Cryofibrinogenemia in PRECOVID-19 and COVID-19 Periods: Single University Study in Northern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
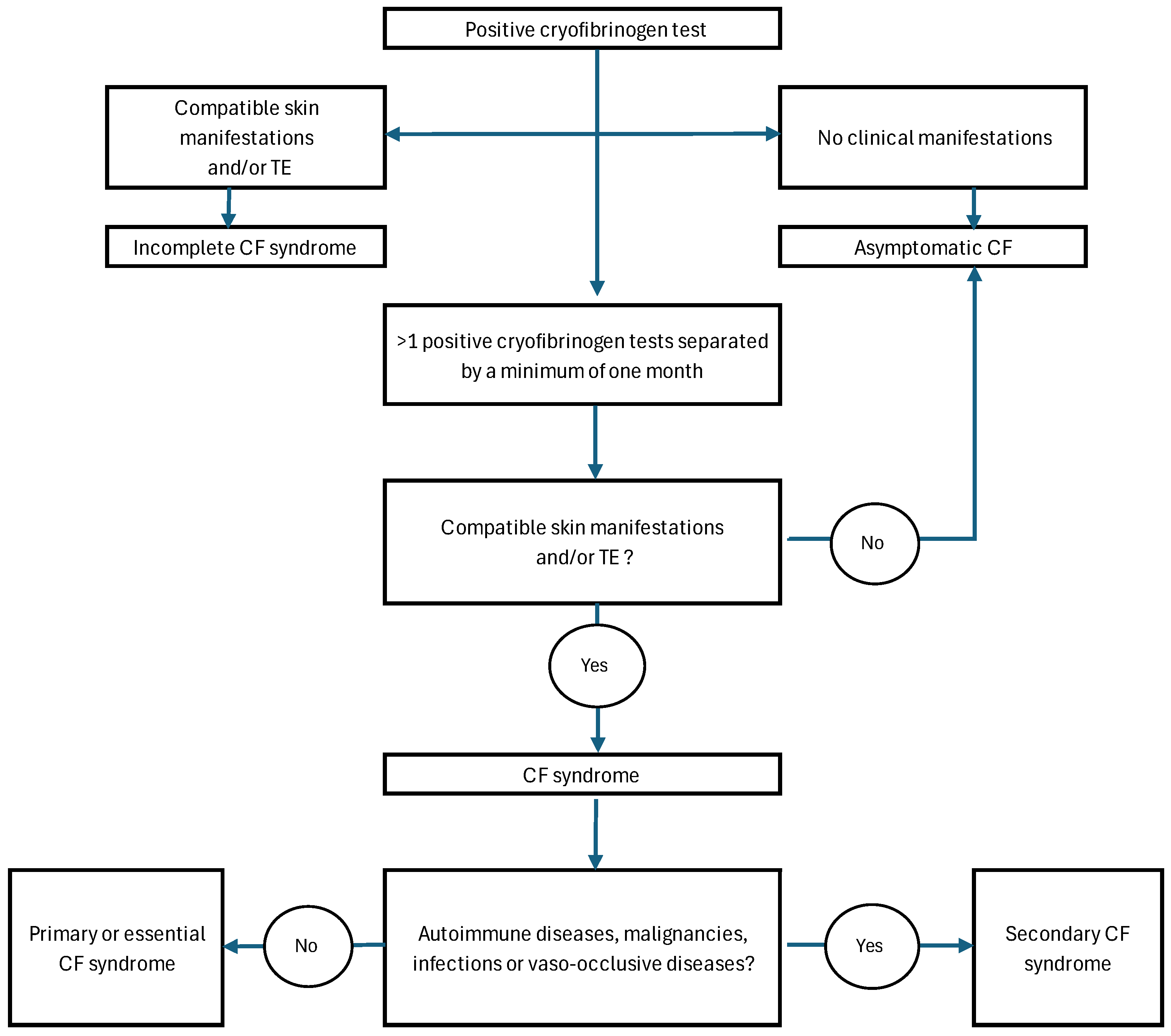
2.3. Clinical Definitions
2.4. Laboratory Tests
2.5. Statistical Analysis
3. Results
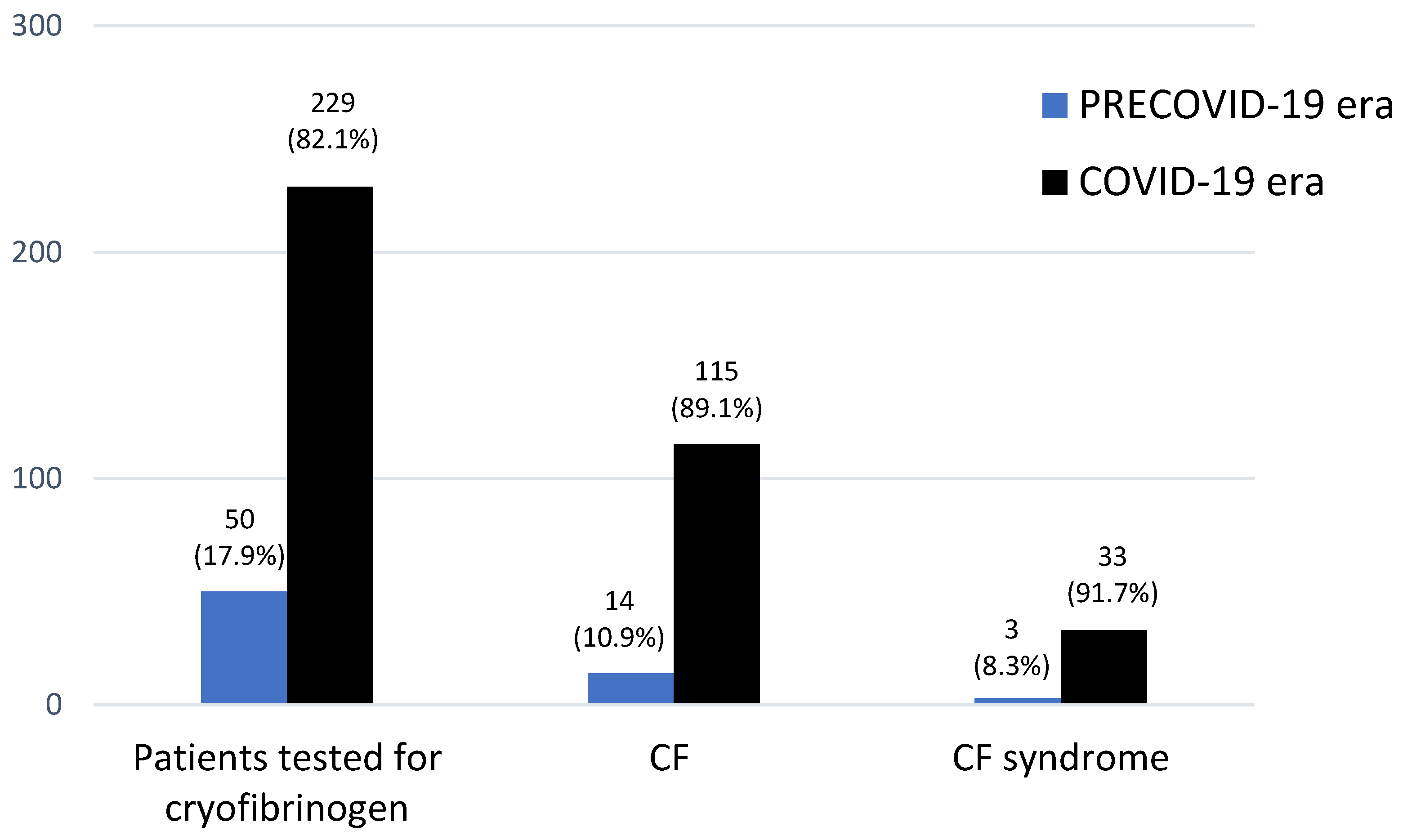
3.1. CF in Both Periods
3.1.1. Frequency, Epidemiology and CVRF
3.1.2. Clinical Features and Severity
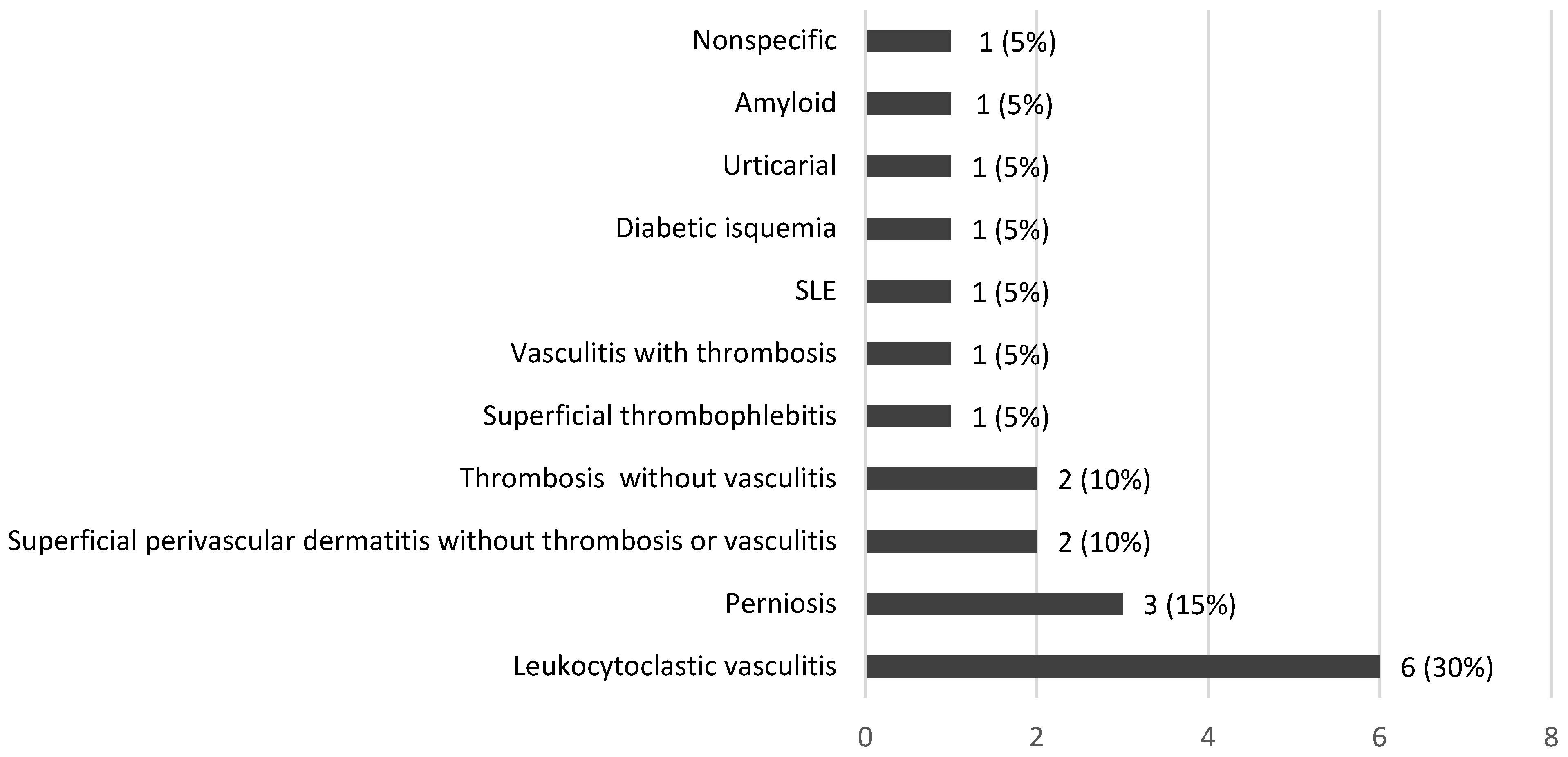
3.1.3. Histopathological Features
3.1.4. Underlying Diseases
3.2. CF Syndrome in Both Periods
3.2.1. Frequency and Epidemiology
3.2.2. Clinical and Histopathological Features and Severity
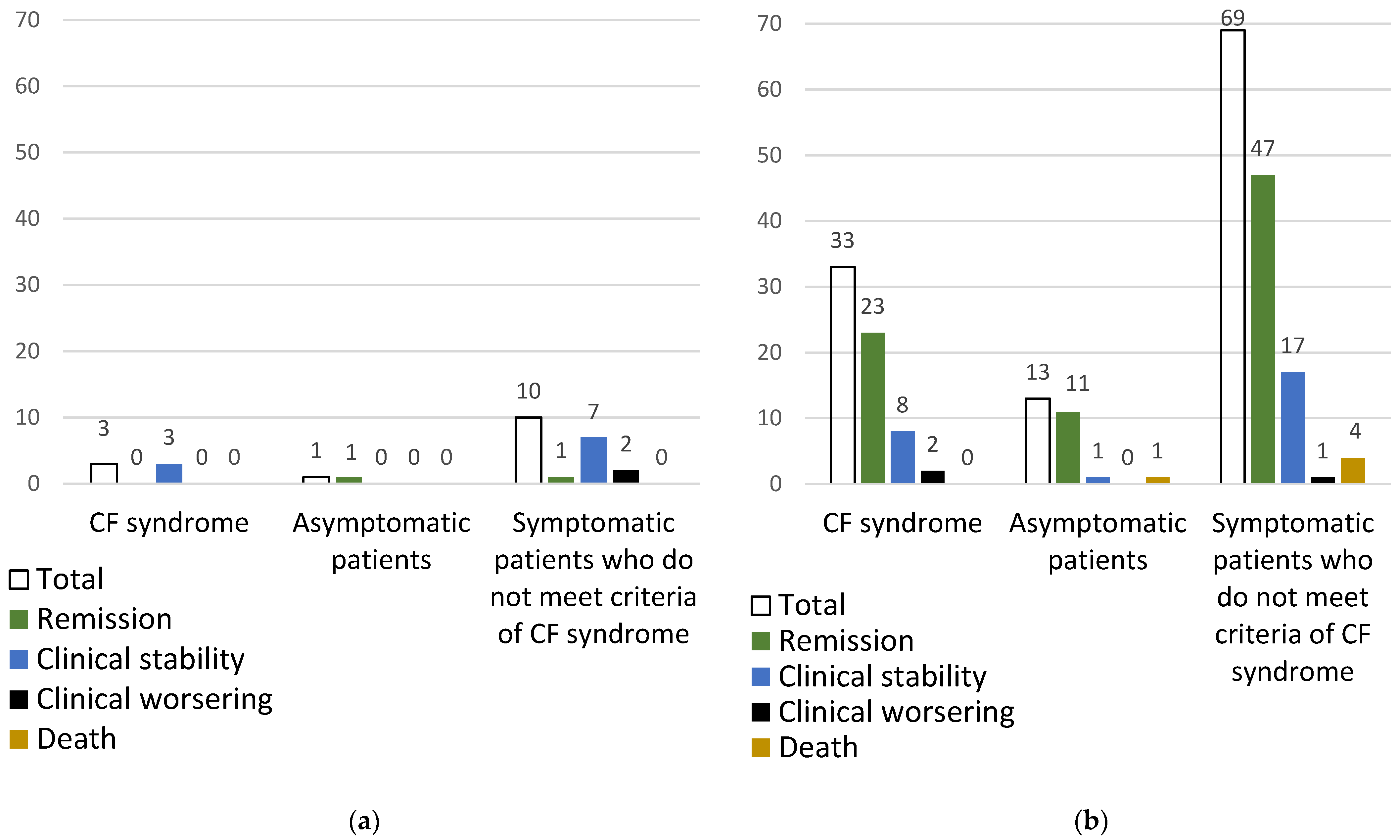
3.2.3. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korst, D.R.; Kratochvil, C.H. Cryofibrinogen in a case of lung neoplasm associated with thrombophlebitis migrans. Blood 1955, 10, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, S.; Luqmani, R.; Novikov, P.; Shevtsova, T. Cryofibrinogenaemia-A neglected disease. Rheumatology 2017, 56, 1445–1451. [Google Scholar] [CrossRef][Green Version]
- Michaud, M.; Pourrat, J. Cryofibrinogenemia. J. Clin. Rheumatol. 2013, 19, 142–148. [Google Scholar] [CrossRef]
- Santiago, M.B.; Melo, B.S. Cryofibrinogenemia: What Rheumatologists Should Know. Curr. Rheumatol. Rev. 2022, 18, 186–194. [Google Scholar] [CrossRef]
- Amdo, T.D.; Welker, J.A. An approach to the diagnosis and treatment of cryofibrinogenemia. Am. J. Med. 2004, 116, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Falanga, V. Cryofibrinogenemia-Induced Cutaneous Ulcers: A Review and Diagnostic Criteria. Am. J. Clin. Dermatol. 2017, 18, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Villani, A.; Fabbrocini, G.; Battista, T. COVID-19 and cutaneous manifestations: A review of the published literature. J. Cosmet. Dermatol. 2023, 22, 4–10. [Google Scholar] [CrossRef]
- Genovese, G.; Moltrasio, C.; Berti, E.; Marzano, A.V. Skin Manifestations Associated with COVID-19: Current Knowledge and Future Perspectives. Dermatology 2021, 237, 1–12. [Google Scholar] [CrossRef]
- Nakashima, C.; Kato, M.; Otsuka, A. Cutaneous manifestations of COVID-19 and COVID-19 vaccination. J. Dermatol. 2023, 50, 280–289. [Google Scholar] [CrossRef]
- Gómez-Fernández, C.; López-Sundh, A.E.; González-Vela, C.; Ocejo-Vinyals, J.G.; Mayor-Ibarguren, A.; Salas-Venero, C.A.; Gutiérrez-Larrañaga, M.; Tejerina-Puente, A.; Fariñas, M.C.; Cabero-Pérez, M.J.; et al. High prevalence of cryofibrinogenemia in patients with chilblains during the COVID-19 outbreak. Int. J. Dermatol. 2020, 59, 1475–1484. [Google Scholar] [CrossRef]
- Polyclonal Rabbit Fibrinogen No de catálogo A0080. Fibrinogen PRA 2010, 2, 44–45.
- Blain, H.; Cacoub, P.; Musset, L.; Costedoat-Chalumeau, N.; Silberstein, C.; Chosidow, O.; Godeau, P.; Frances, C.; Piette, J.C. Cryofibrinogenaemia: A study of 49 patients. Clin. Exp. Immunol. 2000, 120, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Elalamy, I.; Ghillani-Dalbin, P.; Sene, D.; Delluc, A.; Cacoub, P. Cryofibrinogenemia: New insights into clinical and pathogenic features. Am. J. Med. 2009, 122, 1128–1135. [Google Scholar] [CrossRef]
- Belizna, C.C.; Tron, F.; Joly, P.; Godin, M.; Hamidou, M.; Lévesque, H. Outcome of essential cryofibrinogenaemia in a series of 61 patients. Rheumatology 2008, 47, 205–207. [Google Scholar] [CrossRef]
- Soyfoo, M.S.; Goubella, A.; Cogan, E.; Wautrecht, J.C.; Ocmant, A.; Stordeur, P. Clinical significance of cryofibrinogenemia: Possible pathophysiological link with Raynaud’s phenomenon. J. Rheumatol. 2012, 39, 119–124. [Google Scholar] [CrossRef]
- Andina, D.; Noguera-Morel, L.; Bascuas-Arribas, M.; Gaitero-Tristán, J.; Alonso-Cadenas, J.A.; Escalada-Pellitero, S.; Hernández-Martín, Á.; de la Torre-Espi, M.; Colmenero, I.; Torrelo, A. Chilblains in children in the setting of COVID-19 pandemic. Pediatr. Dermatol. 2020, 37, 406–411. [Google Scholar] [CrossRef]
- Smith, S.B.; Arkin, C. Cryofibrinogenemia: Incidence, clinical correlations, and a review of the literature. Am. J. Clin. Pathol. 1972, 58, 524–530. [Google Scholar] [CrossRef]
- McKee, P.A.; Kalbfleisch, J.M. Incidence and significance of cryofibrinogenemia. J. Lab. Clin. Med. 1963, 61, 203–210. [Google Scholar]
- Jager, B.V. Cryofibrinogenemia. N. Engl. J. Med. 1962, 266, 579–583. [Google Scholar] [CrossRef]
- Sarda-Kolopp, M.N.; Miossec, P. Cryofibrinogen-Characteristics and Association with Cryoglobulin: A Retrospective Study out of a Series of 1, 712 Samples over 7 Years. Thromb Haemost. 2022, 123, 669–678. [Google Scholar] [CrossRef]
- Martin, S. Cryofibrinogenemia, monoclonal gammopathy, and purpura. Report of a case and review of the literature. Arch. Dermatol. 1979, 115, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Saida, T.; Hasegawa, R. Cryofibrinogen in the plasma of patients with skin ulcerative lesions on the legs: A complex of fibrinogen and cold insoluble globulin. Thromb. Res. 1976, 9, 541–552. [Google Scholar] [CrossRef]
- Belizna, C.; Loufrani, L.; Subra, J.F.; Godin, M.; Jolly, P.; Vitecocq, O.; Faller, B.; Ghali, A.; Tron, F.; Hamidou, M.; et al. A 5-year prospective follow-up study in essential cryofibrinogenemia patients. Autoimmun. Rev. 2011, 10, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Ireland, T.A.; Werner, D.A.; Rietschel, R.L.; Patterson, J.H.; Spraker, M.K. Cutaneous lesions in cryofibrinogenemia. J. Pediatr. 1984, 105, 67–69. [Google Scholar] [CrossRef]
- Hassan, F.; Khoury, A.; Awad, J.; Jeries, H.; Naffaa, M.E. A very rare cause of blue finger: A case-based review. J. Scleroderma Relat. Disord. 2023, 8, NP1–NP5. [Google Scholar] [CrossRef]
- Terrier, B.; Izzedine, H.; Musset, L.; Ghillani, P.; Deray, G.; Saadoun, D.; Cacoub, P. Prevalence and clinical significance of cryofibrinogenaemia in patients with renal disorders. Nephrol. Dial. Transplant. 2011, 26, 3577–3581. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.G.; Troiano, G.; Cohen, H.; Foadi, M. Acute transient cryofibrinogenemia in infants. J. Pediatr. 1966, 69, 35–39. [Google Scholar] [CrossRef]
- William RGriswold, M.D.; Gilbert Simon, M.D.; Rawle MMcintosh, M.D. Anaphylactoid Purpura, Cold Exposure, and Cryofibrinogenemia. Ann. Intern. Med. 1973, 78, 611. [Google Scholar]
- Rachmilewitz, E.A. Essential cryofibrinogenemia. Clinical, pathological and immunological studies. Isr. J. Med. Sci. 1970, 6, 32–43. [Google Scholar]
- Perry, W.M.; Salame, N.; Swerlick, R.A.; Cheeley, J.T. The clot thickens with COVID-19 and cryofibrinogenemia: A thought-provoking association. JAAD Case Rep. 2022, 24, 24–28. [Google Scholar] [CrossRef]
- Ball, G.V.; Goldman, L.N. Chronic ulcerative colitis, skin necrosis, and cryofibrinogenemia. Ann. Intern. Med. 1976, 85, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.W.; Ross, P., Jr.; Crowson, N.A.; Taylor, J.; Morales, J.E.; Yunger, T.M.; Magro, C. The histopathologic spectrum of cryofibrinogenemia in four anatomic sites: Skin, lung, muscle, and kidney. Am. J. Clin. Pathol. 2003, 119, 114–122. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Gollnick, H.; Weber, S.; Peter, H.H.; Orfanos, C.E. Intravascular coagulation necrosis of the skin associated with cryofibrinogenemia, diabetes mellitus, and cardiolipin autoantibodies. J. Am. Acad. Dermatol. 1991, 25, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kalbfleisch, J.M.; Bird, R.M. Cryofibrinogenemia. N. Engl. J. Med. 1960, 3, 881–886. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 129) | PRECOVID-19 (n = 14) | COVID-19 (n = 115) | p | |
|---|---|---|---|---|
| Sex and age | ||||
| Female/Male, n (%) | 61/68 (47.3/52.7) | 7/7 (50/50) | 54/61 (47/53) | 0.829 |
| Age, years, median [IQR] | 37 [15–67] | 55 [36–75] | 33 [15–67] | 0.054 |
| Cardiovascular Risk Factors, n (%) | 56 (43.4) | 11 (78.6) | 45 (39.1) | 0.005 |
| Hypercholesterolemia | 34 (26.4) | 7 (50) | 27 (23.5) | 0.051 |
| High blood pressure | 33 (25.6) | 6 (42.9) | 27 (23.5) | 0.19 |
| Obesity | 23 (17.8) | 3 (21.4) | 20 (17.4) | 0.715 |
| Hypertriglyceridemia | 19 (14.7) | 3 (21.4) | 16 (13.9) | 0.433 |
| Smoker (past or present) | 16 (12.4) | 4 (28.6) | 12 (10.4) | 0.074 |
| Diabetes Mellitus | 15 (11.6) | 1 (7.1) | 14 (12.2) | 1 |
| Alcohol consumption | 13 (10.1) | 2 (14.3) | 11 (9.7) | 0.633 |
| Other toxics | 2 (1.6) | 0 (0) | 2 (1.7) | 1 |
| Thrombotic Cardiovascular events, n (%) | 17 (13.2) | 2 (14.3) | 15 (13) | 1 |
| VTE (DVT, PE), n (%) | 16 (12.4) | 2 (14.3) | 14 (12.1) | 0.685 |
| AMI, n (%) | 1 (0.8) | 0 (0) | 1 (0.9) | 1 |
| Patients with cutaneous lesions, n (%) | 105 (81.4) | 13 (92.9) | 92 (80) | 0.465 |
| Type of skin lesions | ||||
| Purpuric macules, n (%) | 38 (29.5) | 3 (21.4) | 35 (30.4) | 0.757 |
| Raynaud, n (%) | 30 (23.3) | 6 (42.9) | 24 (20.9) | 0.091 |
| Perniosis, n (%) | 25 (19.4) | 0 (0) | 25 (21.7) | 0.07 |
| Acrocyanosis, n (%) | 19 (14.7) | 1 (7.1) | 18 (15.7) | 0.692 |
| Distal ulceration, n (%) | 14 (10.9) | 5 (35.7) | 9 (7.8) | 0.008 |
| Livedo, n (%) | 5 (3.9) | 2 (14.3) | 3 (2.6) | 0.091 |
| Cold urticaria, n (%) | 1 (0.8) | 0 (0) | 1 (0.9) | 1 |
| Location of skin lesions | ||||
| Feet, n (%) | 38 (29.5) | 3 (21.4) | 35 (30.4) | 0.757 |
| Hands, n (%) | 31 (24) | 4 (28.6) | 27 (23.5) | 0.742 |
| Hands and feets, n (%) | 25 (19.4) | 2 (14.3) | 23 (20) | 1 |
| Other (lower extremities and trunk), n (%) | 11 (8.5) | 4 (28.6) | 7 (6.1) | 0.019 |
| Cold sensitivity | ||||
| Yes, n (%) | 70 (54.3) | 7 (50) | 63 (54.8) | 0.734 |
| No, n (%) | 40 (31) | 7 (50) | 33 (28.7) | 0.129 |
| Not provided, n (%) | 19 (14.7) | 0 (0) | 19 (16.5) | 0.222 |
| Skin biopsy, n (%) | 20 (15.5) | 5 (35.7) | 15 (13) | 0.043 |
| Overall (n = 129) | PRECOVID-19 (n = 14) | COVID-19 (n = 115) | p | |
|---|---|---|---|---|
| Underlying diseases, n (%) | 68 (52.7) | 7 (50) | 61 (53) | 1 |
| SARS-CoV-2 infection, n (%) | 45 (34.9) | 0 (0) | 45 (39.1) | 0.002 |
| Connective tissue disease, n (%) | 13 (10.1) | 2 (14.3) | 11 (9.6) | 0.633 |
| SLE | 1 (0.8) | 1 (7.1) | 0 (0) | 0.109 |
| APS | 2 (1.6) | 1 (7.1) | 1 (0.9) | 0.206 |
| Serological APS | 4 (3.1) | 0 (0) | 4 (3.5) | 1 |
| Undifferentiated | 2 (1.6) | 0 (0) | 2 (1.7) | 1 |
| Under investigation | 4 (3.1) | 0 (0) | 4 (3.5) | 1 |
| Malignancies, n (%) | 7 (5.4) | 1 (7.1) | 6 (5.2) | 1 |
| Vasculitis, n (%) | 5 (3.9) | 3 (21.4) | 2 (1.7) | 0.009 |
| Buerger, n (%) | 2 (1.6) | 2 (14.3) | 0 (0) | 0.011 |
| Henoch-Schoenlein Purpura, n (%) | 3 (1.6) | 1 (7.1) | 2 (1.7) | 0.294 |
| Vaso-occlusive diseases, n (%) | 2 (1.6) | 1 (7.1) | 1 (0.9) | 0.206 |
| Leriche´s syndrome, n (%) | 1 (0.8) | 0 (0) | 1 (0.9) | 1 |
| CRVT, n (%) | 1 (0.8) | 1 (7.1) | 0 (0) | 0.109 |
| Overall (n = 36) | PRECOVID-19 (n = 3) | COVID-19 (n = 33) | p | |
|---|---|---|---|---|
| Sex and age | ||||
| Female/Male, n (%) | 18/18 (50/50) | 2/1 (67/33) | 16/17 (48.5/51.5) | 1 |
| Age, years, median [IQR] | 28.5 [30] | 51 [40–56] | 17 [13–41] | 0.045 |
| Diagnosis of CF syndrome | ||||
| Essential, n (%) | 15 (41.7) | 2 (66.7) | 13 (39.4) | 0.559 |
| Secondary, n (%) | 21 (58.3) | 1 (33.3) | 20 (60.6) | 0.559 |
| Underlying diseases, n (%) | 20 (55.6) | 1 (33.3) | 19 (57.6) | 0.574 |
| SARS-CoV-2 infection, n (%) | 16 (44.4) | 0 (0) | 16 (48.5) | 0.238 |
| Connective tissue disease, n (%) | 3 (8.3) | 0 (0) | 3 (9) | 1 |
| APS, n (%) | 1 (2.7) | 0 (0) | 1 (3) | 1 |
| Others, n (%) | 2 (5.5) | 0 (0) | 2 (6) | 1 |
| Vasculitis, n (%) | 1 (2.7) | 1 (33.3) | 0 (0) | 0.083 |
| Buerger, n (%) | 1 (2.7) | 1 (33.3) | 0 (0) | 0.083 |
| Malignancies, n (%) | 1 (2.7) | 0 (0) | 1 (3) | 1 |
| Thrombotic Cardiovascular events, n (%) | 4 (11.1) | 0 (0) | 4 (12.1) | 1 |
| DVT | 3 (8.3) | 0 (0) | 3 (9) | 1 |
| AMI | 1 (2.7) | 0 (0) | 1 (3) | 1 |
| Patients with cutaneous lesions, n (%) | 35 (97.2) | 3 (100) | 32 (97) | 1 |
| Type of skin lesions | ||||
| Purpuric macules, n (%) | 16 (44.4) | 1 (33.3) | 15 (45.5) | 1 |
| Raynaud, n (%) | 9 (25) | 2 (66.7) | 7 (21.2) | 0.148 |
| Perniosis, n (%) | 7 (19.4) | 0 (0) | 7 (21.2) | 1 |
| Acrocyanosis, n (%) | 7 (19.4) | 0 (0) | 6 (18.2) | 1 |
| Distal ulceration, n (%) | 2 (5.5) | 0 (0) | 2 (6) | 1 |
| Livedo, n (%) | 2 (5.5) | 0 (0) | 2 (6) | 1 |
| Location of skin lesions | ||||
| Feet, n (%) | 18 (51.4) | 0 (0) | 18 (54.5) | 0.229 |
| Hands, n (%) | 9 (25.7) | 3 (100) | 6 (18.2) | 0.012 |
| Hands and feets, n (%) | 8 (22.9) | 0 (0) | 8 (24.2) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez López, M.d.A.; Lasa-Teja, C.; Renuncio-García, M.; Abraira-Meriel, C.; Simón-Coloret, S.; Bertomeu-Genis, I.; Martín-Gutiérrez, A.; Secada-Gómez, C.; González-Vela, C.; Irure-Ventura, J.; et al. Cryofibrinogenemia in PRECOVID-19 and COVID-19 Periods: Single University Study in Northern Spain. Sci 2025, 7, 81. https://doi.org/10.3390/sci7020081
Sánchez López MdA, Lasa-Teja C, Renuncio-García M, Abraira-Meriel C, Simón-Coloret S, Bertomeu-Genis I, Martín-Gutiérrez A, Secada-Gómez C, González-Vela C, Irure-Ventura J, et al. Cryofibrinogenemia in PRECOVID-19 and COVID-19 Periods: Single University Study in Northern Spain. Sci. 2025; 7(2):81. https://doi.org/10.3390/sci7020081
Chicago/Turabian StyleSánchez López, María del Amparo, Carmen Lasa-Teja, Mónica Renuncio-García, Cristina Abraira-Meriel, Saray Simón-Coloret, Inmaculada Bertomeu-Genis, Adrián Martín-Gutiérrez, Carmen Secada-Gómez, Carmen González-Vela, Juan Irure-Ventura, and et al. 2025. "Cryofibrinogenemia in PRECOVID-19 and COVID-19 Periods: Single University Study in Northern Spain" Sci 7, no. 2: 81. https://doi.org/10.3390/sci7020081
APA StyleSánchez López, M. d. A., Lasa-Teja, C., Renuncio-García, M., Abraira-Meriel, C., Simón-Coloret, S., Bertomeu-Genis, I., Martín-Gutiérrez, A., Secada-Gómez, C., González-Vela, C., Irure-Ventura, J., López-Hoyos, M., González-López, M. A., & Blanco, R. (2025). Cryofibrinogenemia in PRECOVID-19 and COVID-19 Periods: Single University Study in Northern Spain. Sci, 7(2), 81. https://doi.org/10.3390/sci7020081







