Abstract
Carbon monoxide (CO) is a major toxic gas emitted from vehicle exhaust, industrial processes, and incomplete fuel combustion, posing serious environmental and health risks. Catalytic oxidation of CO into less harmful CO2 is an effective strategy to reduce these emissions. In this study, we investigated the catalytic performance of platinum (Pt) single atoms doped on C3N monolayers with various vacancy defects, including single carbon (CV) and nitrogen (NV) vacancies, using density functional theory (DFT) calculations. Our results demonstrate that Pt@NV-C3N exhibited the most favorable catalytic properties, with the highest O2 adsorption energy (−3.07 eV). This performance significantly outperforms Pt atoms doped at other vacancies. It can be attributed to the strong binding between Pt and nitrogen vacancies, which contributes to its excellent resistance to Pt aggregation. CO oxidation on Pt@NV-C3N proceeds via the Eley–Rideal (ER2) mechanism with a low activation barrier of 0.41 eV for the rate-determining step, indicating high catalytic efficiency at low temperatures. These findings suggest that Pt@NV-C3N is a promising candidate for CO oxidation, contributing to developing cost-effective and environmentally sustainable catalysts. The strong binding of Pt atoms to the nitrogen vacancies prevents aggregation, ensuring the stability and durability of the catalyst. The kinetic modeling further revealed that the ER2 mechanism offers the highest reaction rate constants over a wide temperature range (273–700 K). The low activation energy barrier also facilitates CO oxidation at lower temperatures, addressing critical challenges in automotive and industrial pollution control. This study provides valuable theoretical insights for designing advanced single-atom catalysts for environmental remediation applications.
1. Introduction
Carbon monoxide (CO) is one of the most common toxic gases in the atmosphere. It originates from car exhausts, industrial processes, and incomplete fuel or petroleum product burning [1,2,3,4,5,6]. CO is a colorless, odorless, tasteless, flammable component in the air, which, however, is toxic and harmful to the human body [1,7,8,9,10]. When inhaled, CO can irreversibly attach to hemoglobin and hence reduce its capacity to transfer oxygen to body tissues. It may even cause personal death, as carbon monoxide gas replaces oxygen by binding to the iron atom in blood hemoglobin (Hb). Hence, Hb becomes unqualified to carry oxygen to the brain [6,10]. Toxicity by CO is accompanied by some symptoms, including nausea, dizziness, loss of consciousness, and headache, and may lead to death. Therefore, developing effective methods to reduce or remove CO emissions from vehicle and industrial sources is critical and requires urgent attention. One of the most widely used methods for removing CO as a highly toxic gas is its conversion into carbon dioxide (CO2), which is significantly less harmful. This conversion commonly occurs via a catalytic oxidation reaction using suitable catalysts.
In recent decades, significant attention has been devoted to low-temperature CO oxidation due to its crucial role in mitigating the environmental pollution associated with harmful emissions. Noble metal catalysts such as Pd, Pt, and Au have been extensively employed in catalytic processes due to their excellent catalytic activities, stability, and reliability in practical applications such as vehicle catalytic converters and industrial reactions [11,12,13,14,15,16,17]. Nevertheless, these traditional noble metal catalysts typically require elevated temperatures to achieve optimal catalytic efficiency, posing substantial drawbacks including high operational costs, limited tunability, scarcity, and availability constraints. Consequently, there is an urgent need for alternative catalysts capable of efficiently catalyzing CO oxidation at lower temperatures and lower material costs. Recently, two-dimensional (2D) materials have emerged as promising candidates for catalytic applications owing to their remarkable physicochemical properties, including exceptional surface reactivity, tunable electronic structures, cost-effectiveness, and environmental compatibility. Among these, carbon nitride (C3N)-based catalysts have attracted considerable research interest for catalytic reactions [1,18,19,20,21,22,23]. However, pristine C3N monolayers exhibit intrinsic chemical inertness toward the activation and adsorption of small molecules such as O2, severely limiting their practical catalytic applications. Recent studies have shown that introducing single atomic vacancies, including carbon vacancies (CV) and nitrogen vacancies (NV), into C3N monolayers can significantly enhance their chemical reactivity. These vacancy sites create unsaturated coordination environments that facilitate stronger adsorption and activation of reactant molecules. Moreover, such vacancies serve as effective anchoring sites for single metal atoms, improving their stability and catalytic performance. Due to their unique structural attributes, single-atom catalysts (SACs), featuring isolated metal atoms anchored onto support materials, have shown remarkable promise. SACs maximize metal utilization efficiency, significantly reducing costs, and exhibit enhanced catalytic properties from well-defined active sites, distinct electronic structures, and minimized aggregation effects. Additionally, SACs offer improved selectivity and stability compared to traditional nanoparticle-based catalysts, making them particularly advantageous in catalytic applications.
Previous studies have categorized the CO oxidation reaction into four primary mechanisms based on extensive theoretical and experimental investigations [24,25,26,27,28,29,30,31]: (i) the Eley–Rideal (ER) mechanism, where gaseous CO directly reacts with adsorbed O2 (O2*(ads) + CO(g)→CO2(g) + O*(ads)), (ii) the Langmuir–Hinshelwood (LH) mechanism, involving the simultaneous adsorption of both CO and O2 molecules onto the catalyst surface before reaction, (O2*(ads) + CO*(ads)→CO2(g) + O*(ads)), (iii) the new Eleye Rideal (NER) mechanism, characterized by two gaseous CO molecules reacting directly with adsorbed O2 (2CO(g) + O2*(ads)→2CO2(g)), and (iv) the termolecular Eley–Rideal (TER) mechanism, in which gaseous O2 reacts directly with two adsorbed CO molecules (2CO*(ads) + O2(g)→2CO2(g)). However, this study specifically focuses on the ER and LH mechanisms, as single-atom metal doping typically exhibits pronounced reaction specificity toward these pathways. Furthermore, prior investigations indicate that CO oxidation on C3N-based surfaces primarily proceeds via these mechanisms, achieving relatively low activation energy barriers. It has also been established that non-metal doping on C3N surfaces predominantly facilitates CO oxidation through the LH mechanism with notably low activation energies [1,21]. Conversely, doping metal atoms onto C3N tends to promote the ER mechanism preferentially [18,20], usually characterized by lower activation barriers than the LH pathway, although somewhat higher than those observed in non-metal doping scenarios. Consequently, a promising research avenue involves incorporating isolated single metal atoms to further reduce the energy barriers for CO oxidation reactions.
Motivated by these insights, the present work theoretically investigates the strategic introduction of nitrogen vacancies into the C3N monolayer, followed by the doping of platinum (Pt) single atoms, to create an innovative catalytic platform. This combined approach aims to exploit synergistic interactions between Pt single atoms and nitrogen-deficient sites on the C3N surface, significantly enhancing the adsorption and activation of reactant molecules. Ultimately, our objective is to achieve an effective low-temperature catalytic oxidation of CO, demonstrating the considerable potential of these modified C3N-based single-atom catalyst (SACs) systems for efficient, cost-effective, and sustainable catalytic technologies.
2. Computational Method
All calculations were performed using the plane-wave-based periodic DFT method implemented in the Vienna ab initio simulation package (VASP) [32,33], where the ionic cores are described by the projector augmented wave (PAW) method [34,35,36]. The exchange and correlation energies are computed using the Perdew–Burke–Ernzerhof (PBE) functional [37] and the dispersion correction to account for the van der Waals interaction (PBE-D3) method [38,39]. To have accurate energies, a plane-wave cutoff energy of 400 eV, the Gaussian electron smearing method with σ = 0.10 eV was used. The Brillouin zone integrations were performed using the Monkhorst–Pack grids of 15 × 15 × 15 and 3 × 3 × 1 for the bulk and surface calculations, respectively. All transition state structures were located using the climbing image nudged elastic band (CI-NEB) method [40]. Geometry optimization was performed until the forces acting on each atom were smaller than 0.05 eV/Å with the electronic self-consistent field iteration threshold of 10−6 eV. A vacuum spacing of 21 Å was applied to prevent spurious interactions between periodic images.
The binding energy (Eb) of a Pt atom doped on the C3N surface was calculated using the following equation.
where is the total energy of the C3N surface with a Pt atom doped at either a carbon vacancy (CV) or a nitrogen vacancy (NV) site; is the total energy of the bare C3N surface; is the total energy of the Pt atom. The stability of the Pt-doped C3N surface was considered by the value. The less-positive Eb value refers to high stability or strong interaction of the promoted Pt on the C3N surface (Pt@CV-C3N or Pt@NV-C3N).
The adsorption energies (Eads) of CO, O2, and CO+O2 adsorption on the Pt@CV-C3N or Pt@NV-C3N surface were calculated using the following equation.
where is the total energy of the CO, O2, and CO+O2 molecule adsorption on a Pt@CV-C3N or Pt@NV-C3N surface; is the total energy of the Pt@CV-C3N or Pt@NV-C3N surface; and is the total energy of the CO, O2, and CO+O2 molecule. The negative Eads value represents the strong interaction between the CO, O2, and CO+O2 molecules and the Pt@CV-C3N or Pt@NV-C3N surface.
The reaction rate constants () of the reaction mechanisms on the Pt@C3N surface were estimated using the standard Arrhenius equation [41,42,43,44,45,46].
where is the reaction rate constant, is the pre-exponential factor, is the activation energy required for the reaction in J/mol, is the gas constant (8.314 J/K−1mol−1), and is the absolute temperature in K.
3. Results
3.1. Structural Model of Pristine C3N
Figure 1a presents the optimized structure of a pristine C3N monolayer, which exhibits a hexagonal lattice and belongs to the P6/mmm space group [47,48,49]. In our study, the pristine C3N was modeled using the 5 × 5 pristine C3N supercell to capture the structural and electronic characteristics of the material accurately. Within this monolayer, two distinct types of hexagonal rings are observed. As shown in Figure 1b, (1) the C-N rings are composed of four carbon and two nitrogen atoms, and (2) the C-C rings comprise six carbon atoms. Both the C-N and C-C bonds exhibit a bond length of approximately 1.41 Å, which aligns well with previously reported values in the literature [19,50,51,52].
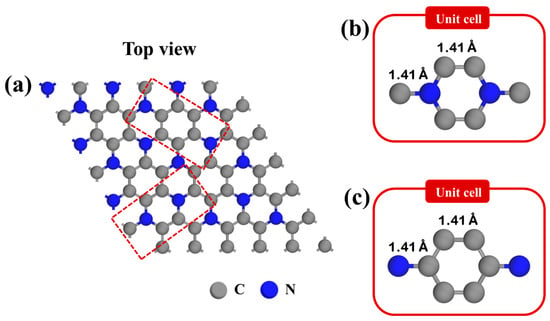
Figure 1.
The optimized structure of (a) pristine C3N surface and unit cell of C3N with (b) C-N rings and (c) C-C rings.
Despite the structural stability and periodicity of pristine C3N, its chemical inertness toward small gas molecules, particularly O2, limits its potential for catalytic applications such as CO oxidation. To address this shortcoming, we further investigate the effect of introducing structural defects, specifically nitrogen vacancies (NV), into the C3N monolayer. Defect engineering has proven to be an effective strategy for tuning the electronic properties and enhancing the surface reactivity of 2D materials. In the case of C3N, NV can create localized electronic states that serve as active sites, improving the adsorption and activation of gas molecules. By focusing on the defective structure of C3N, we aim to explore how NV can synergistically interact with doped Pt single atoms to enhance catalytic activity further. This defect-engineered approach not only increases the number of active sites but also modifies the local electronic environment to favor the stronger binding of reactants and lower energy barriers for the CO oxidation reaction. Therefore, investigating nitrogen-deficient C3N is crucial to designing high-performance, single-atom catalyst systems for low-temperature and energy-efficient CO oxidation.
3.2. The Defective Structure of C3N
Decorating C3N monolayers with single-atom catalysts (SACs) has emerged as a promising strategy to overcome the intrinsic chemical inertness of pristine C3N toward gas molecules. In particular, introducing atomic-scale defects, such as vacancies, can significantly enhance the interaction between the surface and adsorbates by providing reactive sites and modulating the local electronic environment. In this study, we focus on exploring the doping of a Pt atom into the defective C3N monolayer to identify the most thermodynamically stable configurations and to assess their potential for improved gas adsorption and catalytic performance in CO oxidation. We specifically consider single vacancies, which include two types: (i) a carbon vacancy (CV), created by removing a single carbon atom from the C3N monolayer (Figure 2a), and (ii) a nitrogen vacancy (NV), formed by removing a single nitrogen atom (Figure 2b). These vacancy sites serve as anchoring points for Pt atoms, forming Pt-doped C3N systems (Pt@C3N). The introduction of such defects not only alters the electronic structure of the C3N surface but also improves the binding affinity of the Pt atom, thus enhancing its catalytic potential. To evaluate the stability and interaction strength between the Pt atom and the defective C3N monolayer, we calculated the binding energy of the Pt atom at each vacancy site, as defined in Equation (2). A lower binding energy indicates a stronger interaction and higher structural stability. The binding energies for Pt atoms anchored at the CV and NV sites are presented in the upper panel of Figure 3. Based on these results, the most stable Pt@C3N configuration is identified and further analyzed in the context of CO oxidation mechanisms in the subsequent sections. Furthermore, to evaluate the feasibility of defect formation in C3N, we calculated the vacancy formation energies for both carbon (CV) and nitrogen (NV) vacancies using the following equation:
where is the total energy of the C3N monolayer with one vacancy (either CV or NV); is the total energy of the pristine C3N monolayer; and is the chemical potential of the removed atom (C or N). The results show that the formation energy of CV is 5.03 eV, whereas that of NV is 6.21 eV. These positive values indicate that both vacancies require energy input to form, consistent with the chemically stable nature of the C3N surface. However, both vacancies have sufficiently low formation energies to be accessible under realistic experimental conditions, such as thermal annealing. Although nitrogen vacancies are less likely to form spontaneously due to their higher formation energy, subsequent Pt doping stabilizes both vacancy types effectively. As demonstrated in the following section, Pt atoms bind strongly to both carbon and nitrogen vacancies with comparable binding energies, making both defect types viable anchoring sites for single-atom Pt catalysts on the C3N monolayer.
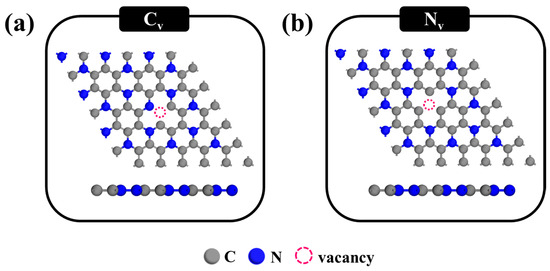
Figure 2.
Optimized atomic structures of vacancy defects on the C3N monolayer: (a) single carbon vacancy (CV), formed by removing one C atom; (b) single nitrogen vacancy (NV), formed by removing one N atom.
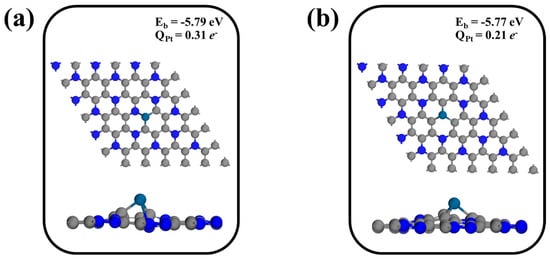
Figure 3.
Optimized structures, binding energies, and Bader charges of Pt atoms doped at different vacancy sites on the C3N monolayer: (a) Pt@CV-C3N and (b) Pt@NV-C3N. Gray, blue, and navy spheres represent C, N, and Pt atoms, respectively.
We then investigated the Pt-doped C3N (Pt@C3N) monolayer for its potential in catalyzing the CO oxidation reaction. The optimized structures and electronic properties of Pt atoms anchored at single-vacancy sites, specifically carbon (CV) and nitrogen (NV) vacancies, are shown in Figure 3. The binding energies (Eb) for Pt@CV-C3N and Pt@NV-C3N configurations are significantly negative, −5.77 and −5.79 eV, respectively, indicating strong interactions between the Pt atom and the defective C3N surface. This strong binding suggests that Pt atoms are thermodynamically stable at these vacancies and are unlikely to migrate or aggregate into clusters, a common issue in nanoparticle-based catalysts. To further assess the electronic characteristics of these systems, we performed a Bader charge analysis to quantify the charge transfer between the Pt atom and the C3N monolayer. The Bader charge analysis (QPt) indicates that the Pt atom exhibits electron donation to the surrounding lattice, with QPt values of 0.31 e− for Pt@CV-C3N and 0.21 e− for Pt@NV-C3N, respectively. This charge transfer confirms that the Pt atom becomes partially oxidized upon incorporation into the defect site, which can play a crucial role in enhancing its catalytic activity by modifying its electronic structure and increasing its interaction with reactant molecules. Given their strong binding and favorable charge characteristics, the Pt@CV-C3N and Pt@NV-C3N configurations are selected for further analysis in the subsequent sections to evaluate their adsorption behavior and catalytic performance in CO oxidation. To evaluate the influence of Pt decoration and carbon or nitrogen vacancies on the electronic structure of C3N monolayers, the partial density of states (PDOS) was calculated and compared among the pristine C3N, and Pt-decorated C3N with carbon or nitrogen vacancies [53,54], as shown in Figure S6. As observed in the pristine C3N monolayer (Figure S6a), a clear band gap exists near the Fermi level, indicating its semiconducting nature. Upon decoration with a Pt single atom (Figure S6b,c), new states emerge near the Fermi level, which are mainly contributed by the Pt d-orbitals. These states significantly enhance the electronic conductivity of the system. Furthermore, the hybridization between the Pt d-orbital and the surrounding N p-orbital or C p-orbital orbitals suggests a strong interaction between the Pt atom and the defective C3N surface, which facilitates the charge transfer processes that are essential for CO oxidation. These electronic modifications are expected to lower the reaction barriers and enhance the overall catalytic performance.
3.3. The O2 and CO Adsorption on Pt-Doped C3N Monolayers with Single Vacancies
In this section, we use DFT calculations to investigate the adsorption behavior of CO and O2 molecules on Pt-doped C3N monolayers containing single vacancies, namely, carbon vacancies (CV) and nitrogen vacancies (NV). The adsorption energy (Eads) was used as the key parameter to assess the interaction strength between gas molecules and the modified C3N surface [55,56] and to help identify possible CO oxidation mechanisms. Only the most stable adsorption configurations, characterized by the most significant Eads values, are discussed herein, though multiple initial configurations were evaluated. For CO adsorption on the Pt@C3N monolayers with CV and NV vacancies, the carbon atom of the CO molecule binds directly to the Pt atom located at the vacancy site (Figure 4a). The Eads were calculated to be −2.52 and −2.24 eV for Pt@CV-C3N and Pt@NV-C3N. These interactions produce strong chemisorption, indicating a robust binding between CO and the Pt site. This strong interaction suggests a favorable environment for the initial step in the CO oxidation process.
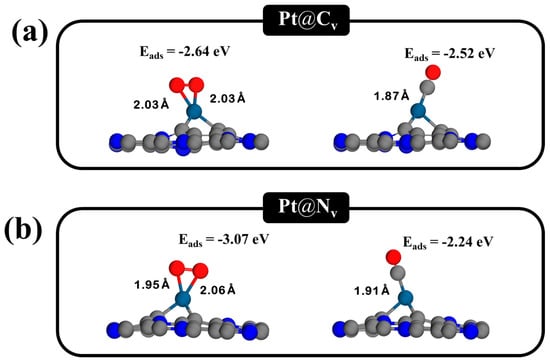
Figure 4.
Optimized structures of CO and O2 adsorption on Pt-doped C3N monolayers at single vacancy sites: (a) Pt@CV-C3N and (b) Pt@NV-C3N.
For O2 adsorption on the same Pt-doped single-vacancy systems, the O2 molecule forms a bidentate configuration with the Pt atom, where both oxygen atoms participate in bonding. These interactions also exhibit strong chemisorption, with Eads of −2.64 and −3.07 eV for Pt@CV-C3N and Pt@NV-C3N, respectively (Figure 4b). The stronger adsorption of O2 compared to CO suggests that the Pt@CV-C3N and Pt@NV-C3N systems satisfy the energetic requirement for the Eley–Rideal (ER) mechanism, where pre-adsorbed O2 reacts with incoming CO molecules. To compare O2 and CO adsorption on various vacancy sites of the C3N monolayer, the adsorption energy was used to identify the most favorable configurations and to determine the possible oxidation mechanism of CO on the C3N monolayer. The criterion for CO oxidation via the ER mechanism is that the adsorption energy of the O2 molecule must be stronger than that of the CO molecule. Our study found that O2 molecules can significantly better adsorb onto Pt-doped single vacancies on the C3N monolayer (Pt@CV-C3N and Pt@NV-C3N) than CO molecules on other surfaces. Therefore, this research focuses exclusively on Pt@CV-C3N and Pt@NV-C3N. Furthermore, co-adsorption of CO and O2 was observed to be thermodynamically favorable on both Pt@CV-C3N and Pt@NV-C3N surfaces, indicating the potential for the Langmuir–Hinshelwood (LH) mechanism, where both reactants are adsorbed before reaction (Figure S1). Among all configurations studied, Pt@CV-C3N and Pt@NV-C3N show the most promising properties for CO oxidation. This is attributed to their significantly stronger O2 adsorption compared to CO, co-adsorption feasibility, and the favorable energetics supporting both ER and LH pathways. These features underscore the catalytic potential of Pt-doped single-vacancy C3N monolayers as efficient, low-temperature CO oxidation catalysts. Therefore, Pt@CV-C3N and Pt@NV-C3N systems have been selected for further in-depth mechanistic investigation, given their superior reactivity and thermodynamic favorability for CO oxidation.
Moreover, prior to investigating the charge density differences, the co-adsorption of CO and O2 on the Pt@CV-C3N and Pt@NV-C3N surfaces was also studied to support the possibility of a Langmuir–Hinshelwood (LH) mechanism. The results indicate that CO+O2 can be chemisorbed on both surfaces, with adsorption energies of −3.44 eV for Pt@CV-C3N and −4.14 eV for Pt@NV-C3N, respectively. These findings imply that the simultaneous adsorption of both reactants on the Pt@CV-C3N and Pt@NV-C3N surfaces is possible, offering initial support for the occurrence of the Langmuir–Hinshelwood (LH) mechanism. Moreover, the charge density differences of the adsorption of CO, O2, and the co-adsorbed CO+O2 species on Pt@CV-C3N and Pt@NV-C3N surfaces are illustrated in Figure 5 and Figure 6, where the yellow and blue regions represent charge accumulation and depletion, respectively. These visualizations reveal pronounced charge redistribution in the CO+O2 co-adsorbed systems, indicating strong electronic interactions between the adsorbates and the Pt-doped C3N surfaces. This observation is further supported by the calculated net charge transfer values ∆QCO+O2, which show substantial electron donation from the surfaces to the co-adsorbed species: −0.97 e− for Pt@CV-C3N and −0.83 e− for Pt@NV-C3N. In comparison, the individual adsorption of O2 leads to moderately lower charge transfer values of −0.18 e− and −0.72 e−, respectively, for the same surfaces. CO adsorption shows the weakest charge transfer, with values of −0.17 e− for Pt@CV-C3N and −0.25 e− for Pt@NV-C3N, indicating relatively weaker interaction. These results suggest a preference for O2 adsorption on the Pt-doped C3N surfaces, which may then undergo a reaction with incoming CO molecules, a behavior characteristic of the Eley–Rideal (ER) mechanism. The stronger interaction and greater electron transfer for O2 compared to CO further support the conclusion that CO oxidation is more likely to proceed effectively via the ER pathway on both Pt@CV-C3N and Pt@NV-C3N surfaces.
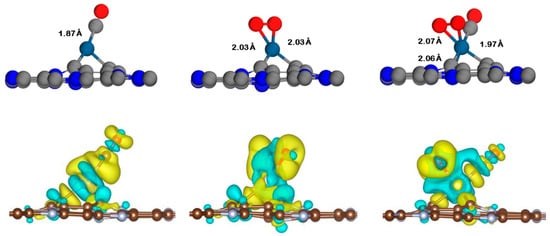
Figure 5.
Charge density differences of the CO, O2, and CO+O2 co-adsorption on the Pt@CV-C3N surface with isovalue of ±0.002 e/Å3. Charge accumulation and depletion are represented by yellow and blue regions, respectively. Gray, blue, navy, and red spheres represent carbon (C), nitrogen (N), platinum (Pt), and oxygen (O) atoms, respectively.
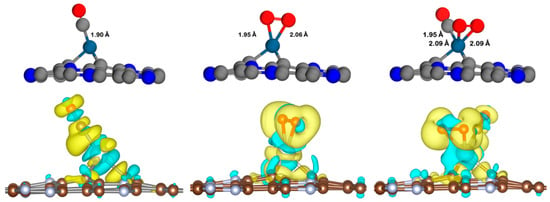
Figure 6.
Charge density differences of the CO, O2, and CO+O2 co-adsorption on the Pt@NV-C3N surface with isovalue of ±0.002 e/Å3. Charge accumulation and depletion are represented by yellow and blue regions, respectively. Gray, blue, navy, and red spheres represent carbon (C), nitrogen (N), platinum (Pt), and oxygen (O) atoms, respectively.
3.4. Reaction Mechanism of CO Oxidation on Pt@NV-C3N
Based on previous theoretical and experimental studies on CO oxidation on C3N-based catalysts, it primarily proceeds via two dominant mechanisms: the Eley–Rideal (ER) and Langmuir–Hinshelwood (LH) pathways. In this work, we evaluated the feasibility of these mechanisms on the Pt@NV-C3N surface by analyzing the adsorption energies of CO, O2, and co-adsorbed CO+O2 species, identifying the most energetically favorable pathways. For the ER mechanism, the reaction begins with the pre-adsorption of an O2 molecule on the Pt active site. The absorbed O2 then reacts directly with a gas-phase CO molecule, resulting in either a transient carbonate-like intermediate (CO3) or the immediate formation of CO2 and a surface-bound oxygen atom (O*). In contrast, the LH mechanism involves the co-adsorption of both CO and O2 molecules at the Pt site on the NV defective C3N surface. This leads to the formation of a peroxo-type intermediate (OO-CO), which subsequently decomposes to produce a CO2 molecule and an activated oxygen atom (O*) remaining on the surface. To better understand the full catalytic process, we examined the detailed stepwise pathways for both mechanisms, including the structural configurations of each intermediate and transition state. The energetic profiles and geometric changes along these reaction pathways are discussed in the following sections to elucidate the role of Pt doping and nitrogen vacancies in facilitating low-energy CO oxidation.
3.4.1. Eley–Rideal Mechanism (ER) on Pt@NV-C3N
The Eley–Rideal (ER) mechanism involves a reaction between a gas-phase CO molecule and a pre-adsorbed O2 molecule on the surface of the catalyst. On the Pt@NV-C3N system, various physisorption configurations of the CO molecule, floating above or approaching different orientations of the adsorbed O2, can significantly influence the reaction pathway and energetics. This study examines two distinct ER reaction pathways: (i) ER1 Mechanism: The CO molecule approaches and reacts with the center of the adsorbed O2 molecule, forming a transient carbonate-like intermediate (CO3). This intermediate subsequently decomposes to yield carbon dioxide (CO2) and a surface-bound activated oxygen atom (O*). The overall reaction can be summarized as: ER1: O2 + CO → CO3 → CO2 + O*.ER2: The CO molecule attacks the side of the adsorbed O2 molecule, bypassing the CO3 intermediate and leading directly to the formation of CO2 and a remaining O* on the surface: ER2: O2 + CO → CO2 + O*. The specific configurations and structural evolution involved in both ER1 and ER2 mechanisms will be illustrated in Figure 7. Corresponding energy profiles detailing the reaction steps and associated energy barriers are presented in Figure 8 and Figure 9, providing insight into the relative favorability and activation energies of each pathway.
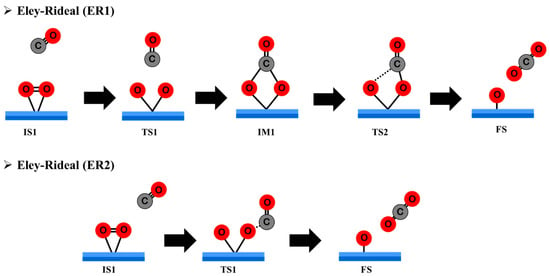
Figure 7.
Proposed reaction mechanisms of ER1 and ER2 on Pt@NV-C3N.
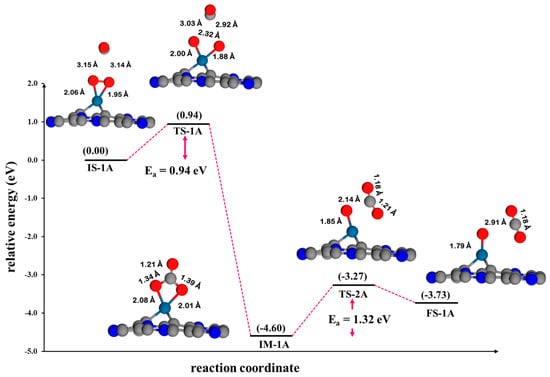
Figure 8.
The potential energy profile and optimized stationary points for the oxidation of CO via the ER1 mechanism on Pt@NV-C3N.
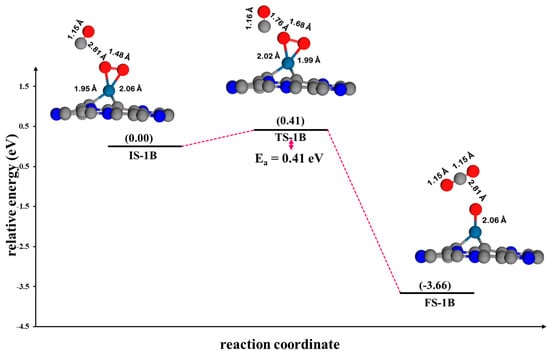
Figure 9.
The potential energy profile and optimized stationary points for the oxidation of CO via the ER2 mechanism on Pt@NV-C3N.
ER1 mechanism: Figure 8 presents the energy profile for the CO oxidation on Pt@NV-C3N via the ER1 mechanism. In this pathway, O2 and CO molecules do not react in a single elementary step. Instead, the reaction proceeds through a carbonate-like intermediate (CO3). The process begins with the physisorption of a CO molecule above pre-adsorbed O2 species on the Pt@Nv-C3N surface (IS-1A), with an initial CO-O2 distance of 3.14 Å, indicating weak initial interaction. The O2 molecule interacts with the surface metal atom through both of its oxygen atoms, exhibiting O–metal bond lengths of 2.06 Å and 1.95 Å, respectively, while the O-O bond remains intact. The reaction progresses as the CO approaches and inserts itself between the O atoms of O2, leading to O-O bond cleavage and the formation of the CO3 intermediate (IM-1A). This step requires an activation energy of 0.94 eV (TS-1A). A vibrational frequency of 428.58i cm−1 is associated with this process, and the direction of this vibrational mode is consistent with the transition state structure and aligns well with the motion along the reaction coordinate. Subsequently, the CO3 structure decomposes into a CO2 molecule and an activated oxygen atom (O*) adsorbed on the Pt@NV-C3N surface (FS-1A). However, this decomposition step is kinetically hindered, as it involves breaking a strong intermolecular bond with a stable CO3 structure. This is reflected in the higher activation energy barrier of 1.32 eV (TS-2A), identifying this step as the rate-limiting step of the ER1 pathway. The calculated imaginary frequency of 280.74i cm−1 corresponds to the transition state and reaction coordinate, indicating that the CO3 bond cleavage is kinetically unfavorable due to the structural stability of the CO3 intermediate. This result is further supported by density of states (DOS) analysis (Figure S2), which reveals significant electronic interaction during CO3 formation and its subsequent breakdown. The strong binding within the intermediate is consistent with the observed energy profile, confirming the critical role of CO3 stability in governing the kinetics of this pathway.
ER2 mechanism: In the CO oxidation on Pt@NV-C3N via the ER2 mechanism (Figure 9), the reaction starts with the physisorption of CO molecules onto pre-adsorbed O2 on the Pt@NV-C3N monolayers and is defined as the initial state (IS-1B) of the ER2 mechanism, where the distance between the O atoms in O2 and the CO molecule in IS-1B is 2.81 Å. Then, the CO molecules react with the O atom of the O2 molecule to form CO2, which desorbs from the surface, leaving an activated O atom on the surface. This contrasts with the ER1 mechanism, where the CO molecule interacts with the terminal site of the O2 molecule. In the ER2 mechanism, the CO molecule will react on the side of the O2 molecule, allowing for a concerted process in which CO2 formation and O-O bond cleavage co-occurs. As a result, the reaction along this pathway is more favorable, consistent with the low energy barrier values of 0.41 eV (TS-1B). This represents a critical point of reaction where bond cleavage and formation occur concurrently. The observed variations in bond lengths are consistent with the structure of the transition state (TS-1B). Specifically, the Pt-O bond length ranges from 1.99 to 2.02 Å, while the O-O bond length is 1.68 Å, suggesting bond elongation and weakening. In addition, the direction and magnitude of the vibrational mode align with the TS structure, as evidenced by a calculated imaginary frequency of 531.28i cm−1. This is consistent with the DOS analysis, which presents a weak interaction between the CO molecule and the side-on adsorbed O2 on the surface, followed by the decomposition into CO2 and atomic O, as illustrated in Figure S3.
3.4.2. Langmuir–Hinshelwood Mechanism (LH) on Pt@NV-C3N
The Langmuir–Hinshelwood (LH) mechanism for CO oxidation on the Pt@NV-C3N surface begins with the co-adsorption of O2 and CO molecules at the Pt active site. After co-adsorption, the O2 molecule interacts with a neighboring CO molecule to form a peroxo-type intermediate species (OC-OO). This intermediate represents a crucial transition state in which the O-O bond is weakened, facilitating the subsequent cleavage. Following the formation of the OC-OO intermediate, the reaction proceeds via decomposition of the peroxo-complex, resulting in the formation of one CO2 molecule and a remaining adsorbed oxygen atom (O*), which remains available for a further reaction with an additional CO molecule in the next catalytic cycle. We will illustrate the specific characteristics of the LH reaction mechanisms in Figure 10, and we will present the energy profiles for CO oxidation via the LH mechanisms in Figure 11.
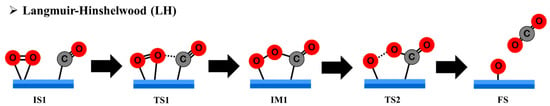
Figure 10.
Proposed reaction mechanisms of LH on Pt@NV-C3N.
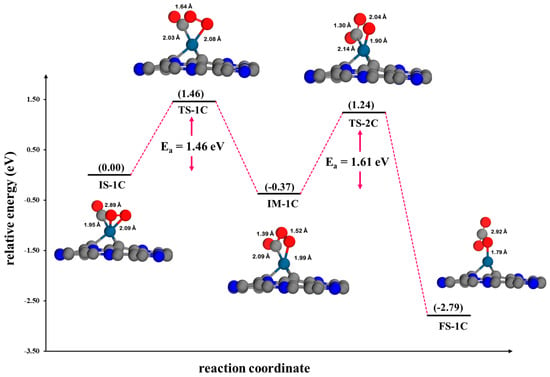
Figure 11.
The potential energy profile and optimized stationary points for the oxidation of CO via the LH mechanism on Pt@NV-C3N.
In the CO oxidation process on Pt@NV-C3N via the LH mechanism (Figure 11), the reaction begins with CO and O2 co-adsorbed on the Pt site of the Pt@NV-C3N monolayer, referred to as the initial state (IS-1C) of the LH mechanism. The distance between the O atom in the O2 molecule and the Pt atom on the surface in IS-1 is calculated to be 2.09 Å, while the distance between the CO molecule and the Pt atom on the surface in IS-1C is 1.95 Å. Then, one O atom from the O2 molecule approaches the CO molecule, forming a peroxo-type OC-OO intermediate (IM-1C). The OC-OO intermediate forms over the Pt atom on the surface, passing through the TS-1C transition state with a high activation energy of 1.46 eV. The direction and magnitude of the vibrational mode are consistent with the structure of this transition state, as indicated by the calculated imaginary frequency of 293.15i cm−1. Afterward, the O-O bond in the O2 molecule decomposes into a CO2 product, and an O atom is adsorbed on the surface (FS-1C). The cleavage of the O-O bond leading to CO2 formation is kinetically hindered, necessitating a high activation energy barrier at this stage. The activation energy required for this reaction step is 1.61 eV (TS-2C), making it unlikely to occur at room temperature. The direction and vibrational mode are consistent with the structure of this transition state, as confirmed by a frequency of 311.21i cm−1 According to the density of states (DOS) analysis, the strong interaction involved in the formation of the peroxo-type OC-OO intermediate inhibits its decomposition into CO2 and O*, indicating that this step is energetically unfavorable, as shown in Figure S4.
3.4.3. Eley–Rideal Mechanism (ER2) on Pt@CV-C3N
Previous studies have shown that the rate-determining step (RDS) for single-atom metal-doped catalysts typically involves an activation energy barrier below 0.80 eV [57,58], indicating that the formation of CO2 can readily occur at room temperature. This observation provides a valuable benchmark for evaluating the catalytic activity of Pt-doped C3N systems investigated in this study. Therefore, we selected only the ER2 mechanism for further study of CO oxidation on Pt@CV-C3N.
ER2 mechanism: In the CO oxidation process on Pt@CV-C3N via the ER2 mechanism, the corresponding energy profile and reaction pathway are illustrated in Figure 12. In this pathway, a CO molecule in the gas phase reacts directly with a pre-adsorbed O2 species on the Pt@CV-C3N surface. The reaction proceeds through the formation of a transient CO-O2 complex, followed by O-O bond cleavage and the subsequent formation of a CO2 molecule and an adsorbed atomic oxygen species (O*). The energy barrier for this step is calculated to be 0.91 eV (TS-1D), which is significantly higher than that of the ER2 mechanism on Pt@NV-C3N (0.41 eV), suggesting that the CV site is less kinetically favorable for CO oxidation. Furthermore, the direction and vibrational mode associated with the imaginary frequency of 697.23i cm−1 are consistent with the transition state structure, confirming its validity. This observation aligns with the DOS analysis, which reveals a weak interaction between the CO molecule and side-on adsorbed O2 on the surface, leading to the formation of CO2 and atomic O*, as illustrated in Figure S5. In comparison, the ER2 mechanism on the Pt@NV-C3N surface exhibits an even weaker interaction, suggesting that the presence of vacancy sites significantly influences the reaction process.
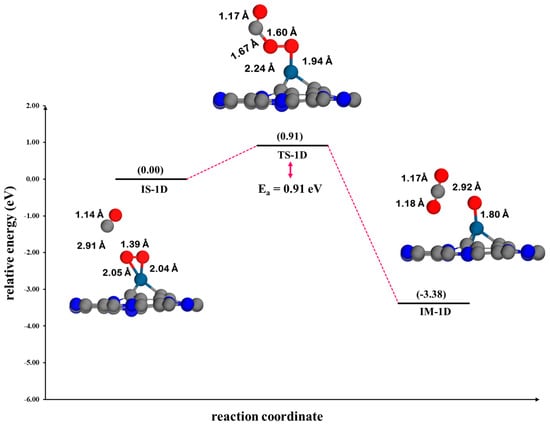
Figure 12.
The potential energy profile and optimized stationary points for the oxidation of CO via the ER mechanism on Pt@CV-C3N.
4. Discussion
4.1. Comparison of the Reaction Mechanism of CO Oxidation on Pt@NV-C3N
To elucidate the reaction mechanism of CO oxidation on Pt-based single-atom catalysts, we systematically investigated four distinct reaction pathways using DFT calculations: ER1-Pt@NV-C3N, ER2-Pt@NV-C3N, LH-Pt@NV-C3N, and ER2-Pt@CV-C3N. These pathways were analyzed based on their energy profiles, focusing on the activation energy barriers (Ea) and the thermodynamic stability of the products, as illustrated in Figure 13. Among the mechanisms explored, the ER2 pathway on Pt@C3N, where a CO molecule reacts with the side of a pre-adsorbed O2 molecule, was found to be the most favorable. This reaction forms a CO2 molecule, desorbing from the surface, leaving behind an adsorbed O atom (O*). This step exhibited the lowest activation barrier, making it the kinetically preferred route for CO oxidation on the Pt@C3N system. In comparison, the ER1 mechanism, which involves the formation of a carbonate-like intermediate (CO3), was found to be less favorable. Although the CO3 intermediate is thermodynamically stable, its decomposition requires relatively high activation energy ( = 1.32 eV) due to the strength of its internal bonds. This is consistent with charge density difference analyses, which show a high degree of localized electron density within the CO3 species, indicating strong electronic stabilization and significant charge redistribution relative to the transition state (TS) in the ER2 mechanism. Furthermore, our analysis highlights that the type of vacancy (CV vs. NV) on the C3N surface plays a critical role in determining the reaction pathway. The ER2 mechanism on Pt@N-C3N emerged as the most favorable route, both kinetically and thermodynamically, among all studied cases. The detailed variations in bond lengths and bond angles for all key structures involved in the CO oxidation process, including reactants, intermediates, transition states, and products, are summarized in Table S1 in the Supporting Information. These findings suggest that engineering nitrogen vacancies on C3N supports can significantly enhance the catalytic activity of Pt single-atom systems for CO oxidation. Thus, tailoring vacancy types offers a promising strategy for the rational design of efficient, low-temperature oxidation catalysts based on 2D materials.
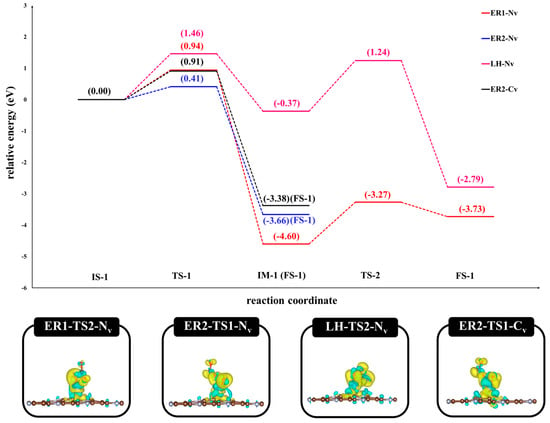
Figure 13.
The potential energy profile and optimized stationary points for the oxidation of CO via the ER1, ER2, and LH pathway on Pt@NV-C3N surface and ER2 pathway on Pt@CV-C3N surface, and charge density differences of the transition state on all surfaces.
To evaluate the catalytic performance of the designed Pt@NV-C3N system for CO oxidation, a comparative analysis was conducted against previously reported transition metal-doped C3N systems, as summarized in Table 1. According to previous research, Fe-C3N with nitrogen vacancies exhibited the lowest activation energy () of 0.47 eV among the reported transition metal-doped systems. These studies also revealed that, in metal-doped C3N surfaces, the CO oxidation reaction generally proceeds via the Eley–Rideal (ER) mechanism. In contrast, our analysis shows that Pt-C3N at nitrogen vacancy sites demonstrates a superior catalytic performance, with an of only 0.41 eV, which is lower than that of Fe-C3N and other reported systems. Furthermore, the corresponding adsorption energies for CO and O2 are −2.24 eV and −3.07 eV, respectively, indicating significantly enhanced adsorption strength, especially for O2 adsorption. This strong adsorption of O2 indicates efficient activation and charge transfer, which are essential steps in the CO oxidation process. Furthermore, to confirm that the CO oxidation reaction proceeds more favorably on nitrogen vacancies than on carbon vacancies on the metal-doped C3N surface, a comparative study was conducted using Pt doped at the carbon vacancy site of the C3N surface (Pt@CV-C3N). The results showed that Pt@CV-C3N exhibits an increased activation energy () of 0.91 eV and significantly stronger adsorption energies (−2.52 eV for CO and −2.64 eV for O2). This may be attributed to overly stable intermediate species forming, which can block the reaction progress and lead to a high energy barrier. In contrast, the Pt@NV-C3N surface offers a more promising balance between activation energy and adsorption strength, making it a highly efficient candidate for low-temperature CO oxidation applications. These results highlight the superior catalytic performance of Pt@NV-C3N compared to previously reported TM-doped C3N systems, especially in terms of the thermodynamics and kinetics that are important for the catalytic activity.

Table 1.
The highest energy barrier of CO oxidation in the best mechanism by various catalysts.
4.2. Kinetic Studies and Microkinetic Modeling
To comprehensively evaluate the temperature dependence of CO oxidation on Pt@C3N systems, we performed kinetic studies based on the Arrhenius equation for the four most relevant pathways: ER1, ER2, LH mechanisms on Pt@NV-C3N, and the ER2 mechanism on Pt@CV-C3N. The results are presented in Figure 14a, which shows the natural logarithm of the rate constant () versus the inverse temperature (1000/). Figure 14b depicts the corresponding reaction rate trends across the temperature range of 273–700 K. As shown in Figure 14a, the slope of each line reflects the activation energy () of the respective mechanism. The ER2 mechanism on the Pt@NV-C3N surface exhibits the shallowest slope, consistent with its lowest activation barrier (0.41 eV), indicating that this pathway is kinetically the most accessible. In contrast, the LH and ER1 mechanisms display steeper slopes due to their higher activation energies (1.46 eV and 1.32 eV, respectively), which correspond to slower reaction rates, particularly at lower temperatures. Figure 14b further reinforces these findings through microkinetic modeling. The ER2 pathway on Pt@NV-C3N demonstrates a higher reaction rate across a broader temperature window, with the peak activity occurring between 500 and 600 K. This peak suggests an optimal operation temperature range for catalytic performance under realistic conditions [61,62]. The LH and ER1 pathways on the same surface show much narrower and lower rate profiles, confirming that their high activation barriers make them less effective at moderate temperatures. Additionally, the ER2 mechanism on Pt@CV-C3N shows an activity peak at a higher temperature (~670 K). This further supports the conclusion that NV creates a more reactive environment for low-temperature CO oxidation than CV. These findings indicate that ER2 on Pt@NV-C3N is the most kinetically favorable pathway across the full temperature range. Pt@CV-C3N requires a significantly higher temperature to achieve comparable catalytic activity. ER1 and LH mechanisms are kinetically limited due to their higher energy barriers and reduced charge transfer during key transition steps. Thus, combining Arrhenius analysis and microkinetic modeling clearly demonstrates that the ER2 mechanism on Pt@NV-C3N offers superior catalytic performance for CO oxidation under both ambient and elevated temperatures. This insight emphasizes the importance of vacancy engineering, specifically nitrogen vacancies, in tuning the activity of single-atom catalysts and guiding the design of next-generation, low-temperature catalytic materials.
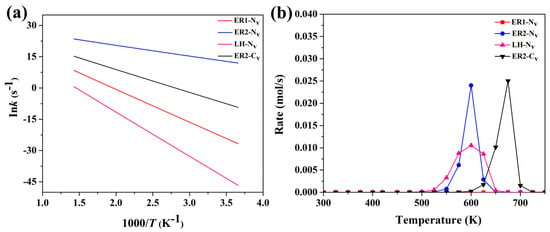
Figure 14.
(a) The reaction rate constants and (b) microkinetic modeling of ER1, ER2, and LH pathway on Pt@NV-C3N surface and ER2 pathway on Pt@CV-C3N during CO oxidation within the 273-700 K temperature range.
5. Conclusions
In this study, the catalytic performance of single platinum (Pt)-doped C3N monolayers for CO oxidation was thoroughly investigated using density functional theory (DFT) calculations. Among all Pt-doped vacancy configurations in C3N monolayers, Pt doped at a nitrogen vacancy (Pt@NV-C3N) exhibited the most favorable adsorption properties for both CO and O2 molecules, making it the most promising catalyst for CO oxidation. Furthermore, mechanistic studies of CO oxidation via the Eley–Rideal (ER1 and ER2) and Langmuir–Hinshelwood (LH) pathways on the Pt@NV-C3N surface revealed that the ER2 pathway is more efficient. In particular, the ER2 mechanism exhibits low energy barriers (rate-limiting step of 0.41 eV) and high reaction rate constants at temperatures between 273 K and 700 K, indicating that CO oxidation via the ER2 route is the most suitable reaction mechanism for CO oxidation on the Pt@NV-C3N surface. Furthermore, microkinetic modeling confirmed that the ER2 mechanism dominates the CO oxidation process at optimal temperatures of 500–600 K. By comparison, we found that Pt@Nv-C3N is predicted to be a potential material for CO oxidation among these materials. Therefore, we hope that Pt@NV-C3N might be a highly effective material for the CO oxidation reaction.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/sci7030101/s1: Figure S1: The adsorption energy (Eads) of CO+O2 on metal decorated Pt-C3N surface; Figure S2: The PDOS analysis CO Oxidation by ER1 mechanism on Pt@NV-C3N surface. Figure S3: The PDOS analysis CO Oxidation by ER2 mechanism on Pt@NV-C3N surface. Figure S4: The PDOS analysis CO Oxidation by LH mechanism on Pt@NV-C3N surface. Figure S5: The PDOS analysis CO Oxidation by ER2 mechanism on Pt@CV-C3N surface. Table S1. Structural parameters (bond lengths and angles) for reactants, intermediates, transition states, and products during CO oxidation on Pt/C3N with nitrogen and carbon vacancies. Figure S6: The band structure of (a) pristine, (b) Pt@CV and (c) Pt@NV on C3N monolayers.
Author Contributions
S.J. and M.K. conceptualized the research. M.K. designed the computational methodology. S.K. performed DFT calculations, conducted data analysis, and wrote the original draft. Y.I. collected data, performed formal analysis, and reviewed and edited the manuscript. N.Y. and R.-Q.Z. performed formal analysis. S.J. supervised the project, contributed to the results discussion, and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the NSRF via the Program Management Unit for Human. Resources & Institutional Development, Research and Innovation, grant number B16F640099.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available on request.
Acknowledgments
This project is partly funded by the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation, Thailand [Grant number B16F640099]. The authors thank the NSTDA Supercomputer Center (ThaiSC) for providing computing resources. S.K. thanks the Thailand Graduate Institute of Science and Technology (TGIST) for financial support. S.J. would like to thank the National Science, Research, and Innovation Fund, Ubon Ratchathani University, and the Center of Excellence for Innovation in Chemistry (PERCH-CIC) for their computing resources.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DFT | Density functional theory |
| LH | Langmuir–Hinshelwood |
| ER | Eley–Rideal |
| Ea | Activation energy barriers |
References
- Esrafili, M.D.; Heydari, S. B-doped C3N monolayer: A robust catalyst for oxidation of carbon monoxide. Theor. Chem. Acc. 2019, 138, 57. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. Boosting graphene reactivity with co-doping of boron and nitrogen atoms: CO oxidation by O2 molecule. Appl. Surf. Sci. 2018, 455, 808–814. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. A DFT study on the possibility of using a single Cu atom incorporated nitrogen-doped graphene as a promising and highly active catalyst for oxidation of CO. Int. J. Quantum Chem. 2019, 119, e25857. [Google Scholar] [CrossRef]
- Freund, H.J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, J.; Chen, Z. Fe-anchored graphene oxide: A low-cost and easily accessible catalyst for low-temperature CO oxidation. J. Phys. Chem. C 2012, 116, 2507–2514. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X. The theoretical study of Rh single atom catalysts decorated C3N monolayer with N vacancy for CO oxidations. Appl. Phys. A 2023, 129, 325. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y. A comparative study of CO oxidation on Cu-doped C3N monolayer with N and C vacancies. J. Phys. Chem. Solids 2023, 180, 111441. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A review on CO oxidation over copper chromite catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, M.G.; Shin, D.; Han, J.W. Design of ceria catalysts for low-temperature CO oxidation. ChemCatChem 2020, 12, 11–26. [Google Scholar] [CrossRef]
- Wallander, H.J.; Gajdek, D.; Albertin, S.; Harlow, G.; Braud, N.; Buß, L.; Krisponeit, J.-O.; Flege, J.I.; Falta, J.; Lundgren, E.; et al. Dynamic Behavior of Tin at Platinum Surfaces during Catalytic CO Oxidation. ACS Catal. 2023, 13, 16158–16167. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Liberman, E.Y.; Naumkin, A.V. Influence of Pt/Pd state on ceria-based support in CO oxidation. J. Rare Earths 2023, 41, 1963–1968. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Li, H.; Xu, R.; Sun, Z. Highly active and water-resistant Lanthanum-doped platinum-cobalt oxide catalysts for CO oxidation. Appl. Catal. B Environ. 2023, 331, 122678. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.-L.; Feng, L.; Hu, J.; Chai, X.; Liu, J.-X.; Li, W.-X. Reactant-Induced Dynamic Stabilization of Highly Dispersed Pt Catalysts on Ceria Dictating the Reactivity of CO Oxidation. ACS Catal. 2024, 14, 3504–3513. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. 2021, 28, 24847–24871. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Lai, C.; Zhang, Y.; Lin, X.; Ding, S. Recent advances in noble metal-based catalysts for CO oxidation. RSC Adv. 2024, 14, 30566–30581. [Google Scholar] [CrossRef]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT Study of the CO Oxidation on the Au (321) Surface. J. Phys. Chem. C 2008, 112, 17291–17302. [Google Scholar] [CrossRef]
- Rao, X.; Si, Q.; Shi, T.; Han, X.; Ma, S. Fe-doped C3N monolayer as a promising SAC for CO oxidation with low temperature and high reactivity. Comput. Theor. Chem. 2021, 1194, 113080. [Google Scholar] [CrossRef]
- Bafekry, A.; Ghergherehchi, M.; Shayesteh, S.F.; Peeters, F. Adsorption of molecules on C3N nanosheet: A first-principles calculations. Chem. Phys. 2019, 526, 110442. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Jia, P. Pd-doped C3N monolayer: A promising low-temperature and high-activity single-atom catalyst for CO oxidation. Appl. Surf. Sci. 2021, 537, 147881. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Heydari, S. An effective approach for tuning catalytic activity of C3N nanosheets: Chemical-doping with the Si atom. J. Mol. Graph. Model. 2019, 92, 320–328. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, Y.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. Transition metal doped C3N monolayer as efficient electrocatalyst for carbon dioxide electroreduction: A computational study. Appl. Surf. Sci. 2021, 542, 148568. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Zhu, L.; Ling, C.; Xue, Q.; Xing, W. Charge-modulated CO2 capture of C3N nanosheet: Insights from DFT calculations. Chem. Eng. J. 2018, 338, 92–98. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Yang, F.; Ma, D.; Yang, Z.; Lu, Z. CO oxidation on single Pd atom embedded defect-graphene via a new termolecular Eley-Rideal mechanism. Carbon 2017, 118, 35–42. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Ding, Y.; Lu, Z.; Ma, D.; Yang, Z. First-principles study on the single Ir atom embedded graphdiyne: An efficient catalyst for CO oxidation. J. Phys. Chem. C 2018, 122, 23481–23492. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Xu, G.; Wang, T.; Ma, D.; Yang, Z.; Yang, L. Single Pt atom stabilized on nitrogen doped graphene: CO oxidation readily occurs via the tri-molecular Eley—Rideal mechanism. Phys. Chem. Chem. Phys. 2015, 17, 20006–20013. [Google Scholar] [CrossRef]
- González-Torres, J.C.; Bertin, V.; Poulain, E.; Olvera-Neria, O. The CO oxidation mechanism on small Pd clusters. A theoretical study. J. Mol. Model. 2015, 21, 279. [Google Scholar] [CrossRef]
- Baskaran, S.; Jung, J. Termolecular Eley–Rideal pathway for efficient CO oxidation on phosphorene-supported single-atom cobalt catalyst. Bull. Korean Chem. Soc. 2022, 43, 1254–1261. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Chen, M.; Liu, C.; Qi, X.; Peera, S.G.; Liu, J.; Liang, T. Theoretical investigation on catalytic mechanisms of oxygen reduction and carbon monoxide oxidation on the MnN x system. New J. Chem. 2020, 44, 15724–15732. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Li, Q.; Bai, X.; Wang, J. Metal single-atom coordinated graphitic carbon nitride as an efficient catalyst for CO oxidation. Nanoscale 2020, 12, 364–371. [Google Scholar] [CrossRef]
- Hussain, S.; Talib, S.H.; Shahzad, S.R.; Muhammad, S.; Mohamed, S.; Qurashi, A.; Wang, H.; Lu, Z. Heterogeneous Co1/P1Mo12O40 single-atom catalyst for CO oxidation via termolecular Eley-Rideal (TER) mechanism. Mol. Catal. 2023, 550, 113539. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J.; Hafner, J. Ab initio force constant approach to phonon dispersion relations of diamond and graphite. Europhys. Lett. 1995, 32, 729. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Först, C.J.; Schimpl, J. Projector augmented wave method: Ab initio molecular dynamics with full wave functions. Bull. Mater. Sci. 2003, 26, 33–41. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 54104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Qin, Z.; Zhang, G.; Yue, H.; Liang, B.; Luo, D. Dissolution behavior and mechanism of low valence vanadium of vanadium-iron spinel in sulfuric acid solution. J. Mater. Res. Technol. 2021, 12, 1391–1402. [Google Scholar] [CrossRef]
- Kilinc, D. Co complex modified on Eupergit C as a highly active catalyst for enhanced hydrogen production. Int. J. Hydrogen Energy 2022, 47, 11894–11903. [Google Scholar] [CrossRef]
- Lv, J.; Hong, J.; Liang, B.; Zhao, E.; Zeng, K.; Chen, M.; Hu, J.; Yang, G. Study of the curing kinetics of melamine/phthalonitrile resin system. Thermochim. Acta 2020, 683, 178442. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xue, S.; Wu, Y.; Li, Z.; Chang, L. Evaluation of the susceptibility of coal to spontaneous combustion by a TG profile subtraction method. Korean J. Chem. Eng. 2016, 33, 862–872. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, F.; Zhu, Z.; Yang, Y.; Zhao, S.; Chen, J.; Lv, X.; Wang, Y.; Xu, J.; Liu, N. The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation. Appl. Catal. B Environ. 2021, 297, 120393. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, M.; Qian, Y.; Miao, Y.; Ao, Z. Excellent sulfur and water resistance for CO oxidation on Pt single-atom-catalyst supported by defective graphene: The effect of vacancy type. Appl. Surf. Sci. 2021, 566, 150624. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Q.; Hou, Y.; Wang, H.; Zhou, A.; Wang, L.; Cao, G. Unexpected ground-state structures and properties of carbon nitride C3N at ambient and high pressures. Mater. Des. 2018, 140, 45–53. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Yang, J. C3N: A two dimensional semiconductor material with high stiffness, superior stability and bending Poisson’s effect. arXiv 2017, arXiv:1703.08754. [Google Scholar]
- Sakhraoui, T. Effect of vacancy defect and strain on the structural, electronic and magnetic properties of carbon nitride 2D monolayers by DFTB method. J. Phys. Condens. Matter 2023, 35, 324003. [Google Scholar] [CrossRef]
- Makaremi, M.; Mortazavi, B.; Singh, C.V. Adsorption of metallic, metalloidic, and nonmetallic adatoms on two-dimensional C3N. J. Phys. Chem. C 2017, 121, 18575–18583. [Google Scholar] [CrossRef]
- Mizuno, S.; Fujita, M.; Nakao, K. Electronic states of graphitic heterocompounds of carbon, boron and nitrogen. Synth. Met. 1995, 71, 1869–1870. [Google Scholar] [CrossRef]
- Mortazavi, B. Ultra high stiffness and thermal conductivity of graphene like C3N. Carbon 2017, 118, 25–34. [Google Scholar] [CrossRef]
- Ragab, A.H.; Khan, I.; Khan, M.; Yousef, T.A.; Abou-Krisha, M.M.; Kenawy, S.H.; Ansari, M.Z. Efficient oxidation of CO over highly active Al-decorated nitrogenated holey 2D-graphene: A DFT perspective. Inorg. Chem. Commun. 2023, 155, 110977. [Google Scholar] [CrossRef]
- Chang, F.; Li, J.; Kou, Y.; Bao, W.; Shi, Z.; Zhu, G.; Kong, Y. The intense charge migration and efficient photocatalytic NO removal of the S-scheme heterojunction composites Bi7O9I3-BiOBr. Sep. Purif. Technol. 2025, 353, 128402. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Chen, M.; Peera, S.G.; Liu, C.; Yang, H.; Qi, X.; Liu, J.; Liang, T. Catalytic oxidation mechanisms of carbon monoxide over single-and double-vacancy Mn-embedded graphene. New J. Chem. 2020, 44, 9402–9410. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, J.; Ao, Z.; Huang, H.; He, H.; Wu, Y. First principles study on the CO oxidation on Mn-embedded divacancy graphene. Front. Chem. 2018, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, Q.; Yang, X.; Zhang, T.; Li, J. A systematic theoretical study on FeOx-supported single-atom catalysts: M1/FeOx for CO oxidation. Nano Res. 2018, 11, 1599–1611. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Chen, Z. 1 + 1′ > 2: Heteronuclear biatom catalyst outperforms its homonuclear counterparts for CO oxidation. Small Methods 2019, 3, 1800480. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Liu, C.; Qi, X.; Chen, M.; Sagar, R.U.R.; Yang, H.; Liang, T. Single–atom manganese and nitrogen co-doped graphene as low-cost catalysts for the efficient CO oxidation at room temperature. Appl. Surf. Sci. 2021, 536, 147809. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Q.; Liu, J.; Lu, Z. The CO oxidation on Mn atom embedded in N vacancy of C3N monolayer: A DFT-D study. Surf. Sci. 2023, 734, 122320. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Fang, S.; Yao, S. Revealing the differences in CO oxidation activity of Fe-CeO2, Fe2O3, and CeO2 using operando CO2-DRIFTS-MS: Carbonate species and desorption process. J. Alloys Compd. 2025, 1010, 177414. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Myint, M.T.Z.; Göthelid, M.A.; Dutta, J. CO oxidation efficiency and hysteresis behavior over mesoporous Pd/SiO2 catalyst. Catalysts 2021, 11, 131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).