Theoretical Study of CO Oxidation on Pt Single-Atom Catalyst Decorated C3N Monolayers with Nitrogen Vacancies
Abstract
1. Introduction
2. Computational Method
3. Results
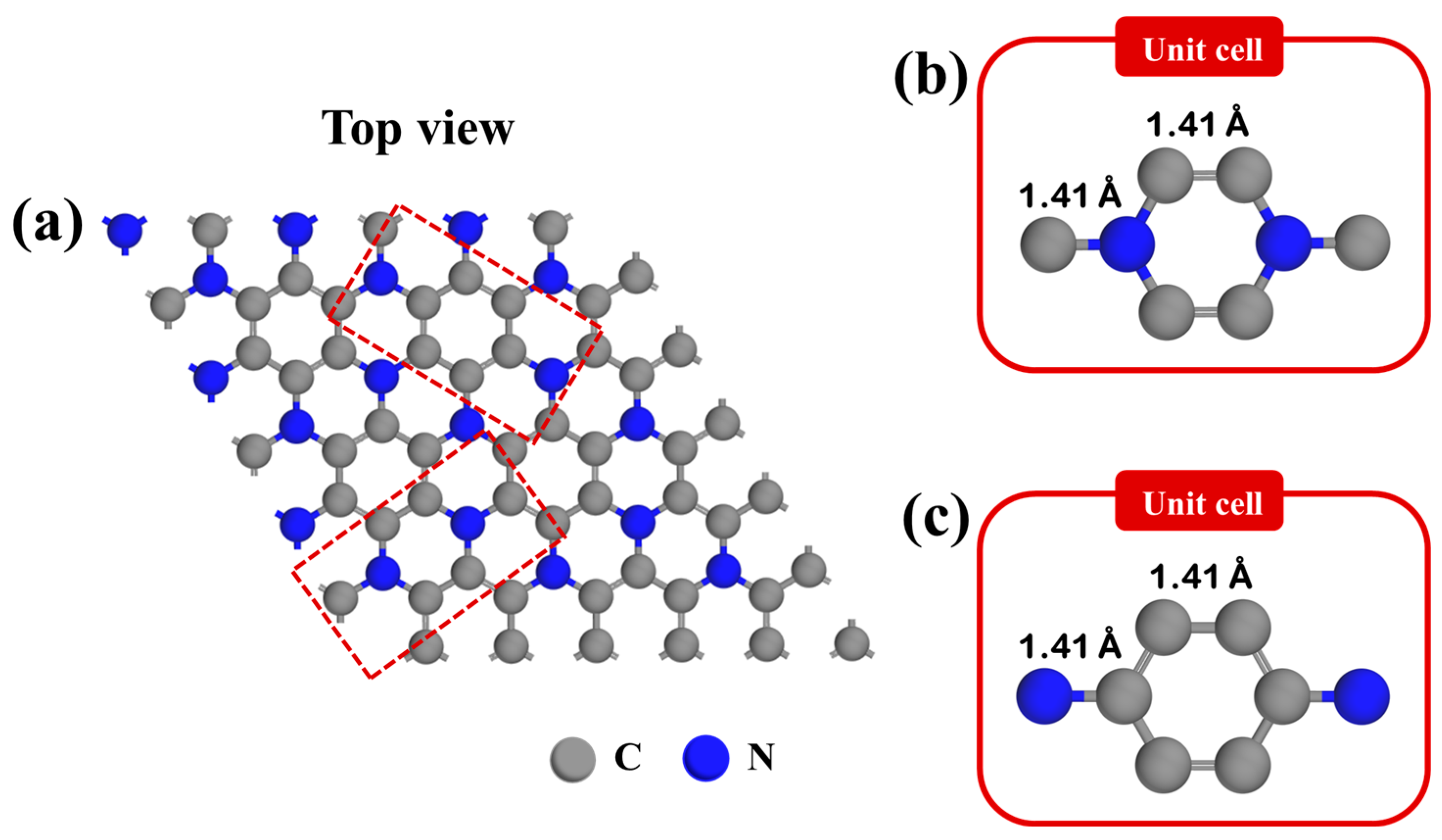
3.1. Structural Model of Pristine C3N
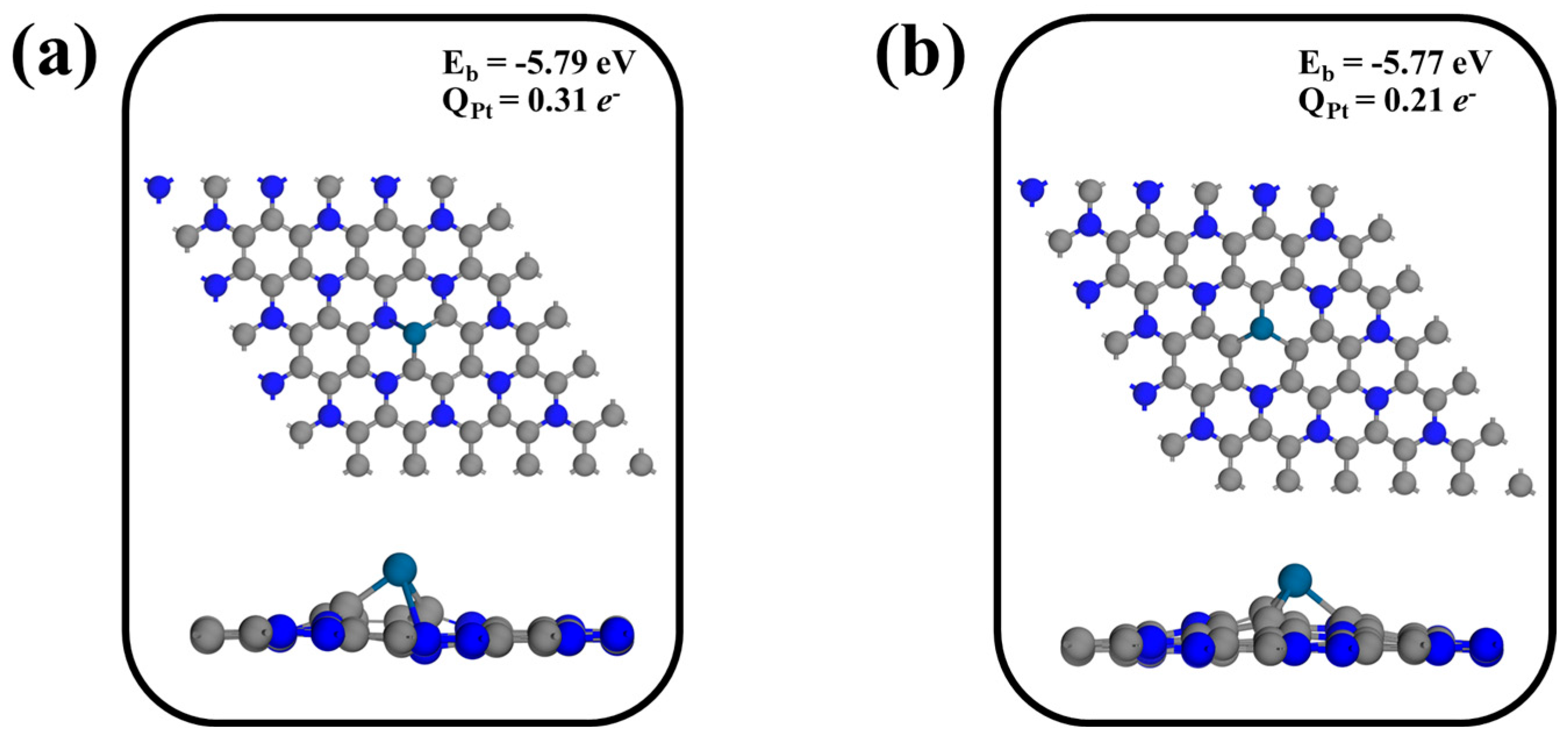
3.2. The Defective Structure of C3N
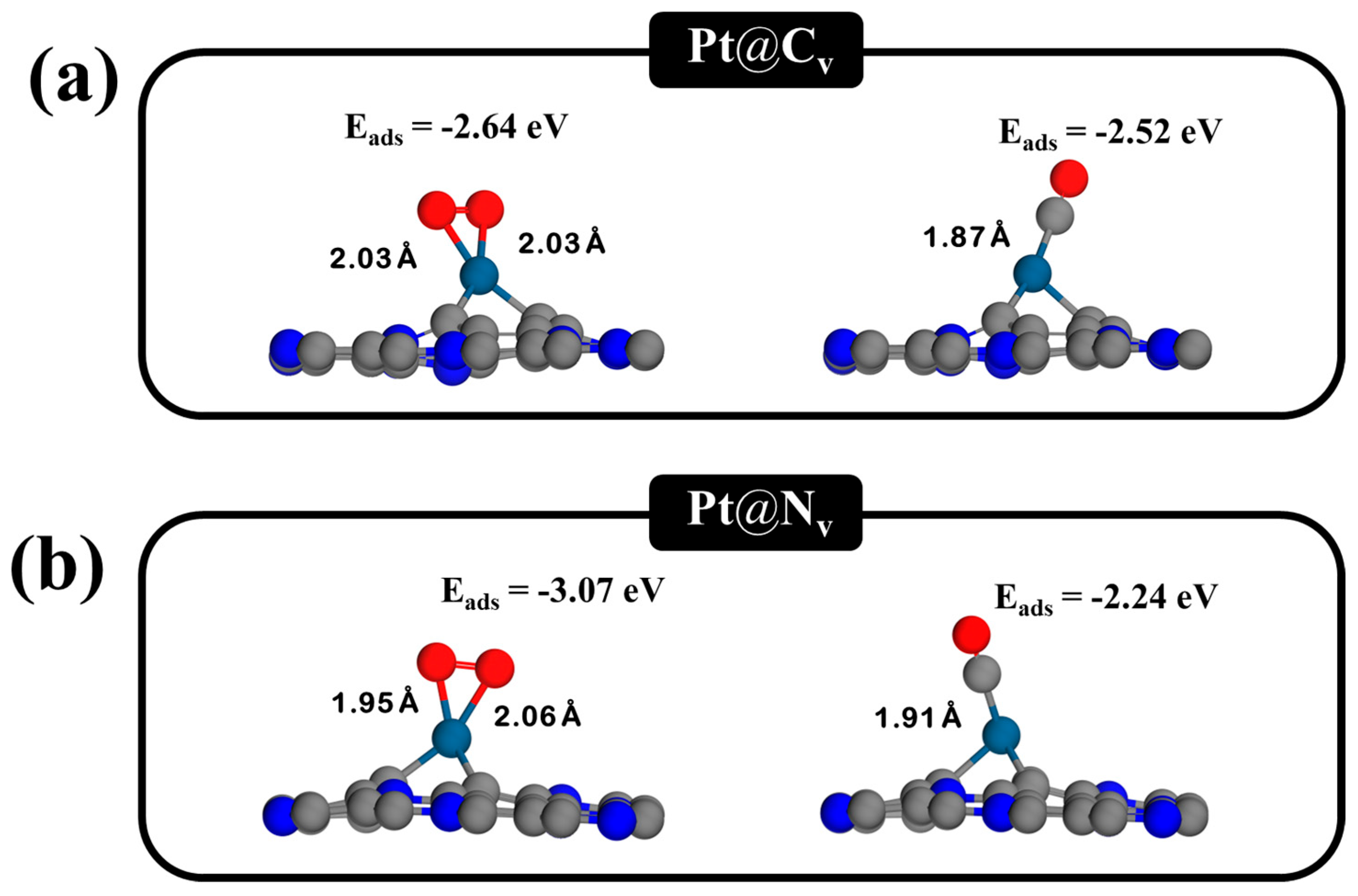
3.3. The O2 and CO Adsorption on Pt-Doped C3N Monolayers with Single Vacancies
3.4. Reaction Mechanism of CO Oxidation on Pt@NV-C3N
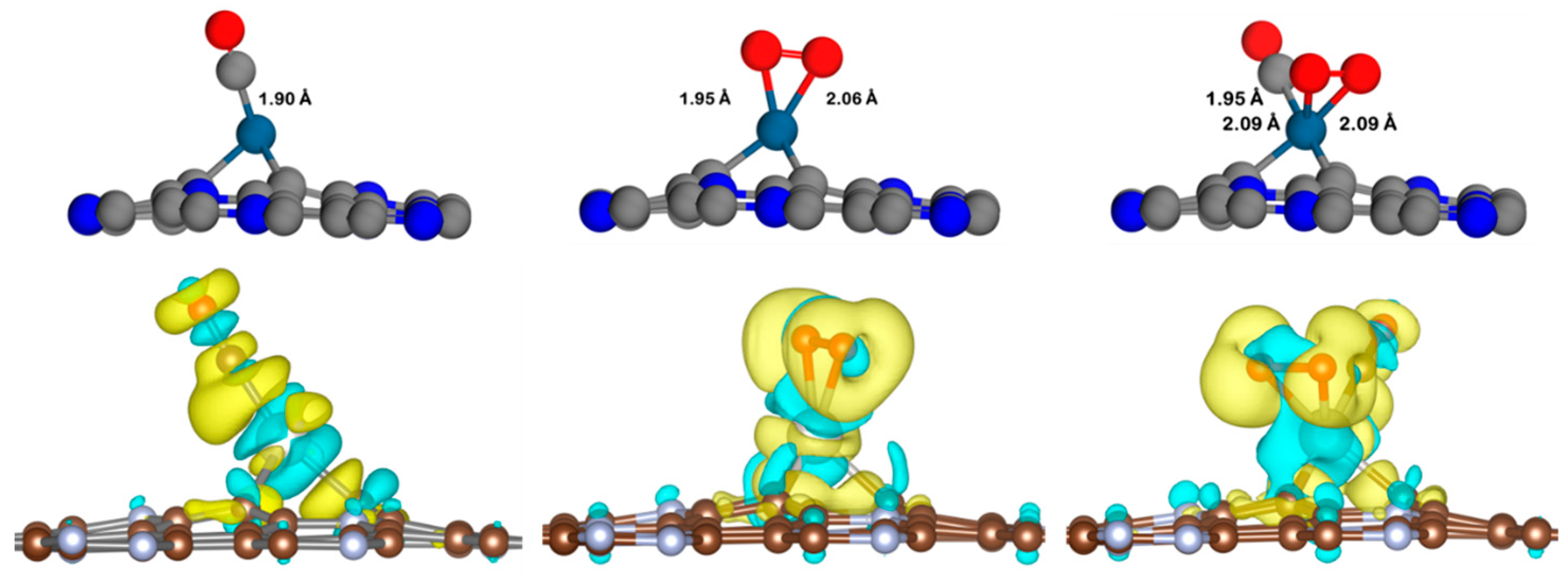
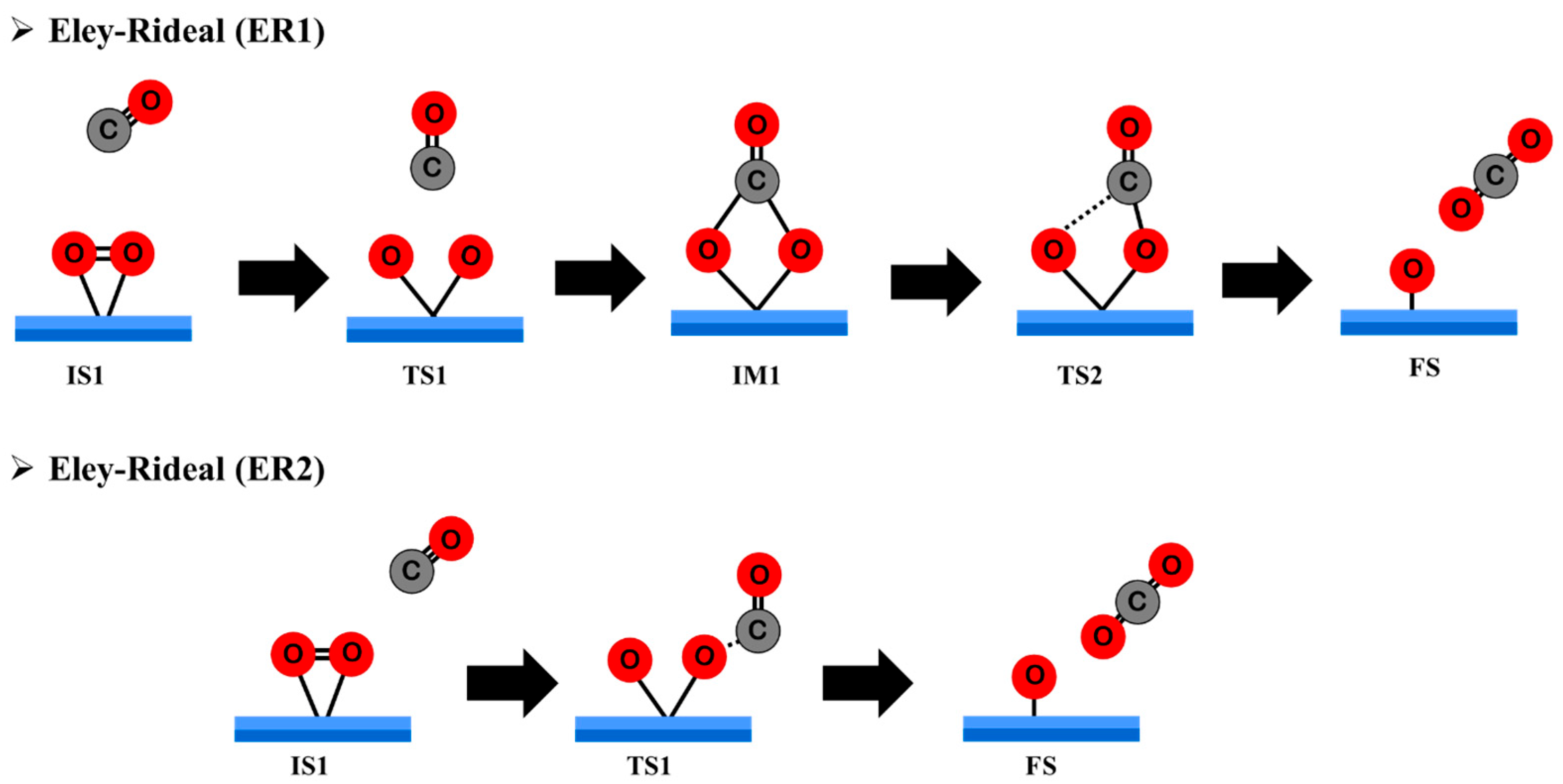
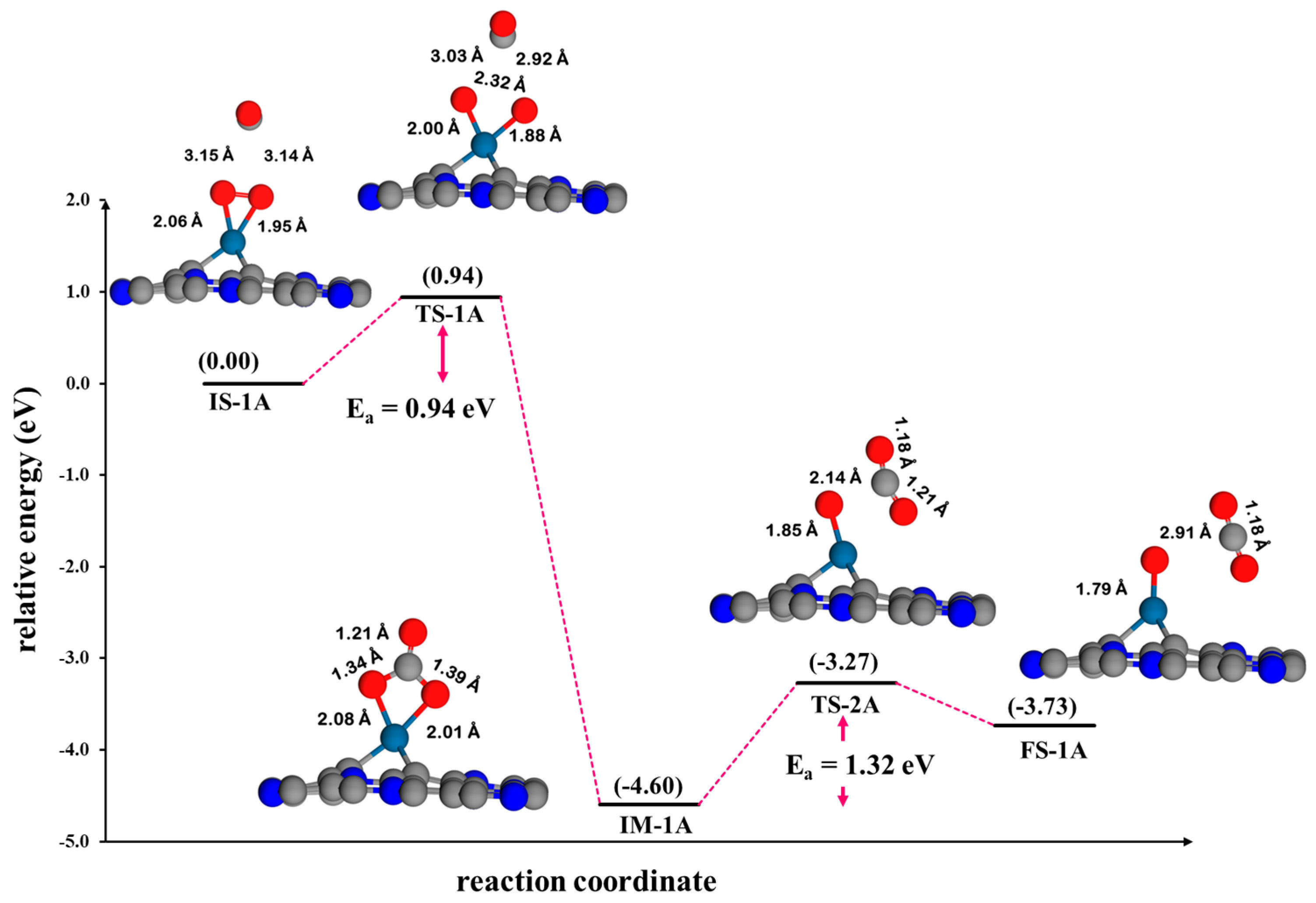
3.4.1. Eley–Rideal Mechanism (ER) on Pt@NV-C3N
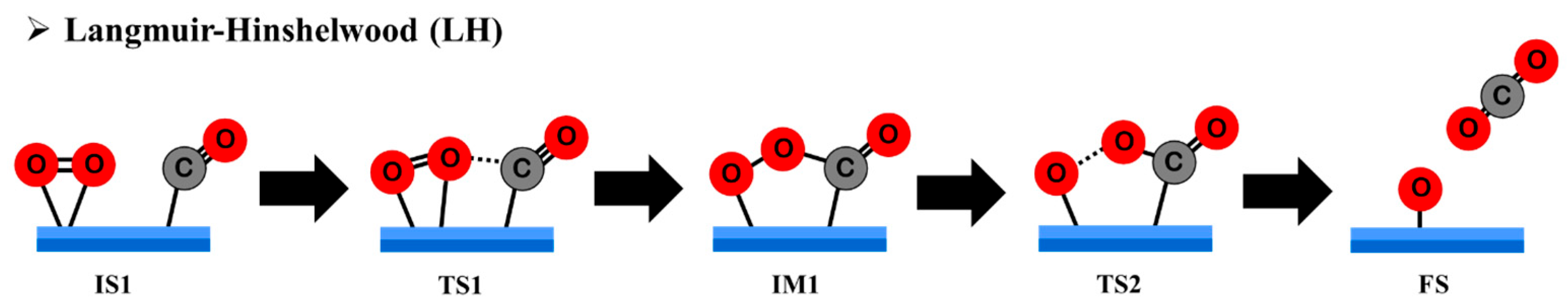
3.4.2. Langmuir–Hinshelwood Mechanism (LH) on Pt@NV-C3N
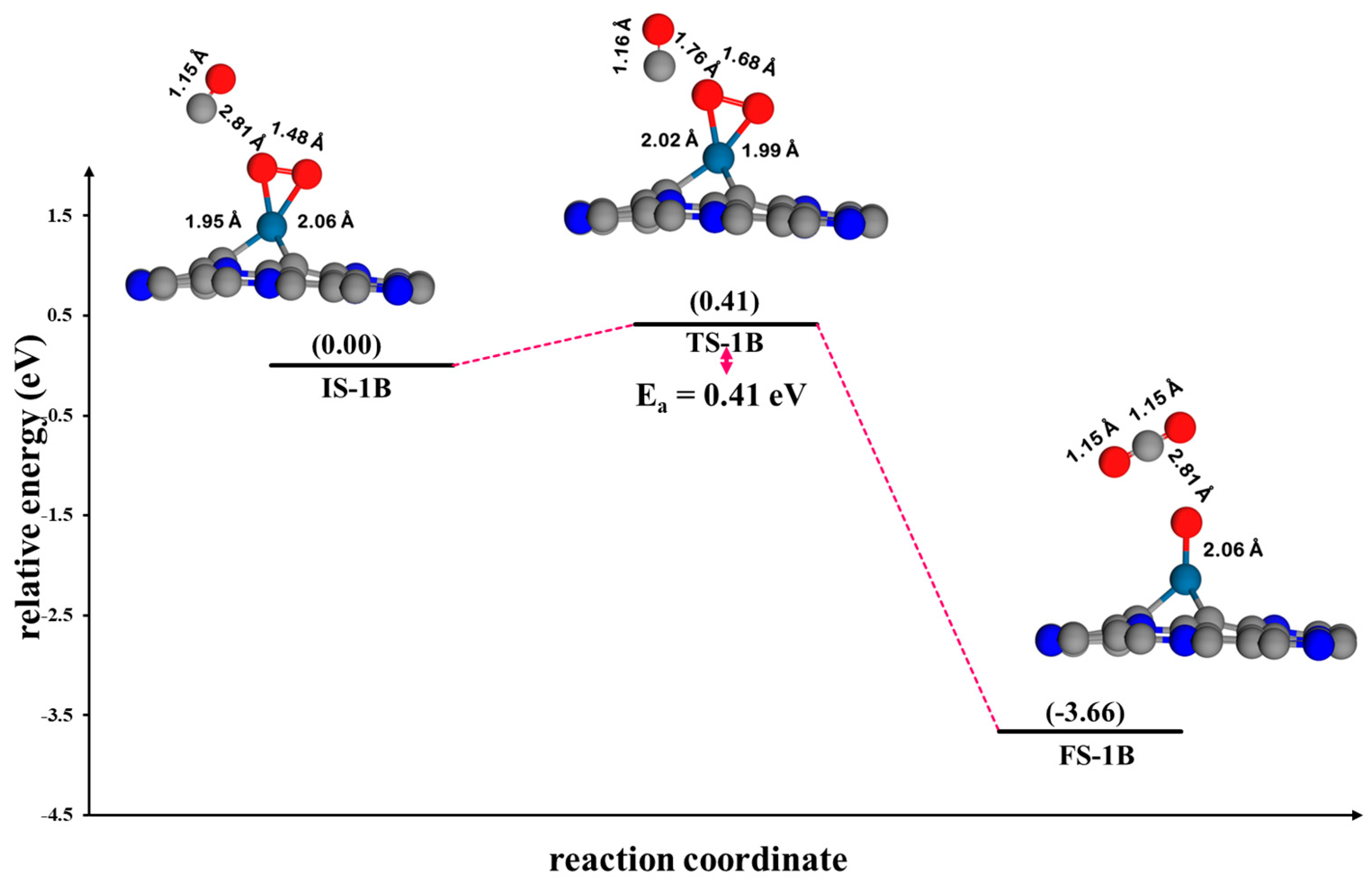
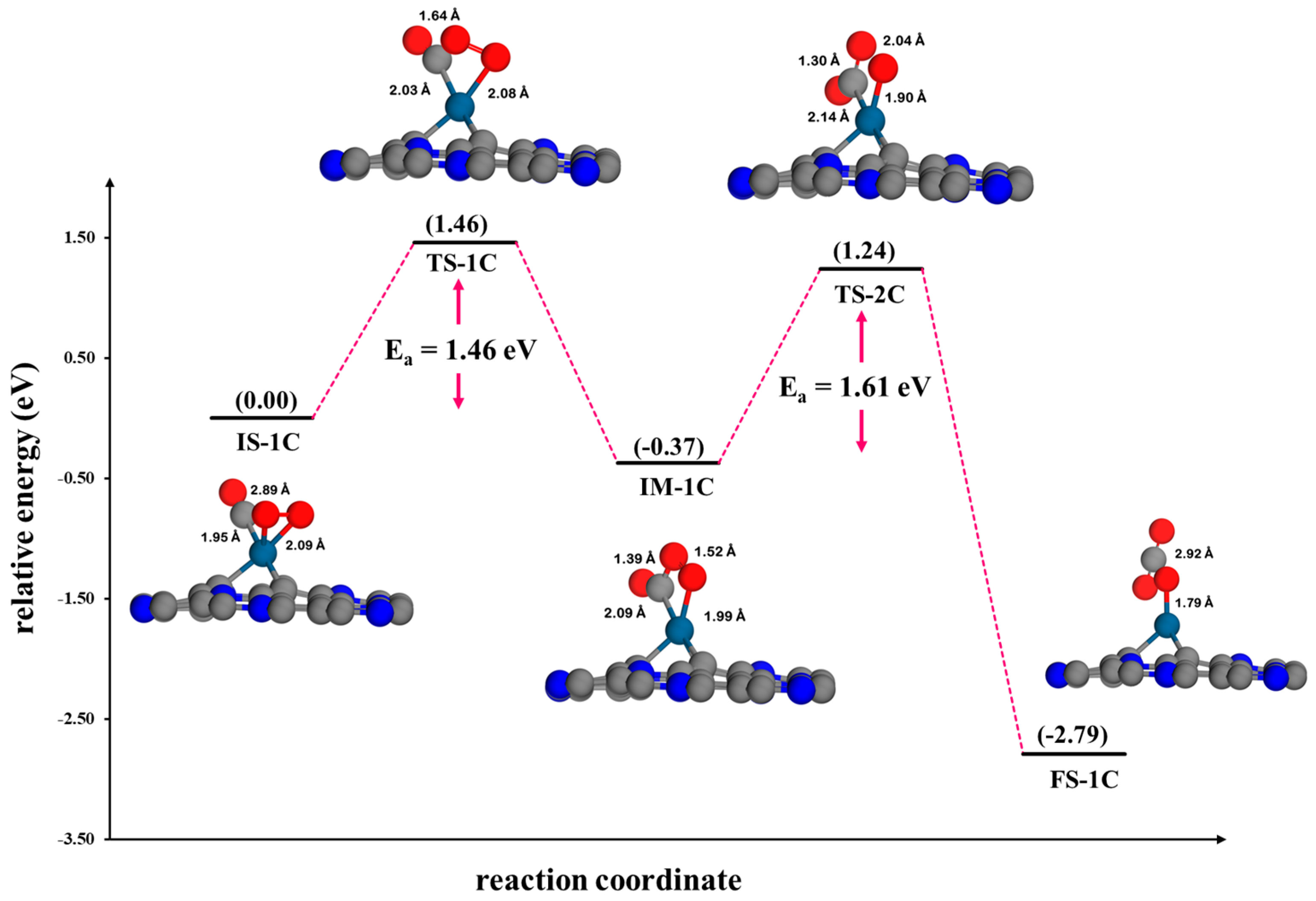
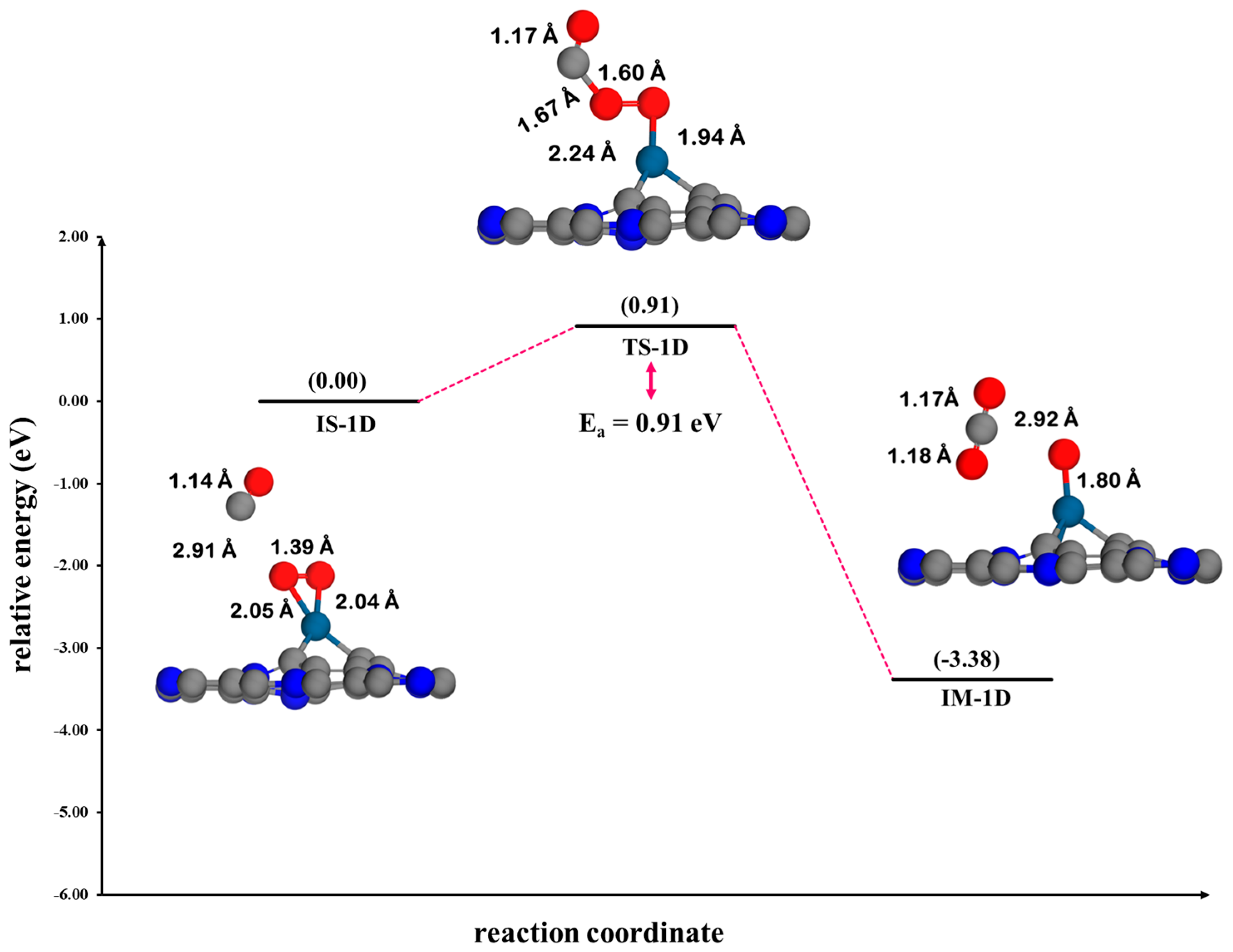
3.4.3. Eley–Rideal Mechanism (ER2) on Pt@CV-C3N
4. Discussion
4.1. Comparison of the Reaction Mechanism of CO Oxidation on Pt@NV-C3N
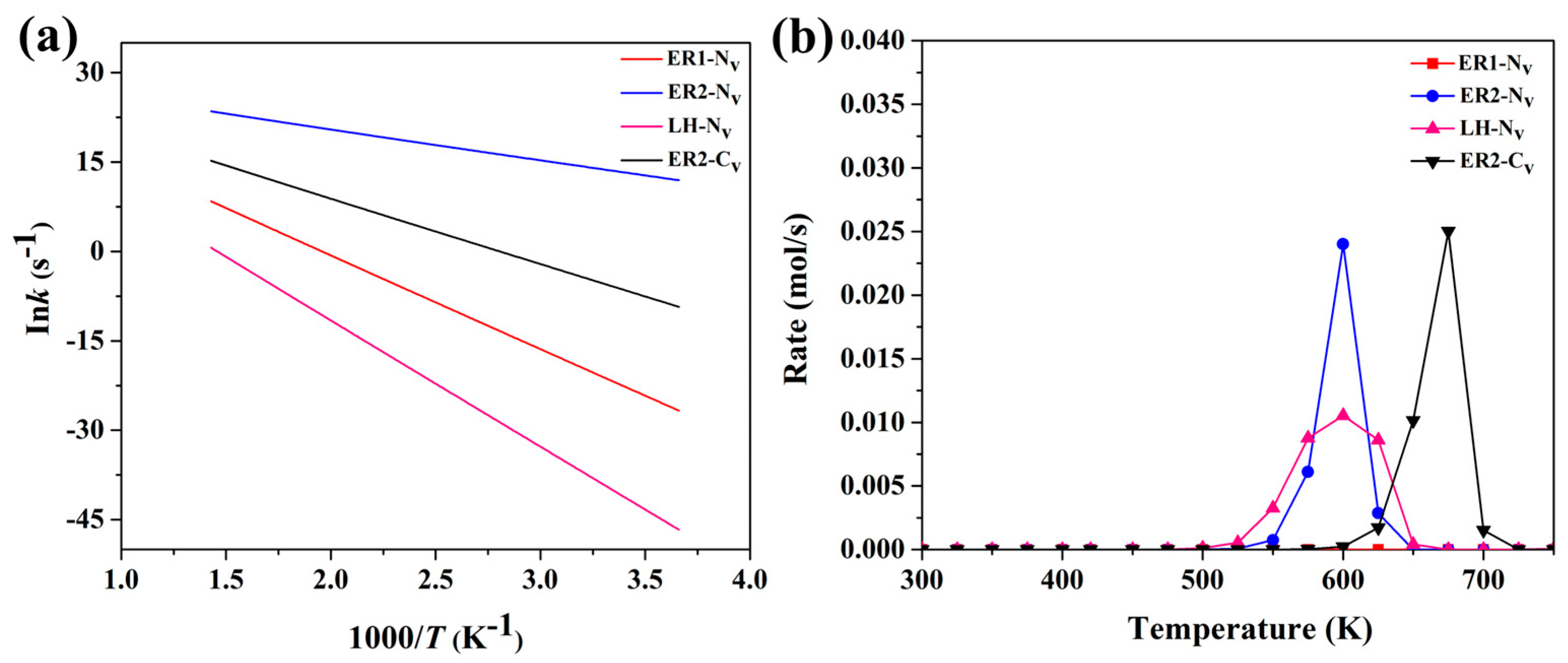
4.2. Kinetic Studies and Microkinetic Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| LH | Langmuir–Hinshelwood |
| ER | Eley–Rideal |
| Ea | Activation energy barriers |
References
- Esrafili, M.D.; Heydari, S. B-doped C3N monolayer: A robust catalyst for oxidation of carbon monoxide. Theor. Chem. Acc. 2019, 138, 57. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. Boosting graphene reactivity with co-doping of boron and nitrogen atoms: CO oxidation by O2 molecule. Appl. Surf. Sci. 2018, 455, 808–814. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. A DFT study on the possibility of using a single Cu atom incorporated nitrogen-doped graphene as a promising and highly active catalyst for oxidation of CO. Int. J. Quantum Chem. 2019, 119, e25857. [Google Scholar] [CrossRef]
- Freund, H.J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, J.; Chen, Z. Fe-anchored graphene oxide: A low-cost and easily accessible catalyst for low-temperature CO oxidation. J. Phys. Chem. C 2012, 116, 2507–2514. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X. The theoretical study of Rh single atom catalysts decorated C3N monolayer with N vacancy for CO oxidations. Appl. Phys. A 2023, 129, 325. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y. A comparative study of CO oxidation on Cu-doped C3N monolayer with N and C vacancies. J. Phys. Chem. Solids 2023, 180, 111441. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A review on CO oxidation over copper chromite catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, M.G.; Shin, D.; Han, J.W. Design of ceria catalysts for low-temperature CO oxidation. ChemCatChem 2020, 12, 11–26. [Google Scholar] [CrossRef]
- Wallander, H.J.; Gajdek, D.; Albertin, S.; Harlow, G.; Braud, N.; Buß, L.; Krisponeit, J.-O.; Flege, J.I.; Falta, J.; Lundgren, E.; et al. Dynamic Behavior of Tin at Platinum Surfaces during Catalytic CO Oxidation. ACS Catal. 2023, 13, 16158–16167. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Liberman, E.Y.; Naumkin, A.V. Influence of Pt/Pd state on ceria-based support in CO oxidation. J. Rare Earths 2023, 41, 1963–1968. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Li, H.; Xu, R.; Sun, Z. Highly active and water-resistant Lanthanum-doped platinum-cobalt oxide catalysts for CO oxidation. Appl. Catal. B Environ. 2023, 331, 122678. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.-L.; Feng, L.; Hu, J.; Chai, X.; Liu, J.-X.; Li, W.-X. Reactant-Induced Dynamic Stabilization of Highly Dispersed Pt Catalysts on Ceria Dictating the Reactivity of CO Oxidation. ACS Catal. 2024, 14, 3504–3513. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. 2021, 28, 24847–24871. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Lai, C.; Zhang, Y.; Lin, X.; Ding, S. Recent advances in noble metal-based catalysts for CO oxidation. RSC Adv. 2024, 14, 30566–30581. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT Study of the CO Oxidation on the Au (321) Surface. J. Phys. Chem. C 2008, 112, 17291–17302. [Google Scholar] [CrossRef]
- Rao, X.; Si, Q.; Shi, T.; Han, X.; Ma, S. Fe-doped C3N monolayer as a promising SAC for CO oxidation with low temperature and high reactivity. Comput. Theor. Chem. 2021, 1194, 113080. [Google Scholar] [CrossRef]
- Bafekry, A.; Ghergherehchi, M.; Shayesteh, S.F.; Peeters, F. Adsorption of molecules on C3N nanosheet: A first-principles calculations. Chem. Phys. 2019, 526, 110442. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Jia, P. Pd-doped C3N monolayer: A promising low-temperature and high-activity single-atom catalyst for CO oxidation. Appl. Surf. Sci. 2021, 537, 147881. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Heydari, S. An effective approach for tuning catalytic activity of C3N nanosheets: Chemical-doping with the Si atom. J. Mol. Graph. Model. 2019, 92, 320–328. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, Y.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. Transition metal doped C3N monolayer as efficient electrocatalyst for carbon dioxide electroreduction: A computational study. Appl. Surf. Sci. 2021, 542, 148568. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Zhu, L.; Ling, C.; Xue, Q.; Xing, W. Charge-modulated CO2 capture of C3N nanosheet: Insights from DFT calculations. Chem. Eng. J. 2018, 338, 92–98. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Yang, F.; Ma, D.; Yang, Z.; Lu, Z. CO oxidation on single Pd atom embedded defect-graphene via a new termolecular Eley-Rideal mechanism. Carbon 2017, 118, 35–42. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Ding, Y.; Lu, Z.; Ma, D.; Yang, Z. First-principles study on the single Ir atom embedded graphdiyne: An efficient catalyst for CO oxidation. J. Phys. Chem. C 2018, 122, 23481–23492. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Xu, G.; Wang, T.; Ma, D.; Yang, Z.; Yang, L. Single Pt atom stabilized on nitrogen doped graphene: CO oxidation readily occurs via the tri-molecular Eley—Rideal mechanism. Phys. Chem. Chem. Phys. 2015, 17, 20006–20013. [Google Scholar] [CrossRef]
- González-Torres, J.C.; Bertin, V.; Poulain, E.; Olvera-Neria, O. The CO oxidation mechanism on small Pd clusters. A theoretical study. J. Mol. Model. 2015, 21, 279. [Google Scholar] [CrossRef]
- Baskaran, S.; Jung, J. Termolecular Eley–Rideal pathway for efficient CO oxidation on phosphorene-supported single-atom cobalt catalyst. Bull. Korean Chem. Soc. 2022, 43, 1254–1261. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Chen, M.; Liu, C.; Qi, X.; Peera, S.G.; Liu, J.; Liang, T. Theoretical investigation on catalytic mechanisms of oxygen reduction and carbon monoxide oxidation on the MnN x system. New J. Chem. 2020, 44, 15724–15732. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Li, Q.; Bai, X.; Wang, J. Metal single-atom coordinated graphitic carbon nitride as an efficient catalyst for CO oxidation. Nanoscale 2020, 12, 364–371. [Google Scholar] [CrossRef]
- Hussain, S.; Talib, S.H.; Shahzad, S.R.; Muhammad, S.; Mohamed, S.; Qurashi, A.; Wang, H.; Lu, Z. Heterogeneous Co1/P1Mo12O40 single-atom catalyst for CO oxidation via termolecular Eley-Rideal (TER) mechanism. Mol. Catal. 2023, 550, 113539. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J.; Hafner, J. Ab initio force constant approach to phonon dispersion relations of diamond and graphite. Europhys. Lett. 1995, 32, 729. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Först, C.J.; Schimpl, J. Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull. Mater. Sci. 2003, 26, 33–41. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 54104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Qin, Z.; Zhang, G.; Yue, H.; Liang, B.; Luo, D. Dissolution behavior and mechanism of low valence vanadium of vanadium-iron spinel in sulfuric acid solution. J. Mater. Res. Technol. 2021, 12, 1391–1402. [Google Scholar] [CrossRef]
- Kilinc, D. Co complex modified on Eupergit C as a highly active catalyst for enhanced hydrogen production. Int. J. Hydrogen Energy 2022, 47, 11894–11903. [Google Scholar] [CrossRef]
- Lv, J.; Hong, J.; Liang, B.; Zhao, E.; Zeng, K.; Chen, M.; Hu, J.; Yang, G. Study of the curing kinetics of melamine/phthalonitrile resin system. Thermochim. Acta 2020, 683, 178442. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xue, S.; Wu, Y.; Li, Z.; Chang, L. Evaluation of the susceptibility of coal to spontaneous combustion by a TG profile subtraction method. Korean J. Chem. Eng. 2016, 33, 862–872. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, F.; Zhu, Z.; Yang, Y.; Zhao, S.; Chen, J.; Lv, X.; Wang, Y.; Xu, J.; Liu, N. The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation. Appl. Catal. B Environ. 2021, 297, 120393. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, M.; Qian, Y.; Miao, Y.; Ao, Z. Excellent sulfur and water resistance for CO oxidation on Pt single-atom-catalyst supported by defective graphene: The effect of vacancy type. Appl. Surf. Sci. 2021, 566, 150624. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Q.; Hou, Y.; Wang, H.; Zhou, A.; Wang, L.; Cao, G. Unexpected ground-state structures and properties of carbon nitride C3N at ambient and high pressures. Mater. Des. 2018, 140, 45–53. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Yang, J. C3N: A two dimensional semiconductor material with high stiffness, superior stability and bending Poisson’s effect. arXiv 2017, arXiv:1703.08754. [Google Scholar]
- Sakhraoui, T. Effect of vacancy defect and strain on the structural, electronic and magnetic properties of carbon nitride 2D monolayers by DFTB method. J. Phys. Condens. Matter 2023, 35, 324003. [Google Scholar] [CrossRef]
- Makaremi, M.; Mortazavi, B.; Singh, C.V. Adsorption of metallic, metalloidic, and nonmetallic adatoms on two-dimensional C3N. J. Phys. Chem. C 2017, 121, 18575–18583. [Google Scholar] [CrossRef]
- Mizuno, S.; Fujita, M.; Nakao, K. Electronic states of graphitic heterocompounds of carbon, boron and nitrogen. Synth. Met. 1995, 71, 1869–1870. [Google Scholar] [CrossRef]
- Mortazavi, B. Ultra high stiffness and thermal conductivity of graphene like C3N. Carbon 2017, 118, 25–34. [Google Scholar] [CrossRef]
- Ragab, A.H.; Khan, I.; Khan, M.; Yousef, T.A.; Abou-Krisha, M.M.; Kenawy, S.H.; Ansari, M.Z. Efficient oxidation of CO over highly active Al-decorated nitrogenated holey 2D-graphene: A DFT perspective. Inorg. Chem. Commun. 2023, 155, 110977. [Google Scholar] [CrossRef]
- Chang, F.; Li, J.; Kou, Y.; Bao, W.; Shi, Z.; Zhu, G.; Kong, Y. The intense charge migration and efficient photocatalytic NO removal of the S-scheme heterojunction composites Bi7O9I3-BiOBr. Sep. Purif. Technol. 2025, 353, 128402. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Chen, M.; Peera, S.G.; Liu, C.; Yang, H.; Qi, X.; Liu, J.; Liang, T. Catalytic oxidation mechanisms of carbon monoxide over single-and double-vacancy Mn-embedded graphene. New J. Chem. 2020, 44, 9402–9410. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, J.; Ao, Z.; Huang, H.; He, H.; Wu, Y. First principles study on the CO oxidation on Mn-embedded divacancy graphene. Front. Chem. 2018, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, Q.; Yang, X.; Zhang, T.; Li, J. A systematic theoretical study on FeOx-supported single-atom catalysts: M1/FeOx for CO oxidation. Nano Res. 2018, 11, 1599–1611. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Chen, Z. 1 + 1′ > 2: Heteronuclear biatom catalyst outperforms its homonuclear counterparts for CO oxidation. Small Methods 2019, 3, 1800480. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Liu, C.; Qi, X.; Chen, M.; Sagar, R.U.R.; Yang, H.; Liang, T. Single–atom manganese and nitrogen co-doped graphene as low-cost catalysts for the efficient CO oxidation at room temperature. Appl. Surf. Sci. 2021, 536, 147809. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Q.; Liu, J.; Lu, Z. The CO oxidation on Mn atom embedded in N vacancy of C3N monolayer: A DFT-D study. Surf. Sci. 2023, 734, 122320. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Fang, S.; Yao, S. Revealing the differences in CO oxidation activity of Fe-CeO2, Fe2O3, and CeO2 using operando CO2-DRIFTS-MS: Carbonate species and desorption process. J. Alloys Compd. 2025, 1010, 177414. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Myint, M.T.Z.; Göthelid, M.A.; Dutta, J. CO oxidation efficiency and hysteresis behavior over mesoporous Pd/SiO2 catalyst. Catalysts 2021, 11, 131. [Google Scholar] [CrossRef]



| Surface | Position | Type of CO Oxidation Reaction | Calculation |
|---|---|---|---|
| Si-C3N [21] | C vacancy | LH ( = 0.38 eV) | Dmol3 |
| B-C3N [1] | C vacancy | LH ( = 0.32 eV) | Dmol3 |
| Gr-BCN2 [2] | C vacancy | LH ( = 0.46 eV) | Dmol3 |
| Gr-BN3 [2] | C vacancy | LH ( = 0.01 eV) | Dmol3 |
| CuN3-Gr [3] | C vacancy | ER ( = 3.20 eV) | Dmol3 |
| Mn-Gr [59] | C vacancy | ER ( = 0.83 eV) | Dmol3 |
| Pd-C3N [20] | N vacancy | ER ( = 0.64 eV) | Dmol3 |
| Mn-C3N [60] | N vacancy | ER ( = 0.57 eV) | Dmol3 |
| Fe-C3N [18] | N vacancy | ER ( = 0.47 eV) | Dmol3 |
| Pt-C3N* | C vacancy | ER ( = 0.91 eV) | VASP |
| Pt-C3N* | N vacancy | ER ( = 0.41 eV) | VASP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamchompoo, S.; Injongkol, Y.; Yodsin, N.; Zhang, R.-Q.; Kunaseth, M.; Jungsuttiwong, S. Theoretical Study of CO Oxidation on Pt Single-Atom Catalyst Decorated C3N Monolayers with Nitrogen Vacancies. Sci 2025, 7, 101. https://doi.org/10.3390/sci7030101
Kamchompoo S, Injongkol Y, Yodsin N, Zhang R-Q, Kunaseth M, Jungsuttiwong S. Theoretical Study of CO Oxidation on Pt Single-Atom Catalyst Decorated C3N Monolayers with Nitrogen Vacancies. Sci. 2025; 7(3):101. https://doi.org/10.3390/sci7030101
Chicago/Turabian StyleKamchompoo, Suparada, Yuwanda Injongkol, Nuttapon Yodsin, Rui-Qin Zhang, Manaschai Kunaseth, and Siriporn Jungsuttiwong. 2025. "Theoretical Study of CO Oxidation on Pt Single-Atom Catalyst Decorated C3N Monolayers with Nitrogen Vacancies" Sci 7, no. 3: 101. https://doi.org/10.3390/sci7030101
APA StyleKamchompoo, S., Injongkol, Y., Yodsin, N., Zhang, R.-Q., Kunaseth, M., & Jungsuttiwong, S. (2025). Theoretical Study of CO Oxidation on Pt Single-Atom Catalyst Decorated C3N Monolayers with Nitrogen Vacancies. Sci, 7(3), 101. https://doi.org/10.3390/sci7030101







