J.R.; Schauder, K.B.; Anderson, A.W.; Heacock, J.L.; Foss-Feig, J.H.; Newsom, C.R.; Loring, W.A.; Cascio, C.J. White matter correlates of sensory processing in autism spectrum disorders. Neuroimage Clin. 2014, 6, 379–387. [Google Scholar] [CrossRef]
- Qazi, M.R.; Xia, Z.; Bogdanska, J.; Chang, S.-C.; Ehresman, D.J.; Butenhoff, J.L.; Nelson, B.D.; Depierre, J.W.; Abedi-Valugerdi, M. The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptor-alpha (PPARα). Toxicology 2009, 260, 68–76. [Google Scholar] [CrossRef]
- Torres, L.; Redko, A.; Limper, C.; Imbiakha, B.; Chang, S.; August, A. Effect of Perfluorooctanesulfonic acid (PFOS) on immune cell development and function in mice. Immunol. Lett. 2021, 233, 31–41. [Google Scholar] [CrossRef]
- Malek, T.R. The main function of IL-2 is to promote the development of T regulatory cells. J. Leukoc. Biol. 2003, 74, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Kooten, C.V.; Banchereau, J. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 1997, 9, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Audzevich, T.; Bashford-Rogers, R.; Mabbott, N.A.; Frampton, D.; Freeman, T.C.; Potocnik, A.; Kellam, P.; Gilroy, D.W. Pre/pro-B cells generate macrophage populations during homeostasis and inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E3954–E3963. [Google Scholar] [CrossRef]
- Liebig, T.M.; Fiedler, A.; Klein-Gonzalez, N.; Shimabukuro-Vornhagen, A.; Von Bergwelt-Baildon, M. Murine Model of CD40-activation of B cells. J. Vis. Exp. 2010, 37, 1734. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Liu, S.; Lai, H.; Tu, W. Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish. J. Hazard. Mater. 2022, 425, 127950. [Google Scholar] [CrossRef]
- Clark, K.L.; Shukla, M.; George, J.W.; Gustin, S.; Rowley, M.J.; Davis, J.S. An environmentally relevant mixture of per- and polyfluoroalkyl substances (PFAS) impacts proliferation, steroid hormone synthesis, and gene transcription in primary human granulosa cells. Toxicol. Sci. 2024, 200, 57–69. [Google Scholar] [CrossRef]
- Bitran, D.; Shiekh, M.; McLeod, M. Anxiolytic Effect of Progesterone is Mediated by the Neurosteroid Allopregnanolone at Brain GABAA Receptors. J. Neuroendocrinol. 1995, 7, 171–177. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Z.-Y.; Liu, Q.; Liu, H.; Gu, A. Estrogen receptor beta mediates hepatotoxicity induced by perfluorooctane sulfonate in mouse. Environ. Sci. Pollut. Res. 2017, 24, 13414–13423. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Zuloaga, K.L.; Hinds, L.R.; Carbone, D.L.; Handa, R.J. Estrogen receptor β expression in the mouse forebrain: Age and sex differences. J. Comp. Neurol. 2014, 522, 358–371. [Google Scholar] [CrossRef]
- Sharma, P.K.; Thakur, M.K. Expression of estrogen receptor (ER) α and β in mouse cerebral cortex: Effect of age, sex and gonadal steroids. Neurobiol. Aging 2006, 27, 880–887. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Ji, S.; Lai, K.-P.; Wan, H.-T.; Wong, C.K.C.; Li, L. Perfluorooctanesulfonic acid exposure altered hypothalamic metabolism and disturbed male fecundity. Sci. Total Environ. 2022, 844, 156881. [Google Scholar] [CrossRef] [PubMed]
- Makris, G.; Agorastos, A.; Chrousos, G.P.; Pervanidou, P. Stress System Activation in Children and Adolescents With Autism Spectrum Disorder. Front. Neurosci. 2022, 15, 756628. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Antke, C.; Beu, M.; Müller, H.-W. Cortical GABA, Striatal Dopamine and Midbrain Serotonin as the Key Players in Compulsive and Anxiety Disorders-Results from In Vivo Imaging Studies. Rev. Neurosci. 2010, 21, 119–140. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, J.; Lee, A.R.; Jo, S.V.; Park, C.H.; Han, D.S.; Eun, C.S. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J. Nutr. Biochem. 2022, 101, 108926. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; O’Sullivan, B.; Mondal, T.K.; Uddin, M.N.; Manley, K.; Graf, J.; Lawrence, D.A. Mitochondrion-Mediated Metabolism and Microbiome Biodiversity Influence Autism-alike Behaviors. J. Neurol. Transl. Neurosci. 2025, 10, 1100. [Google Scholar]

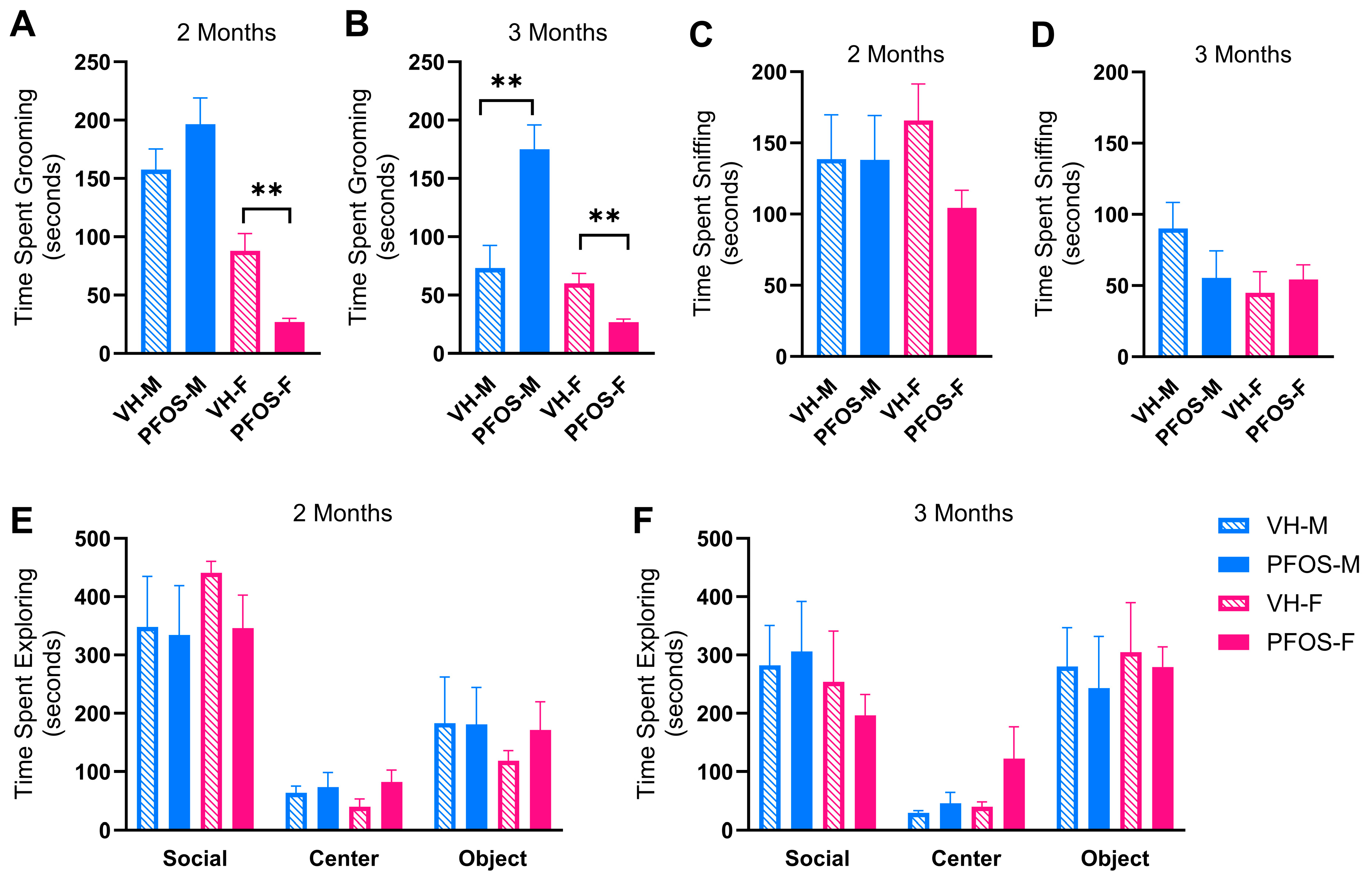
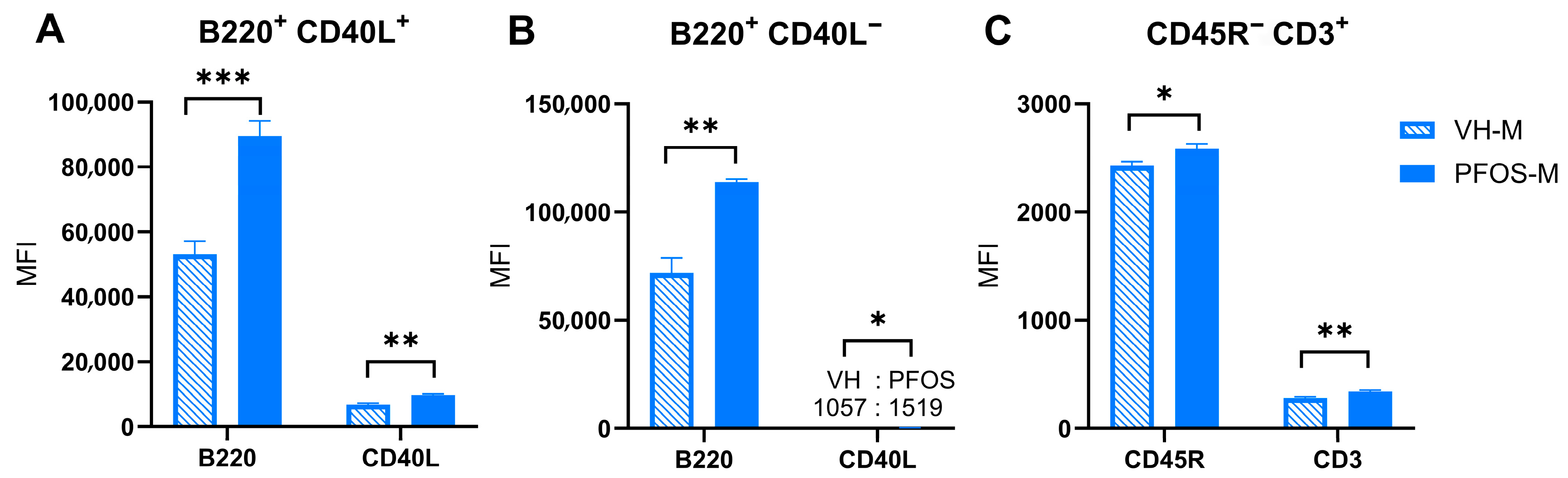
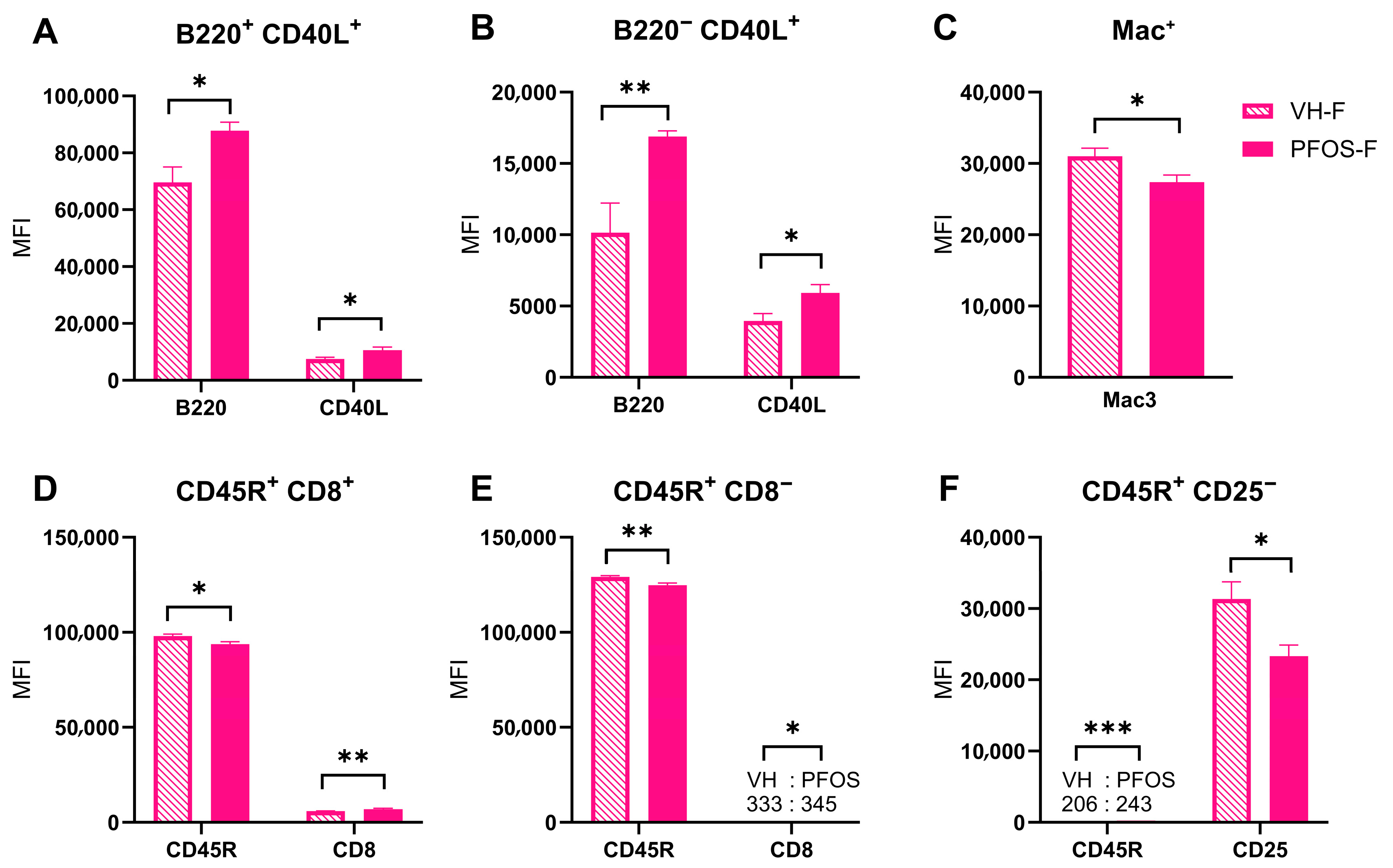
| VH-M | PFOS-M | VH-M (%) | PFOS-M (%) | |
|---|---|---|---|---|
| Body Weight | 39.65 ± 1.09 | 40.17 ± 1.62 | N.A. | N.A. |
| Liver | 2089.50 ± 56.44 | 3042.83 ± 83.08 *** | 5.28 ± 0.14 | 7.60 ± 0.17 *** |
| Spleen | 95.83 ± 11.15 | 88.17 ± 6.57 | 0.24 ± 0.02 | 0.22 ± 0.01 |
| Brain | 419.50 ± 9.81 | 423.33 ± 7.49 | 1.06 ± 0.03 | 1.06 ± 0.03 |
| Gastrointestinal Tract | 4687.00 ± 118.90 | 4629.00 ± 170.57 | 11.84 ± 0.24 | 11.56 ± 0.37 |
| Kidneys | 663.67 ± 17.02 | 650.50 ± 32.60 | 1.68 ± 0.04 | 1.62 ± 0.02 |
| Heart and Lungs | 567.83 ± 18.12 | 643.67 ± 31.30 | 1.44 ± 0.06 | 1.61 ± 0.08 |
| Thymus | 58.17 ± 4.71 | 55.50 ± 7.61 | 0.15 ± 0.01 | 0.14 ± 0.02 |
| Pancreas | 251.00 ± 15.02 | 242.83 ± 21.44 | 0.63 ± 0.03 | 0.60 ± 0.04 |
| VH-F | PFOS-F | VH-F (%) | PFOS-F (%) | |
|---|---|---|---|---|
| Body Weight | 35.38 ± 1.35 | 35.18 ± 0.86 | N.A. | N.A. |
| Liver | 1806.50 ± 58.05 | 2492.80 ± 67.68 *** | 5.12 ± 0.12 | 7.09 ± 0.09 *** |
| Spleen | 113.17 ± 4.27 | 97.00 ± 3.78 * | 0.32 ± 0.01 | 0.28 ± 0.01 ** |
| Brain | 431.00 ± 6.74 | 442.00 ± 3.00 | 1.22 ± 0.03 | 1.26 ± 0.03 |
| Gastrointestinal Tract | 4465.83 ± 146.23 | 4379.20 ± 108.07 | 12.66 ± 0.42 | 12.47 ± 0.36 |
| Kidneys | 498.00 ± 26.30 | 511.40 ± 12.62 | 1.41 ± 0.04 | 1.45 ± 0.02 |
| Heart and Lungs | 556.33 ± 27.00 | 527.00 ± 21.00 | 1.57 ± 0.04 | 1.50 ± 0.04 |
| Thymus | 59.00 ± 3.71 | 53.00 ± 4.88 | 0.17 ± 0.01 | 0.15 ± 0.02 |
| Pancreas | 253.30 ± 14.66 | 232.20 ± 9.07 | 0.72 ± 0.03 | 0.66 ± 0.02 |
| Antibodies | Group | +/+ (%) | +/− (%) | −/+ (%) | −/− (%) |
|---|---|---|---|---|---|
| B220/CD40L | VH-M | 1.32 ± 0.20 | 14.04 ± 2.02 | 1.14 ± 0.11 | 83.48 ± 2.18 |
| PFOS-M | 1.77 ± 0.13 | 25.70 ± 0.72 | 1.70 ± 0.29 | 70.85 ± 0.95 *** | |
| CD24/CD5 | VH-M | 4.13 ± 0.16 | 62.33 ± 1.96 | 28.38 ± 1.79 | 5.20 ± 0.27 |
| PFOS-M | 4.29 ± 0.25 | 62.08 ± 1.38 | 27.22 ± 1.41 | 6.40 ± 0.48 * | |
| GR1/F480 | VH-M | 1.85 ± 0.09 | 4.28 ± 0.26 | 3.49 ± 0.25 | 90.38 ± 0.15 |
| PFOS-M | 1.99 ± 0.24 | 3.32 ± 0.32 * | 3.93 ± 0.20 | 90.75 ± 0.57 | |
| CD8/CD25 (CD3+) | VH-M | 4.55 ± 0.30 | 7.89 ± 0.35 | 17.37 ± 1.13 | 70.17 ± 1.10 |
| PFOS-M | 6.65 ± 1.19 | 9.82 ± 1.25 | 18.22 ± 0.60 | 65.33 ± 1.86 * | |
| Mac-3+/CD45R | VH-M | 3.38 ± 0.19 | 0.19 ± 0.02 | 37.42 ± 1.58 | 59.03 ± 1.72 |
| PFOS-M | 4.22 ± 0.23 * | 0.17 ± 0.02 | 42.17 ± 2.21 | 53.45 ± 2.39 | |
| Mac-3+ | VH-M | 3.27 ± 0.44 | |||
| PFOS-M | 3.92 ± 0.23 ** | ||||
| Antibodies | Group | +/+ (%) | +/– (%) | –/+ (%) | –/– (%) |
|---|---|---|---|---|---|
| B220/CD40L | VH-F | 1.83 ± 0.30 | 18.49 ± 2.64 | 1.34 ± 0.17 | 78.35 ± 2.94 |
| PFOS-F | 2.00 ± 0.35 | 23.82 ± 0.33 * | 1.23 ± 0.17 | 72.96 ± 0.75 | |
| CD8/CD4 | VH-F | 2.98 ± 0.32 | 10.77 ± 0.63 | 11.79 ± 0.72 | 68.02 ± 1.43 |
| PFOS-F | 3.33 ± 0.52 | 12.92 ± 2.16 | 14.04 ± 0.47 * | 62.74 ± 3.04 | |
| CD8/CD25 (CD3+) | VH-F | 4.48 ± 0.19 | 7.39 ± 0.36 | 15.00 ± 0.75 | 73.10 ± 1.13 |
| PFOS-F | 6.24 ± 1.14 * | 8.46 ± 1.07 | 18.90 ± 0.56 ** | 66.40 ± 1.69 ** | |
| CD4/CD25 (CD3+) | VH-F | 17.53 ± 0.89 | 14.23 ± 0.85 | 2.24 ± 0.09 | 65.98 ± 1.51 |
| PFOS-F | 22.48 ± 0.56 ** | 13.42 ± 0.54 | 3.00 ± 0.19 ** | 61.12 ± 1.07 * | |
| CD4/CD45R | VH-F | 6.18 ± 0.37 | 10.61 ± 0.62 | 31.43 ± 1.12 | 51.78 ± 1.88 |
| PFOS-F | 7.24 ± 0.69 | 12.48 ± 0.40 * | 34.68 ± 1.30 | 45.64 ± 2.05 | |
| Mac-3/CD45R | VH-F | 4.58 ± 0.20 | 0.37 ± 0.03 | 34.07 ± 1.44 | 60.97 ± 1.47 |
| PFOS-F | 4.74 ± 0.22 | 0.25 ± 0.02 ** | 38.24 ± 1.74 | 56.78 ± 1.85 | |
| CD45R/CD3 | VH-F | 8.68 ± 0.39 | 32.92 ± 1.23 | 15.62 ± 0.49 | 42.80 ± 1.21 |
| PFOS-F | 8.58 ± 0.37 | 37.48 ± 1.60 * | 13.12 ± 0.32 ** | 40.82 ± 1.70 | |
| CD45R/CD25 | VH-F | 24.63 ± 1.10 | 10.65 ± 0.59 | 9.65 ± 0.51 | 55.10 ± 1.77 |
| PFOS-F | 29.72 ± 0.93 ** | 9.80 ± 0.81 | 12.38 ± 0.24 ** | 48.06 ± 1.90 * | |
| CD3/CD25 | VH-F | 4.55 ± 0.19 | 19.93 ± 0.52 | 37.75 ± 1.44 | 37.80 ± 1.31 |
| PFOS-F | 5.30 ± 0.15 * | 16.60 ± 0.16 ** | 45.78 ± 1.03 ** | 32.36 ± 1.08 * | |
| Mac-3+ | VH-F | 3.96 ± 0.18 | |||
| PFOS-F | 4.87 ± 0.34 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.Q.Y.; Eldefrawy, F.; Navarro, J.I.; Guo, T.L. Sex-Specific Metabolic, Immunologic, and Behavioral Effects of Perfluorooctane Sulfonic Acid (PFOS) in BTBR-mtB6 Mice. Sci 2025, 7, 118. https://doi.org/10.3390/sci7030118
Jiang DQY, Eldefrawy F, Navarro JI, Guo TL. Sex-Specific Metabolic, Immunologic, and Behavioral Effects of Perfluorooctane Sulfonic Acid (PFOS) in BTBR-mtB6 Mice. Sci. 2025; 7(3):118. https://doi.org/10.3390/sci7030118
Chicago/Turabian StyleJiang, Danielle Qiu Yun, Fatma Eldefrawy, Jarissa Isabel Navarro, and Tai L. Guo. 2025. "Sex-Specific Metabolic, Immunologic, and Behavioral Effects of Perfluorooctane Sulfonic Acid (PFOS) in BTBR-mtB6 Mice" Sci 7, no. 3: 118. https://doi.org/10.3390/sci7030118
APA StyleJiang, D. Q. Y., Eldefrawy, F., Navarro, J. I., & Guo, T. L. (2025). Sex-Specific Metabolic, Immunologic, and Behavioral Effects of Perfluorooctane Sulfonic Acid (PFOS) in BTBR-mtB6 Mice. Sci, 7(3), 118. https://doi.org/10.3390/sci7030118







